Abstract
Chlamys farreri is primarily cultivated in Japan, China, and South Korea. Although mass mortality of scallops has been occurring recently, likely caused by high temperatures or infectious diseases, the underlying cause remains unclear. Little is known regarding the viral diseases affecting them. Therefore, we explored DNA virus diversity in the mid-gut gland of C. farreri and compared it with that of seawater. C. farreri was cultivated at depths below 5 m from the sea surface in the coastal waters of South Korea and sampled from May to August 2018. Different DNA viral communities were observed in both C. farreri and seawater. In C. farreri, prevalent groups included Mimiviridae (7%), Poxviridae (6%), and Phycodnaviridae (5%). Conversely, the dominant groups in seawater were Autographiviridae (20%), Kyanoviridae (12%), and Zobellviridae (10%). We identified C. farreri-specific viral communities and potentially infectious viruses, such as Ostreid herpesvirus 1 and Abalone herpesvirus Victoria/AUS/2009. Furthermore, C. farreri acts as a reservoir for various viruses, which impact microbial community dynamics and disease transmission in marine ecosystems. Understanding these viral communities is crucial to protecting and restoring coastal ecosystems by highlighting their role in the transmission of potential avian- and bivalve-specific viruses.
1. Introduction
Bivalves, especially clams, mussels, and oysters, are crucial in global aquatic food production [1], and 2.1 million tonnes (USD 5.3 million) of scallops were cultured through marine aquaculture in 2016 [2]. However, changes in the environment and mass farming lead to mass mortality in coastal aquaculture, and the emergence of pathogens through anthropic activities is one of the primary causes of mortality. For example, mass mortality of scallops began in China in the 1980s and continued for several years in the 1990s [3,4]. The bivalve shellfish farming industry has experienced enormous economic damage owing to various diseases. For example, infection with Herpes-like viruses has been reported in multiple marine bivalve molluscs worldwide [5,6,7,8,9], and mass mortality has recently increased in the Republic of Korea and China [10,11].
Chlamys farreri [12] is one of the largest cultured bivalves in north Asian coastal areas such as China, Japan, and Korea [13,14,15]. Various factors, such as viral infection, climate change, and physiological stress caused by mass farming, increase the mortality rate of C. farreri [16]. The mortality of shellfish is caused by various pathogens [17,18,19,20]. For example, Acute Viral Necrosis Virus, Scallop Picorna-like virus, Gill necrosis virus, Hemocyte infection virus, Oyster velar virus disease, and Haliotid herpesvirus-1 are known to infect bivalve organisms [21,22,23]. Herpesviruses are ubiquitously distributed worldwide and can infect marine bivalve molluscs. These viruses have adapted their infection mechanisms to a single host after a long period of co-evolution [24]; however, the host specificity of these viruses is unknown [25,26]. Ostreid herpesvirus 1 (OsHV-1) belongs to the Herpesviridae family and can kill various bivalve molluscs such as oysters (Crassostrea gigas), scallops (Chlamys farreri), and clams (Scapharca brokenonii) [22,27,28,29]. Thus, these herpesviruses can cause mass scallop mortality because of viral bioaccumulation in the digestive glands through filter feeding [30]. However, research on how viruses in seawater differ based on their concentration in the digestive tract of filter-feeding bivalve molluscs is limited. The epidemiology of infectious viruses in bivalves was studied by (1) studying the viruses in ambient seawater to determine whether they are transmitted from scallop to other scallops and (2) studying changes in infectious viruses in the scallop. Therefore, the aim of this study was to elucidate the epidemiological importance of DNA viruses in the gastrointestinal microbiome of C. farreri by investigating the monthly changes in DNA viruses in scallops and the differences in the diversity of ambient seawater viruses through metagenomics.
