Abstract
Genital Mycoplasmas are implicated in adverse pregnancy outcomes and the development of infertility. However, the role of Mycoplasma fermentans in these outcomes has not been adequately studied; therefore, its participation in these sufferings requires further investigation. This study aimed to evaluate the prevalence of M. fermentans in pregnant and non-pregnant women. End-point PCR was used to analyze two hundred and twenty-eight endocervical samples for M. hominis, M. genitalium, M. fermentans, M. pirum, Ureaplasma urealyticum, and U. parvum diagnoses. The prevalence of Mycoplasma spp. was as follows: U. parvum was found in 83 samples (36.4%), U. urealyticum in 39 instances (17.1%), M. hominis in 36 (15.7%), M. fermentans in 32 (14%), M. genitalium in 15 (6.6%), and M. pirum in 0 samples. No association was found between the Mycoplasma spp. and some infertility conditions or adverse pregnancy. However, M. fermentans and M. hominis were found to be associated with bacterial vaginosis (RR = 3.4 CI 95% 1.85–6.3, p < 0.005). In conclusion, M. fermentans and M. hominis were isolated more often in women with bacterial vaginosis, which suggests that these bacteria could contribute to the development of this pathology.
1. Introduction
Mycoplasma genitalium and Mycoplasma fermentans have been implicated in various human diseases [1,2]. Both bacteria are more difficult to culture in artificial media than other pathogenic Mycoplasmas, which contributes to their diagnosis being determined using molecular techniques [3,4]. Moreover, both M. genitalium and M. fermentans have been detected in the genitourinary tract of adults [5,6] and can, therefore, be sexually transmitted. The prevalence of M. genitalium infection globally in men and women in the “open population”, which refers to individuals who are not in a specific high-risk group, is about 1% to 6.4% [5]. However, the prevalence of M. fermentans is unknown despite it being considered a pathogenic Mycoplasma. In Mexico, the prevalence of both of the above-mentioned microorganisms has yet to be fully elucidated.
M. genitalium infection in women has been associated with the development of cervicitis, endometritis, salpingitis, and tubal factor infertility [5,7]. M. fermentans has been associated with respiratory infections, arthritis, genitourinary tract infections, and chronic fatigue syndrome and has been linked to the development of human immunodeficiency viruses [1,8,9,10]. The present study determined the prevalence of M. fermentans, M. genitalium, and other Mycoplasmas in women with cervicitis who attended a tertiary health institute in Mexico City. The results of this study highlight the need for further research.
2. Materials and Methods
2.1. Study Design
The study design consisted of a descriptive and cross-sectional study carried out in Mexico City.
2.2. Study Population
The population consisted of women between the ages of 15 and 45. All study participants were diagnosed using clinical data on endocervical infection and attended the tertiary care hospital in Mexico City for diagnosis and treatment. The study period was from September 2022 to March 2023.
2.3. Cervicovaginal Fluid Samples
Two hundred and forty-three cervicovaginal samples were obtained from women who attended the gynecology service at the tertiary care hospital in Mexico City. The patients met the following requirements: (a) sexual abstinence for a minimum of 72 h, (b) had not ingested antibiotics or applied vaginal suppositories at the time of sample collection, (c) presented to the sample collection well cleaned, (d) were not in their menstrual period, and (e) were not using any method of family planning. All patients signed the institutional informed consent form before participating in this study.
2.4. Microbiological Culture
Three swabs were obtained for each cervicovaginal sample: the first for culture on artificial media and Gram stain smear, the second for a wet mount examination, and the third for placement on a universal transport medium (UTM®, Copan Diagnostics, Inc., Murrieta, CA, USA). A drop of this suspension was placed between a slide and the coverslip for wet mount examination. The observation was performed using a Karl-Zeiss microscope with a 40× objective, searching for clue cells, yeast cells, pseudohyphae, and Trichomonas vaginalis. The smear was stained with Gram stain, and a 100× objective was used for evaluating the Nugent criteria. The artificial culture medium used was chocolate agar (Columbia CNA Agar supplemented with 5% horse or human blood). The samples were streaked using the cross-streak technique and incubated at 37 °C with 5% CO2 for 48 h. The presence of beta-hemolytic and grayish colonies with a diameter of 1 mm was considered to indicate possible Gardnerella vaginalis. Biochemical tests were performed using the Vitek Compat 2 automated system using the NH card to confirm biochemical identification.
2.5. Amsel Criteria and Nugent Scoring
The Amsel criteria analyzed were pH > 4.5; the presence of gray, homogeneous, and adherent vaginal discharge; the release of a fishy odor upon the addition of 10% potassium hydroxide to the secretion; and the presence of clue cells. The presence of three of the above parameters is considered to indicate bacterial vaginosis (BV). Different morphotypes were counted for the Nugent criteria, and a value was determined depending on the quantity observed. Finally, the scores were summed. If the sum was between 7 and 10, the diagnosis was BV (Supplementary Materials Table S1).
2.6. Mycoplasma Culture
Two hundred microliters of each sample (placed in universal transport medium) was deposited in tubes containing 0.5 mL of base medium for Mycoplasmas (BBL®, Becton Dickinson, Cockeysville, MD, USA) supplemented with 20% horse serum (HyClone™, Cytiva, Marlborough, MA, USA), 1% glucose (Merck®, Darmstadt, Germany), 3% arginine (Research Organics Inc., Cleveland, OH, USA), or 5% urea (Merck®). The tubes were incubated at 37 °C until the pH indicator turned alkaline.
2.7. Mycoplasma Strains
The reference Mycoplasma strains U. parvum ATCC 27815, U. urealyticum ATCC 27618, M. genitalium ATCC 33350, M. hominis ATCC 15488, and M. fermentans ATCC 19989 were used as positive controls. The reference strains were provided by the Molecular and Cellular Bioimmunology Laboratory.
2.8. Nucleic Acid Extraction
Nucleic acid was obtained using the phenol-chloroform technique. An amount of 200 µL of lysis regulator for white cells was added to 200 µL of the sample. Next, the sample was vortexed for 15 s and transferred to a water bath at 56 °C for 1 h. After the incubation time, 200 µL of phenol and 200 µL of chloroform were added, homogenized in a vortex for 1 min, and centrifuged at 1500× g for 10 min; then, the supernatant was recovered in a new Eppendorf tube. Next, 200 µL of chloroform was added to the supernatant, which was left for 15 min in the vortex and then centrifuged at 1500× g for 10 min. The supernatant was recovered in a new tube, and 40 µL of 1 M NaCl and 1 mL of absolute molecular biology-grade alcohol at 4 °C were added. Next, the tubes were centrifuged for 15 min at 14,500× g. Finally, the supernatant was decanted, and the nucleic acid was hydrated with 40 µL of DNAse- and RNAse-free water (Invitrogen by Thermo Fisher Scientific, Austin, TX, USA).
2.9. Mycoplasma Detection Using PCR
PCR analysis was performed on endocervical exudate samples and cultures suspected of Mycoplasmas. A reaction mixture of 12.5 µL of 2× concentrated Master mix containing Taq DNA polymerase and dNTPs (Thermo Fisher) was used. All samples were examined for the presence of U. parvum, U. urealyticum, M. fermentans, M. genitalium, M. hominis, and M. pirum, according to the described protocols of Wang [11], Grau [12], de Barbeyrac [4], and Kong [13]. Table 1 reports the characteristics of the primers of the reaction protocols used to identify each of the Mycoplasmas. The PCR products were identified on a 2% agarose gel. The gel was stained with ethidium bromide and visualized using a Multi-Image™ (Alpha Innotech Corporation, San Leandro, CA, USA) transilluminator. The Mycoplasma species were identified according to the amplification product size. Figure S1 shows the PCR products obtained from the strains used as positive controls. The PCR results were as follows: U. urealyticum of 443 bp, M. hominis of 150 bp, M. genitalium of 280 bp, M. fermentans of 209 bp, and Ureaplasma parvum of 403 bp.

Table 1.
Primer used and lengths of the amplified fragment.
2.10. Statistical Analysis
Fisher’s exact non-parametric test was used to determine the association between Mycoplasma infections and the categorical variables. The magnitude of the associations among the variables was expressed as the relative risk (RR) with a confidence interval (CI) of 95%. A p-value of less than 0.05 was considered statistically significant. SPSS statistics software for Windows, version 20.0 (IBM Corp, Armonk, NY, USA), was used for the analysis.