2. Materials and Methods
2.1. Study Design and Sample Collection
In situ experiments were performed at the Tongyeong Megacosm test station (34°46′11.0″ N 128°22′40.6″ E) in the Southern Sea of South Korea (Figure 1). This C. farreri aquaculture area has clean water and a muddy and sandy bottom layer with a water depth of approximately 30 m. C. farreri were acclimatised in lantern nets at a depth of 5 m from the sea surface. Each net contained 60 individuals. The juveniles had an initial mean shell height of 3.7 ± 0.56 cm at the start of the experiment (January 2018). Culturing was performed without collection from February to April after initiating C. farreri farming in January, considering growth. Eight nets were installed each month (May to December 2018) to minimise the stress caused by air exposure when collecting C. farreri. After deployment, C. farreri were sampled monthly. The scallops were placed in plastic bags after the attached organisms and particles in the shell surface, such as mud, attached seaweeds, and other organisms, were removed. C. farreri were then stored in a portable freezer at −20 °C and transported to the laboratory. In the laboratory, the surface of the shell was washed with sterilised seawater, and the height and width of the shell were measured using vernier calliper (Table S1). The mid-intestinal glands (MGs), the digestive organs of C. farreri, from 15 individuals were pulverised using a homogeniser after freezing with liquid nitrogen to determine viral communities in the MGs of live C. farreri. In total, 30 individuals were obtained for triplicate experiments. The samples collected from the MGs were stored at −20 °C for no longer than two days. Ambient seawater samples at a depth of 5 m under the surface obtained from the cultivation sampling site were collected using a 10 L Niskin-type water sampler (General Oceanics, Miami, FL, USA) from May to December 2018. To analyse the DNA viral community, 25 L of seawater was collected for each experiment, and the experiments were performed in triplicate. The seawater was placed in sterile polypropylene bottles and stored in a 4 °C ice cooler until transported to the laboratory (Travel time: approximately 1 h). MGs were collected, and genomic DNA (gDNA) in the MG samples was extracted within two days. The EXO2 Multiparameter Sonde (YSI, Yellow Springs, OH, USA) measured water temperature and salinity at the sampling site. DNA viruses were harvested by collecting 25 L of seawater using FeCl3-based flocculation, filtration, and resuspension methods. To aggregate DNA viruses with Fe3+ ions, the mixture was maintained in a reservoir (cylindrical, made of black-coloured acrylic material) at 20 °C for 1 h. The DNA viruses aggregated with Fe3+ ions were collected onto a 0.2 μm polycarbonate membrane (Ca No. 111106; 47 mm; Whatman, Buckinghamshire, UK) for gDNA extraction from the DNA virus population (Figure 1C) [31,32]. The membranes were stored at 4 °C, and gDNA in membrane samples were extracted within two days.

Figure 1.
Photos of the field cultivation site of Chlamys farreri at the Tongyeong Megacosm Test Station at the Korea Institute of Ocean Science and Technology (A) and sampling photos of C. farreri acclimating to the lantern net in May (top photo) and August (down photo) (B). Flow chart illustrating the metavirome analysis process of seawater and C. farreri from sample collection to bioinformatics analysis (C).
2.2. Metavirome Analyses of Chlamys farreri and Seawater
The DNA metavirome analysis in seawater and scallops was performed by following our previous results [32]. The total viral gDNA from C. farreri and seawater was extracted using the Viral Gene-spin Viral DNA/RNA Extraction Kit (iNtRON Biotechnology, Seoul, Republic of Korea). Metagenomic libraries were constructed using the NEBNext Ultra II DNA Library Prep Kit (Illumina, San Diego, CA, USA) and sequenced using the Illumina HiSeq 2500 platform. The raw sequence data were trimmed to remove inappropriate sequences using CLC Genomics Workbench v. 20.0.4 (Qiagen, Hilden, Germany). Reads were assembled using metaSPAdes v. 3.13.0 [33], and viral contigs of >1000 bp were extracted from the total contigs using Check V v. 1.0.1 [34]. These viral contig sequences with >95% nucleotide identity were selected using VSEARCH [35]. Read mapping was performed with BBMap v. 38.51 using a 95% minimum alignment identity [36]. The quality-checked viral contigs were subjected to a virus taxonomy analysis using Basic Local Alignment Search Tool (BLASTn) analysis with the Microbial Genomic Module in the CLC Genomics Workbench with the Viral RefSeq database (Release 221) of the National Center for Biotechnology Information.
2.3. Statistical Analysis
The results are presented as the mean of replicate samples. Hierarchical cluster analysis and non-metric multi-dimensional scaling (NMDS) using Bray–Curtis dissimilarity were performed using PRIMER 6 (v 6.1.13) to elucidate the difference in the DNA viral community between C. farreri and seawater. A Venn diagram was created using the ggplot2 function [37] in R Studio to analyse the differences in DNA virus composition between seawater and scallops as well as those between different months of sample collection. A heatmap of the relative abundance of common DNA viruses (mean value: >0.1%) between C. farreri and seawater was created to visualise the differences using ggplot2. The Shannon diversity index was analysed using the vegan package (v 2.6-6.1) in R Studio. A Wilcoxon test was performed to examine the differences among groups of herpesviruses and Ostreid herpesvirus 1.
3. Results and Discussion
Changes in the viral community were investigated through intestinal microbiome analysis along with the growth of C. farreri from May to December 2018, but all scallops died in September; therefore, the experiment was conducted until August (Figure 1B). However, the viral community in seawater was observed continuously until December. Taxonomic analysis of contigs in the genome fragments confirmed their identity as DNA viruses. In total, 1263 and 9525 DNA virus contigs were detected in C. farreri and seawater, respectively, through metavirome analysis (Table S2). The metagenomic analyses were based on triplicate experiments, but some samples were excluded during the study because they failed the quality check. NMDS analysis was used to classify the samples at a similarity of 40% between DNA viral communities of C. farreri and of seawater (Figure 2A). In seawater, the most highly occurring classified viral communities (at the family level) were Autographiviridae (mean: 20%), Kyanoviridae (12%), and Zobellviridae (10%). In comparison, viruses in C. farreri were primarily Mimiviridae (7%), Poxviridae (6%), and Phycodnaviridae (5%). These results indicate a remarkable difference in the viral community between seawater and C. farreri. In the Venn diagram, 270 viral operational taxonomic units (vOTUs) commonly appeared in seawater and C. farreri, 1077 vOTUs only appeared in seawater, and 421 vOTUs only appeared in C. farreri (Figure 2B). In addition, the diversity index of the viral community in scallops was 5.60, which was higher than that in seawater (4.66) (Figure 2C). The high viral diversity observed in scallops aligns with previous findings [22,38,39]. As filter feeders, scallops consume various eukaryotic planktons and microorganisms found in seawater, resulting in a heightened detection of virus types compared to that in seawater. Destoumieux-Garzón [40] reported that bivalves concentrate microorganisms from the surrounding seawater. The microbial community within bivalves is highly distinct from the microbiome found in the surrounding seawater.
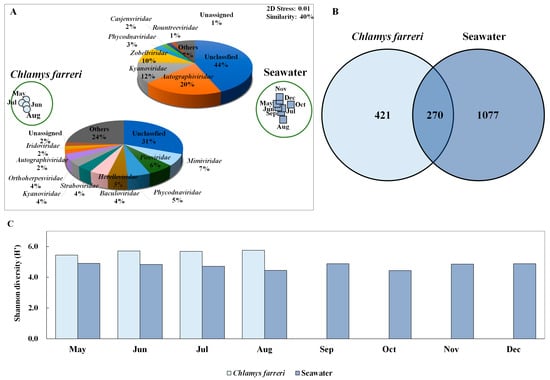
Figure 2.
Non-metric multi-dimensional scale (NMDS) plot based on the results of Bray–Curtis dissimilarity analysis showing the diversity of the marine dsDNA virus community in Chlamys farreri and seawater (A). Pie charts depicting the high-ranking taxonomic distribution at the family level for the viral community. (B) Venn diagram showing the shared and unique total scallop and marine viral operational taxonomic units (vOTUs) (C). Shannon diversity index illustrating changes in dsDNA virus community in C. farreri and seawater.
In C. farreri, the viral community was divided into two groups of “in May” and “between June and August”. The major families in the two groups of viruses were the same; however, their proportions differed (Figure 3A). The common viruses that appeared > 0.1% in the viral community were 302 vOTUs, which accounted for 80.54% of the total relative abundances. The predominant viruses were Acanthamoeba polyphaga moumouvirus, Orpheovirus IHUMI-LCC2, Megavirus chiliensis, A. polyphaga mimivirus, and Cafeteria roenbergensis virus BV-PW1. These viruses belong to Mimiviridae and Orpheoviridae. (Figure 3B). These viruses typically infect eukaryotic plankton [30]. We investigated the hosts of viruses belonging to known infectious families but found no viruses known to infect bivalves. However, members of the Iridoviridae family are known to infect amoebas, and recent studies suggest that the genera Ranavirus and Megalocytivirus may be associated with infections in various marine species [41]. Notably, particles resembling an irido-like virus have been observed in oysters, specifically Crassostrea angulate [42]. These findings suggest that not only the Herpesviridae but also Iridoviridae and Poxviridae may have pathogenic potential in scallops. A hypothesis explaining their dominance in the MGs is that the primary food sources of scallops are plankton of various sizes, which, when concentrated through filter feeding, may promote viral proliferation in the scallop intestines due to eukaryotic plankton viral infections. Bivalve tissues comprise complex systems with distinct microbiome compartments, including viral, bacterial, and eukaryotic communities [43]. Viruses within bivalves form specific communities in the microbiome [30,40], with external environmental factors remarkably influencing viral composition differences [44]. The results suggest that the viral community originates from seawater and is transmitted to scallops, closely associated with bivalve host lineages. For example, the abundance of species belonging to Phycodnaviridae, which have a strong relationship with phytoplankton, observed in C. farreri suggests that they may have entered the intestine through filter feeding of the bivalve. Moreover, these predominant viruses may favour scallops as their preferred habitat. Filter-feeding bivalves serve as potential reservoirs for disseminating infectious marine viruses [30]. The viruses within bivalves are not random but inhabit specific populations. Imbalance in the host’s health and environmental conditions can lead to disease and death in the host [44,45].