3. Results
3.1. Frequency of Mycoplasma spp. and Co-Infections
Two hundred and forty-three samples of cervicovaginal exudates were obtained. However, fifteen samples were eliminated because the patients had received antimicrobial treatment for a chronic Ureaplasma infection. So, two hundred and eight samples were analyzed to identify Mycoplasma strains. The results show that the prevalence of Mycoplasma spp. infection was as follows: Ureaplasma parvum was detected in 83 (36.4%) samples, followed by U. urealyticum in 39 (17.1%), M. hominis in 36 (15.7%), M. fermentans in 32 (14%), and M. genitalium in 15 (6.6%). M. pirum was not detected in any samples (Table 2). Other identified STI pathogens were Chlamydia trachomatis (2.2%), Trichomonas vaginalis (1.3%), and Neisseria gonorrhoeae (0.0%).

Table 2.
Frequency and co-infection of Mycoplasma genera with other microorganisms.
A significant co-infection of M. fermentans and Ureaplasma urealyticum (RR = 2.2; CI 95% 1.14–4.3, p < 0.039) or Gardnerella vaginalis (RR = 4.7; CI 95% 2.6–8.5, p < 0.001) was demonstrated. Figure 1 shows several combinations of co-infections between the Mycoplasma spp. observed in this study.
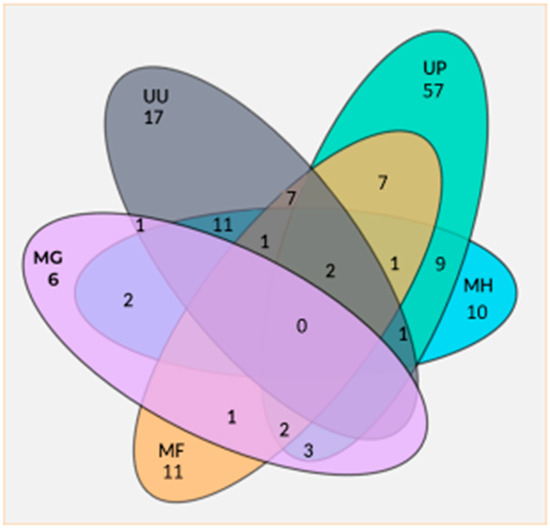
Figure 1.
Co-infection numbers of several Mycoplasma spp. UU = Ureaplasma urealyticum; UP = Ureaplasma parvum; MH = Mycoplasma hominis; MG = Mycoplasma genitalium; MF = Mycoplasma fermentans.
3.2. Prevalence of Mycoplasma spp. Colonization in Pregnant and Infertile Women
All the participants presented some symptoms or clinical signs, such as a change in color, odor, or amount of vaginal discharge as well as itching, vaginal irritation, pain during sex or urination, and light vaginal bleeding or spotting. The age range of the patients was from 15 to 45 years, with a mean age of 30.9 ± 6.8 years. A total of 103 of the patients were women who had infertility problems, and 125 were pregnant. The prevalence percentages of M. fermentans and M. genitalium in infertile women were 10.7% and 4.9%, respectively (Table 3 and Table 4). The prevalence percentages of U. parvum, M. hominis, and U. urealyticum were 29.1%, 21.4%, and 18.4%, respectively (Tables S1–S3). This research highlights that the most critical cause of infertility was a uterine factor in 59.6%, followed by an endocrine–ovarian factor in 57.8%, a masculine factor in 45%, and a tubal factor infertility in 23.9%. The prevalence percentages of M. fermentans and M. genitalium in pregnant women were 16.8% and 8%, respectively. For other Mycoplasma spp., the prevalence results showed U. parvum at 42.4%, M. hominis at 11.2%, and U. urealyticum at 16%. None of the causes of infertility were associated with Mycoplasma spp. infections or with miscarriage, preterm birth, or newborn respiratory illness in pregnant women in this study. However, a significant colonization frequency of M. hominis was observed in infertile patients (RR = 1.13; CI 95% (1.0–1.27, p < 0.045)) (Table S4). However, in pregnant women, a significant frequency of colonization with U. parvum was found (RR = 1.46; 1.0–2.1, p < 0.04) (Table S2).

Table 3.