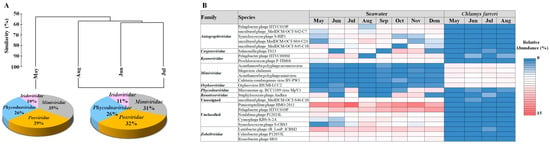
Figure 3.
Hierarchical agglomerative clustering performed based on the group abundance of the Chlamys farreri (A). Pie charts indicate the high-ranking taxonomic distribution for the viral community. (B) Heatmap displaying the relative abundance of dominant taxa occurring at 1% or greater in the virus community.
Herpesviruses infect scallops and have high host specificity for these organisms [22,23]. In C. farreri and seawater, herpesviruses were detected at 36 and 17 vOTUs, respectively (Table S3). The mean relative abundances of Herpesviridae in C. farreri and seawater were 4.30% and 0.34%, respectively. The prevalence of Herpesviridae increased in the “June to August” group compared to in May (Figure 4A,B), suggesting that the infectivity and transmissibility of herpesviruses escalate with rising water temperatures [46]. While research on marine herpesviruses in bivalves is commonly conducted in the laboratory [47], field studies on viral immunology, specific primers, and bivalve microbiomes are scarce in marine ecological studies. Investigating the environmental interplay between scallops and seawater will yield crucial insights into infection transmission and potential infectious viruses. Furthermore, the emergence and activity of any virus can considerably affect disease progression in its host and reflect the growth and overall health status of the host [32].
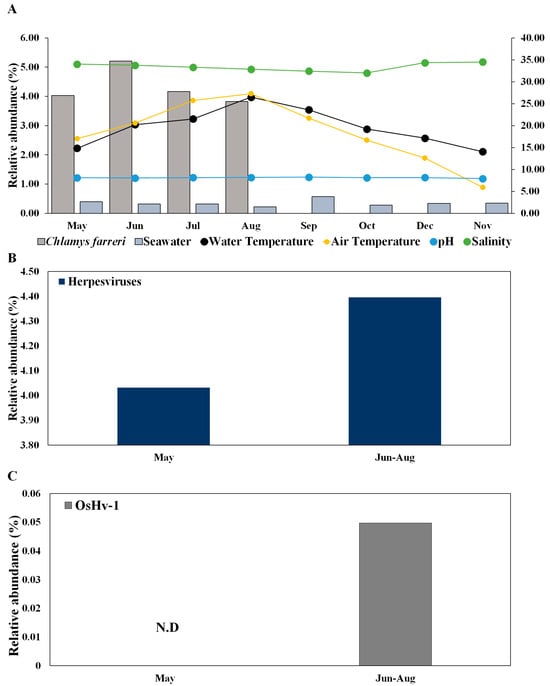
Figure 4.
Change in the relative abundance of herpesviruses in Chlamys farreri and seawater along with environmental factors (A) and comparison between “May” group and “June to August” group of herpesviruses (B) and Ostreid herpesvirus 1 (C) in Chlamys farreri. There was no significant difference between the two groups using the Wilcoxon test in (B,C).
Herpesviruses are particularly increased during summer mass mortality [18,27,48,49]. Herpesviruses were not detected in seawater, but these viruses detected in C. farreri exhibited high substrate specificity and were ubiquitously distributed throughout the study area. This study provides novel insights into the significant associations between C. farreri and seawater in terms of ecological emergence. OsHV-1 occurs primarily in response to elevations in temperature and light intensity caused by increased seawater temperature [19]. OsHV-1 is known to infect bivalves, which has negative implications for aquaculture [28,50,51]. In addition to OsHV-1, Abalone herpesvirus Victoria/AUS/2009 emerged. These species are known to infect bivalves such as abalone, and our results suggest that they emerged in May and may be potential infectious viruses [52]. Although the roles of scallops as reservoirs of infection for subsequent mortality outbreaks remain unclear, OsHV-1 was observed in C. farreri during the summer months (July), and all C. farreri died in September owing to the combined effects of high temperature and infection with OsHV-1 in this study (Figure 4C).
In conclusion, in this study, we suggest that C. farreri is a reservoir of high viral diversity, and its mass mortality is affected by the environment and viral infections in mass farming [53]. The detection of bacteriophages strongly suggests the presence of specific bacteria in the gut of scallops. Since bacteriophages have a close relationship with their bacterial hosts, this indicates that these bacteria may be actively involved in the gut microbiome of the scallops [54]. Furthermore, due to the filter feeding behaviour of scallops, various microorganisms and eukaryotic plankton (e.g., bacteria and algae) can accumulate from seawater, which may have significant impacts on the physiological or ecological status of the scallops [30]. Bacteriophages may also alter this gut ecosystem or interact with specific bacteria, potentially leading to pathological or health-related changes [55]. Furthermore, discovering various potential algae viruses highlights their possible role as infection hotspots. Therefore, studies on the structure and function of viral communities in bivalves will significantly enhance our understanding of their role in microbial community regulation, disease transmission, and the potential to protect and restore coastal ecosystems. This study preliminarily identified specific viral characteristics in C. farreri by comparing viral communities between scallops and seawater. However, further studies are planned to conduct an in-depth viral characterisation, including identifying specific viruses and their genetic makeup, exploring virus–host interactions such as disease mechanisms and potential control strategies, as well as investigating the ecological impact of viral infections in bivalves and their food sources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13110935/s1, Table S1: Size meausrment of Chlamys farreri (Width, Height and Weight); Table S2: Summary of metavirome data (the total bases, reads, GC (%), Q20 (%) and Q30 (%), contigs); Table S3: Presented lists of herpesviruses and their relative abundance (%) found in Chlamys farreri and seawater, along with species identification information (Average Contigs and Average Similarity).
Author Contributions
J.W.S.: writing—original draft, methodology, visualisation, formal analysis, and data curation. K.E.K.: writing—original draft, methodology, visualisation, formal analysis, and data curation. J.S.P.: writing—review and editing and methodology. M.-J.K.: writing—review and editing, methodology, and data curation. T.-K.L.: writing—review and editing, methodology, and funding acquisition, conceptualisation. H.-J.K.: writing—review and editing, methodology, and data curation. Y.J.K.: methodology and data curation. S.M.K.: methodology and data curation. S.W.J.: writing—original draft, project administration, methodology, investigation, funding acquisition, and conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a research project (PEA0204) funded by the Korea Institute of Ocean Science and Technology of the Republic of Korea and the Korea Institute of Marine Science & Technology Promotion (KIMST), both funded by the Ministry of Oceans and Fisheries, Korea (RS-2021-KS211475).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequencing data (Fastq files) are available on the Sequence Read Archive public database of NCBI under the project number PRJNA1149925 (in scallops) and PRJNA1131106 (in seawater).
Acknowledgments
We would like to thank Young Wook Lee, Seogil Jang, and Ilhyung Jung for help with the in situ cultivation of Chlamys farreri at the Tongyeong Megacosm Test Station of the Korea Institute of Ocean Science and Technology, Republic of Korea. The genomic DNA samples were stored in the Library of Marine Samples of KIOST.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Koelmans, B.; Pahl, S.; Backhaus, T.; Bessa, F.; van Calster, G.; Contzen, N.; Cronin, R.; Galloway, T.; Hart, A.; Henderson, L. Science Advice for Policy by European Academies: A Scientific Perspective on Microplastics in Nature and Society; SAPEA: Berlin, Germany, 2019. [Google Scholar]