Gynecological or obstetrical data of patients with vaginal infection by Mycoplasma fermentans.

Table 4.
Gynecological or obstetrical data of patients with vaginal infection by Mycoplasma genitalium.
3.3. Bacterial Vaginosis and Candidiasis
Thirty-three cases of bacterial vaginosis were documented in this study. The results show that twenty-one of the cases were identified as a mix of Gardnerella vaginalis and Mycoplasma spp. (RR = 24.3; CI 95% 9.1–65, p < 0.001), by G. vaginalis caused eight cases, and four cases were caused by Mycoplasma spp. The Mycoplasma spp. percentages associated with G. vaginalis were a mixed combination as follows: 45.5% (15 cases) for U. parvum; 33.3% (11 cases) for M. fermentans; 30.3% (10 cases) for M. hominis; 18.2% (6 cases) for U. urealyticum; and 6.1% (2 cases) for M. genitalium. Twenty-five cases of candidiasis were documented. Of these 25 cases, Candida albicans caused 18, and 7 were caused by Candida glabrata. Interestingly, U. parvum (13 cases) and M. fermentans (six cases) were also observed in these patients.
4. Discussion
Mycoplasmas are considered part of the vaginal microbiome and an opportunistic pathogen. However, the conditions for one or the other case are still being elucidated. M. fermentans has been identified in different patients, including those who are healthy, those who are immunocompromised, and those with chronic-degenerative diseases [1,8,9]. Moreover, M. fermentans DNA has been detected in the lung, synovial fluid, peripheral blood, and vaginal tissues [8]. Despite the above, the pathogenesis of M. fermentans is not yet completely understood, and its prevalence in the genital tract is unknown. In this study, the prevalence of M. fermentans was 14% higher than that of M. genitalium, which was 6.6%, and much lower than that of U. parvum (36.4%). A study by Rivera A. et al. [14] demonstrated that M. fermentans can produce biofilm on copper intrauterine devices (T 380 A). This finding suggests that M. fermentans could be considered an opportunistic pathogen, mainly when using intrauterine devices.
Another pathogen is M. genitalium, which is generally detected in the lower urogenital tract of asymptomatic men and women, occurring in 1% to 6.4% of the general population [5,15]. However, M. genitalium is more prevalent in people with urogenital symptoms (sometimes significantly at more than 20%) and those attending sexually transmitted infection clinics [5,16]. In Mexico, more detailed information is required. The prevalence of M. genitalium in Mexico has been reported to be between 0.5% and 13.8%, depending on the type of patients studied [17,18,19]. In this study, the prevalence of M. genitalium was within the value reported worldwide.
Studies in infertile women have associated M. genitalium as the cause of pelvic inflammatory disease (PID) and tubal occlusion [5,7]. However, other studies failed to demonstrate a significant association between infection and progression to PID [5,20]. Similarly, studies have not been able to verify M. genitalium as a cause of tubal infertility [21,22]. In this study, only five infertile women had M. genitalium infection, and one of them showed tubal factor infertility. Regarding M. fermentans, eleven patients were infertile, and only three showed tubal occlusion. It should be noted that the sexual partners of these women showed teratozoospermia (<4%) and vacuoles in the heads of the spermatozoa (between 7 and 11%), which suggests M. fermentans as a possible cause of this pathology, as has been shown to occur with M. hominis, U. urealyticum, and M. genitalium [23,24,25].
Regarding pregnant women infected with M. genitalium, several studies have reported a prevalence of 1 to 4% [26,27]; however, more recent studies describe a prevalence of mostly 12 to 18% [28,29]. In this study, the prevalence result of M. genitalium was 7.4%. M. genitalium infection has been associated with preterm birth [30,31]; however, other studies have not found an association with preterm birth or pregnancy loss [32,33]. In this study, all pregnant women had deliveries at term. Moreover, in pregnant women, the prevalence of M. fermentans was 16.4%, and it was identified in any trimester of gestation.