- FAO. Food and Agriculture Organization of the United Nations Statistical Database (FAOSTAT); FAO: Rome, Italy, 2018. [Google Scholar]
- Xiao, J.; Ford, S.E.; Yang, H.; Zhang, G.; Zhang, F.; Guo, X. Studies on Mass Summer Mortality of Cultured Zhikong Scallops (Chlamys farreri Jones et Preston) in China (Chlamys farreri Jones et Preston). Aquaculture 2005, 250, 602–615. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, C.; Li, C.; Wang, C.; Xu, Z.; Yan, P. Haemocyte Protein Expression Profiling of Scallop Chlamys farreri Response to Acute Viral Necrosis Virus (AVNV) Infection. Dev. Comp. Immunol. 2011, 35, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Farley, C.A.; Banfield, W.G.; Kasnic, G.; Foster, W.S. Oyster Herpes-Type Virus. Science 1972, 178, 759–760. [Google Scholar] [CrossRef]
- Hine, P.; Thorne, T. Replication of Herpes-Like Viruses in Haemocytes of Adult Flat Oysters Ostrea Angasi: An Ultrastructural Study. Dis. Aquat. Organ. 1997, 29, 189–196. [Google Scholar] [CrossRef]
- Renault; Arzul. Herpes-Like Virus Infections in Hatchery-Reared Bivalve Larvae in Europe: Specific Viral DNA Detection by PCR. J. Fish Dis. 2001, 24, 161–167. [Google Scholar] [CrossRef]
- Vásquez-Yeomans, R.; Cáceres-Martínez, J.; Huerta, A.F. Herpes-Like Virus Associated with Eroded Gills of the Pacific Oyster Crassostrea gigas in Mexico. J. Shellfish Res. 2004, 23, 417–419. [Google Scholar]
- Chongluo, F.; Weibo, S.; Yun, L. Monoclonal antibodies developed for detection of an epizootic virus associated with mass mortalities of cultured scallop Chlamys farreri. Dis Aquat Organ 2005, 65, 17–22. [Google Scholar] [CrossRef]
- Song, W.B.; Wang, C.M.; Wang, X.H.; Li, Y. New Research Porgress on Massive Mortality of Cultured Scallop Chlamys farreri. Mar. Sci. 2001, 25, 23–27. [Google Scholar]
- Evans, O.; Hick, P.; Whittington, R.J. Distribution of Ostreid herpesvirus-1 (OsHV-1) Microvariant in Seawater in a Recirculating Aquaculture System. Aquaculture 2016, 458, 21–28. [Google Scholar] [CrossRef]
- Jones, K.H.; Preston, H.B. List of Mollusca Collected During the Commission of H.M.S. “Water Witch” in the China Seas, 1900–1903, with Description of New Species. J. Molluscan Stud. 1904, 6, 138–151. [Google Scholar] [CrossRef][Green Version]
- Zhan, A.H.; Hu, J.; Hu, X.; Zhou, Z.; Hui, M.; Wang, S.; Peng, W.; Wang, M.; Bao, Z. Fine-Scale Population Genetic Structure of Zhikong Scallop (Chlamys farreri): Do Local Marine Currents Drive Geographical Differentiation? Mar. Biotechnol. 2009, 11, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pan, L.; Yang, Y.; Zhou, Y. Characterizing Transcriptome in Female Scallop Chlamys farreri Provides New Insights into the Molecular Mechanisms of Reproductive Regulation During Ovarian Development and Spawn. Gene 2020, 758, 144967. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-W.; Oh, S.-Y.; Park, J.J.C.; Jung, Y.-H.; Kim, H.-J.; Choi, D.M.; Choi, Y.-U.; Han, J. Offshore Wind Farms in South Korea: A Potential Site for Scallop Culture. J. Mar. Sci. Eng. 2023, 11, 1988. [Google Scholar] [CrossRef]
- Tang, B.; Liu, B.; Wang, X.; Yue, X.; Xiang, J. Physiological and Immune Responses of Zhikong Scallop Chlamys farreri to the Acute Viral Necrobiotic Virus Infection. Fish Shellfish Immunol. 2010, 29, 42–48. [Google Scholar] [CrossRef]
- Burge, C.A.; Griffin, F.J.; Friedman, C.S. Mortality and Herpesvirus Infections of the Pacific Oyster Crassostrea gigas in Tomales Bay, California, USA. Dis. Aquat. Organ. 2006, 72, 31–43. [Google Scholar] [CrossRef]
- Sauvage, C.; Pépin, J.F.; Lapègue, S.; Boudry, P.; Renault, T. Ostreid Herpes Virus 1 Infection in Families of the Pacific Oyster, Crassostrea gigas, During a Summer Mortality Outbreak: Differences in Viral DNA Detection and Quantification Using Real-Time PCR. Virus Res. 2009, 142, 181–187. [Google Scholar] [CrossRef]
- Garcia, C.; Thébault, A.; Dégremont, L.; Arzul, I.; Miossec, L.; Robert, M.; Chollet, B.; François, C.; Joly, J.-P.; Ferrand, S.; et al. Ostreid Herpesvirus 1 Detection and Relationship with Crassostrea gigas Spat Mortality in France Between 1998 and 2006. Vet. Res. 2011, 42, 73. [Google Scholar] [CrossRef]
- Pernet, F.; Barret, J.; Le Gall, P.; Corporeau, C.; Dégremont, L.; Lagarde, F.; Pépin, J.F.; Keck, N. Mass Mortalities of Pacific Oysters Crassostrea gigas Reflect Infectious Diseases and Vary with Farming Practices in the Mediterranean Thau Lagoon, France. Aquacult. Environ. Interact. 2012, 2, 215–237. [Google Scholar] [CrossRef]
- Bai, C.; Wang, C.; Xia, J.; Sun, H.; Zhang, S.; Huang, J. Emerging and Endemic Types of Ostreid Herpesvirus 1 Were Detected in Bivalves in China. J. Invertebr. Pathol. 2015, 124, 98–106. [Google Scholar] [CrossRef]
- Arzul, I.; Corbeil, S.; Morga, B.; Renault, T. Viruses Infecting Marine Molluscs. J. Invertebr. Pathol. 2017, 147, 118–135. [Google Scholar] [CrossRef]
- Corbeil, S. Abalone Viral Ganglioneuritis. Pathogens 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Cocilovo, A.; Dall’Ara, P.; Martino, P.A.; Ponti, W. Microbiologiae Immunologia Veterinaria; UTET Scienze Mediche: Torino, Italy, 2005; Volume 5. [Google Scholar]
- Meyers, T.R.; Burton, T.; Evans, W.; Starkey, N. Detection of Viruses and Virus-Like Particles in Four Species of Wild and Farmed Bivalve Molluscs in Alaska, U.S.A., from 1987 to 2009. Dis. Aquat. Organ. 2009, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Pépin, J.F.; Arzul, I.; Morga, B.; Faury, N.; Renault, T. Detection and Description of a Particular Ostreid Herpesvirus 1 Genotype Associated with Massive Mortality Outbreaks of Pacific Oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010, 153, 92–99. [Google Scholar] [CrossRef]
- Carolyn, S.F.; Robyn, M.E.; Nancy, A.S.; Colleen, A.B.; John, S.H.; Bruce, J.B.; Ralph, A.E.; Eugene, M.B.; Kimberly, S.R. Herpes virus in juvenile Pacific oysters Crassostrea gigas from Tomales Bay, California, coincides with summer mortality episodes. Dis Aquat Organ 2005, 63, 33–41. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The Order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Yeomans, R.; García-Ortega, M.; Cáceres-Martínez, J. Gill Erosion and Herpesvirus in Crassostrea gigas Cultured in Baja California, Mexico. Dis. Aquat. Organ. 2010, 89, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Z.; Fang, Y.-F.; Wei, H.-Y.; Zhu, P.; Liu, M.; Yuan, W.-G.; Yang, L.-L.; Guo, Y.-X.; Jin, T.; Shi, M.; et al. A Remarkably Diverse and Well-Organized Virus Community in a Filter-Feeding Oyster. Microbiome 2023, 11, 2. [Google Scholar] [CrossRef]
- John, S.G.; Mendez, C.B.; Deng, L.; Poulos, B.; Kauffman, A.K.M.; Kern, S.; Brum, J.; Polz, M.F.; Boyle, E.A.; Sullivan, M.B. A Simple and Efficient Method for Concentration of Ocean Viruses by Chemical Flocculation. Environ. Microbiol. Rep. 2011, 3, 195–202. [Google Scholar] [CrossRef]
- Kim, K.E.; Joo, H.M.; Lee, T.-K.; Kim, H.-J.; Kim, Y.J.; Kim, B.K.; Ha, S.-Y.; Jung, S.W. Covariance of Marine Nucleocytoplasmic Large DNA Viruses with Eukaryotic Plankton Communities in the sub-Arctic Kongsfjorden Ecosystem: A Metagenomic Analysis of Marine Microbial Ecosystems. Microorganisms 2023, 11, 169. [Google Scholar] [CrossRef]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. MetaSPAdes: A New Versatile Metagenomic Assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef]
- Nayfach, S.; Camargo, A.P.; Schulz, F.; Eloe-Fadrosh, E.; Roux, S.; Kyrpides, N.C. CheckV Assesses the Quality and Completeness of Metagenome-Assembled Viral Genomes. Nat. Biotechnol. 2021, 39, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner; LBNL Report LBNL-7065E; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2014. [Google Scholar]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Brum, J.R.; Ignacio-Espinoza, J.C.; Roux, S.; Doulcier, G.; Acinas, S.G.; Alberti, A.; Chaffron, S.; Cruaud, C.; de Vargas, C.; Gasol, J.M.; et al. Ocean Plankton. Patterns and Ecological Drivers of Ocean Viral Communities. Science 2015, 348, 1261498. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Swan, B.K.; Wilson, W.H. Marine Viruses, a Genetic Reservoir Revealed by Targeted Viromics. ISME J. 2013, 8, 1079–1088. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Montagnani, C.; Dantan, L.; Nicolas, N.d.S.; Travers, M.-A.; Duperret, L.; Charrière, G.M.; Toulza, E.; Mitta, G.; Cosseau, C.; et al. Cross-Talk and Mutual Shaping Between the Immune System and the Microbiota During an Oyster’s Life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2024, 379, 20230065. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Li, Y.; Li, P.-T.; Jiang, J.; Zeng, W.-H.; Ye, K.; Wang, Y.-L.; Zou, P.-F. Genetic and Pathogenic Characterization of an Iridovirus from the Cultured Largemouth Bass Micropterus salmoides. Fishes 2024, 9, 314. [Google Scholar] [CrossRef]
- Renault, T.; Novoa, B. Viruses Infecting Bivalve Molluscs. Aquat. Living Resour. 2004, 17, 397–409. [Google Scholar] [CrossRef]
- Dupont, S.; Lokmer, A.; Corre, E.; Auguet, J.C.; Petton, B.; Toulza, E.; Montagnani, C.; Tanguy, G.; Pecqueur, D.; Salmeron, C.; et al. Oyster Hemolymph Is a Complex and Dynamic Ecosystem Hosting Bacteria, Protists and Viruses. Anim. Microbiome 2020, 2, 12. [Google Scholar] [CrossRef]
- Nguyen, V.K.; King, W.L.; Siboni, N.; Mahbub, K.R.; Dove, M.; O’Connor, W.; Seymour, J.R.; Labbate, M. The Sydney Rock Oyster Microbiota Is Influenced by Location, Season and Genetics. Aquaculture 2020, 527, 735472. [Google Scholar] [CrossRef]
- Pathirana, E.; Fuhrmann, M.; Whittington, R.; Hick, P. Influence of Environment on the Pathogenesis of Ostreid herpesvirus-1 (OsHV-1) Infections in Pacific Oysters (Crassostrea gigas) Through Differential Microbiome Responses. Heliyon 2019, 5, e02101. [Google Scholar] [CrossRef] [PubMed]
- Delisle, L.; Petton, B.; Burguin, J.F.; Morga, B.; Corporeau, C.; Pernet, F. Temperature Modulate Disease Susceptibility of the Pacific Oyster Crassostrea gigas and Virulence of the Ostreid Herpesvirus Type 1. Fish Shellfish Immunol. 2018, 80, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Evans, O.; Dhand, N.K.; Whittington, R.J. Experimental Infections of Pacific Oyster Crassostrea gigas Using the Australian Ostreid herpesvirus-1 (OsHV-1) µVar Strain. Dis. Aquat. Organ. 2015, 113, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.A.; Judah, L.R.; Conquest, L.L.; Griffin, F.J.; Cheney, D.P.; Suhrbier, A.; Vadopalas, B.; Olin, P.G.; Renault, T.; Friedman, C.S. Synner Seed Mortality of the Pacific Oyster, Crassostrea gigas Thunberg Grown in Tomales Bay, California, USA: The Influence of Oyster Stock, Planting Time, Pathogens, and Environmental Streessors. J. Shellfish Res. 2007, 26, 163–172. [Google Scholar] [CrossRef]
- Lynch, S.A.; Carlsson, J.; Reilly, A.O.; Cotter, E.; Culloty, S.C. A Previously Undescribed Ostreid Herpes Virus 1 (OsHV-1) Genotype Detected in the Pacific Oyster, Crassostrea gigas, in Ireland. Parasitology 2012, 139, 1526–1532. [Google Scholar] [CrossRef]
- Hick, P.; Evans, O.; Looi, R.; English, C.; Whittington, R.J. Stability of Ostreid herpesvirus-1 (OsHV-1) and Assessment of Disinfection of Seawater and Oyster Tissues Using a Bioassay. Aquaculture 2016, 450, 412–421. [Google Scholar] [CrossRef]
- Rosani, U.; Gaia, M.; Delmont, T.O.; Krupovic, M. Tracing the Invertebrate Herpesviruses in the Global Sequence Datasets. Front. Mar. Sci. 2023, 10, 1159754. [Google Scholar] [CrossRef]
- Agius, J.R.; Corbeil, S.; Helbig, K.J. Immune Control of Herpesvirus Infection in Molluscs. Pathogens 2020, 9, 618. [Google Scholar] [CrossRef]
- Coleman, S.; Kiffney, T.; Tanaka, K.R.; Morse, D.; Brady, D.C. Meta-analysis of Growth and Mortality Rates of Net Cultured Sea Scallops Across the Northwest Atlantic. Aquaculture 2022, 546, 737392. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Lian, C.; Cao, L.; Guo, Y.; Wang, M.; Zhong, Z.; Li, M.; Zhang, H.; Li, C. Insights into Phage-Bacteria Interaction in Cold Seep Gigantidas Platifrons Through Metagenomics and Transcriptome Analyses. Sci. Rep. 2024, 14, 10540. [Google Scholar] [CrossRef]
- Sutton, T.D.S.; Hill, C. Gut Bacteriophage: Current Understanding and Challenges. Front. Endocrinol. 2019, 10, 784. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).