Ureaplasma spp. is part of the vaginal microbiota and has an average colonization rate of 40 to 80% [34]. However, this bacterium has been associated with infertility, non-gonococcal urethritis, and prostatitis [35,36]. Ureaplasma spp. can be detected in the cervicovaginal samples of healthy and diseased women; therefore, it is considered an opportunistic pathogen. Moreover, published studies on Ureaplasma spp. infection in pregnant women have associated the bacterium with preterm labor, premature rupture of membranes (PROMs), and clinical chorioamnionitis [6,37,38]. The presence of Ureaplasma spp. in the lower genital tract has been linked to increased matrix metalloproteinases, prostaglandins, and cytokines associated with the precipitation of preterm labor and PROMs [39]. In this study, 53.5% of the participants showed colonization by Ureaplasma spp. The molecular identification of the species showed U. parvum in 36.4% and U. urealyticum in 17.1% of the patients.
Although the association of U. urealyticum with urogenital infections is well established, the role of U. parvum in these infections is still undetermined. The presence of U. parvum in the vagina of many healthy, non-pregnant women complicates understanding of the potential role of this bacterium in adverse pregnancy outcomes [38] and disorders of the reproductive system [35]. In this study, U. parvum was detected in 42.4% of pregnant women and 29.1% of infertile women.
Mycoplasma hominis can infect different parts of the female reproductive tract and lead to infertility. The prevalence of Mycoplasma hominis has been reported to be between 1.2% and 52% worldwide. Diverse studies are considered to be responsible for up to 10% of causes of pelvic inflammatory disease, presenting as either endometritis, pelvic adhesions, or salpingitis. In this study, the general prevalence of Mycoplasma hominis was 15.8%. Moreover, in infertile women, the prevalence of Mycoplasma hominis was 21.4%, and no association was observed with the tubal factor infertility.
In pregnant women, vaginal colonization with M. hominis is associated with premature rupture of membranes (PROMs), preterm delivery, and spontaneous miscarriage, and its reported prevalence is 11.2%. However, a 2021 study on South African pregnant women who were HIV-positive found a 48% prevalence rate for M. hominis [39]. The prevalence of M. hominis found in this study was 11.2%, and no association was observed in some gestation trimesters.
Bacterial vaginosis (BV) is one of the most common vaginal syndromes in infertile women. The worldwide prevalence of BV ranges from 20 to 60% [40]; in this study, the prevalence of BV was 20.4%. Other studies in Mexico have reported between 8.5 and 60% in the same Mexican population [41,42,43]. Gardnerella vaginalis is more frequently the etiological agent in BV; however, in this study, a mixed infection of Gardnerella vaginalis and Mycoplasma spp. was observed in 63% of BV cases, mainly with the combination of U. parvum, M. fermentans, and M. hominis.
Despite the above, a weakness of this study is that all the vaginal microbiota participating were not identified in patients who showed colonization by M. fermentans and bacterial vaginosis, which would have allowed us to make a conclusion about the participation of M. fermentans in this pathology.
Today, the prevalence of genital Mycoplasmas in patients with BV is higher than in those without BV. Keane FE et al. reported a 53% carriage rate of Mycoplasma hominis in women with BV using PCR analysis compared with women without BV, in which Mycoplasma hominis was undetected [44]. Lendamba et al. reported that 60.2% of women with BV were genital Mycoplasma carriers: 33.12% for U. urealyticum; 1.95% for M. hominis; and 25.11% for mixed infections (U. urealyticum and M. hominis) [45]. Although BV is treated with metronidazole (MTZ) and clindamycin, there is a treatment failure between 10% and 15% of patients one month after medication, as well as BV recurrence rates of 30% at 3 months and 50 to 80% per year after therapy with either drug [46,47]. Studies of the vaginal microbiota after MTZ treatment using metagenomics have indicated an increase in other biofilm-producing microbial populations such as Prevotella, Gardnerella, Atopobium, Sneathia, Mycoplasma, and Ureaplasma [46,48,49]. These biofilm-producing bacteria are known for their ability to adhere to surfaces and resist antibiotic treatment [50,51]; this emphasizes the need for a deeper understanding of these bacterial genera and their role in treatment failure. Therefore, detecting and identifying several Mycoplasma spp. and other bacterial species is essential for future studies in patients with BV. Consequently, it is advisable to provide therapeutic prescriptions based on laboratory results identifying antimicrobial resistance genes.
5. Conclusions
In this study, the prevalence values of M. fermentans and M. genitalium in samples from patients with vaginitis were 14% and 6.6%, respectively. Furthermore, a significant association between G. vaginalis and several Mycoplasma spp. was observed in patients with bacterial vaginosis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens13111004/s1: Table S1: Morphotypes based on the Nugent criteria for bacterial vaginosis; Table S2: Gynecological or obstetrical data of patients with vaginal infection by Ureaplasma parvum; Table S3: Gynecological or obstetrical data of patients with vaginal infection by Ureaplasma urealyticum; Table S4: Gynecological or obstetrical data of patients with vaginal infection by Mycoplasma hominis; Figure S1: Amplicons ATCC Mycoplasma strains that were obtained through PCR using specific primers. Lane (1) 100 bp DNA ladder; Lane (2) PCR-amplicon of 429 bp of Ureaplasma spp; Lane (3) Amplicon of 150 bp of M. hominis; Lane (4) Amplicon of 280 bp of M. genitalium, and Lane (5) Amplicon of 209 bp of M. fermentans.
Author Contributions
All authors contributed to the development and research of this study. M.L.-H., S.G.-C. and F.M.G.-I., research conceptualization; J.R.V.-Z. and M.V.-R., data curation; M.R.E.-G., M.L.-H., and F.M.G.-I., research validation; A.D.B.-L., J.R.V.-Z., and M.V.-R.: methodology; A.D.B.-L., S.G.-C., and F.M.G.-I., wrote and produced original draft; M.L.-H. and F.M.G.-I., formal analysis; M.L.-H., S.G.-C., and F.M.G.-I., review and editing; F.M.G.-I., supervision; F.M.G.-I., project administration; F.M.G.-I., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external or specific grants from any funding agency, commercial or not-for-profit sectors.
Institutional Review Board Statement
This study was approved by the research, ethics, and biosafety committees of the National Institute of Perinatology “Isidro Espinosa de los Reyes”, registration numbers 2022-1-16 and 2022-1-19. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Informed Consent Statement
The authors obtained written informed consent from the patients or subjects mentioned in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all the individuals who took part in this study. The authors also wish to thank Laura M. Marqués-Valdelamar of the National Biodiversity Laboratory of the Institute of Biology of the UNAM for sequencing the PCR amplicons analyzed for the diagnosis of Mycoplasmas.
Conflicts of Interest
The authors declare that this study was conducted without any commercial or financial relationships that could potentially create conflicts of interest. All authors have read and agreed to the published version of the manuscript.
References
- Ainsworth, J.G.; Hourshid, S.; Webster, A.D.; Gilroy, C.B.; Taylor-Robinson, D. Detection of Mycoplasma fermentans in healthy students and patients with congenital immunodeficiency. J. Infect. 2000, 40, 138–140. [Google Scholar] [CrossRef]
- Blanchard, A.; Hamrick, W.; Duffy, L.; Baldus, K.; Cassell, G.H. Use of the Polymerase Chain Reaction for Detection of Mycoplasma fermentans and Mycoplasma genitalium in the Urogenital Tract and Amniotic Fluid. Clin. Infect. Dis. 1993, 17 (Suppl. S1), S272–S279. [Google Scholar] [CrossRef]
- Blanchard, A.; Hentschel, J.; Duffy, L.; Baldus, K.; Cassell, G.H. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin. Infect. Dis. 1993, 17 (Suppl. S1), S148–S153. [Google Scholar] [CrossRef]
- de Barbeyrac, B.; Bernet-Poggi, C.; Fébrer, F.; Renaudin, H.; Dupon, M.; Bébéar, C. Detection of Mycoplasma pneumoniae and Mycoplasma genitalium in clinical samples by polymerase chain reaction. Clin. Infect. Dis. 1993, 17 (Suppl. S1), S83–S89. [Google Scholar] [CrossRef]
- Waites, K.B.; Crabb, D.M.; Ratliff, A.E.; Geisler, W.M.; Atkinson, T.P.; Xiao, L. Latest Advances in Laboratory Detection of Mycoplasma genitalium. J. Clin. Microbiol. 2023, 61, e0079021. [Google Scholar] [CrossRef]
- Taylor-Robinson, D.; Lamont, R.F. Mycoplasmas in pregnancy: Mycoplasmas in pregnancy. Br. J. Obstet. Gynaecol. 2011, 118, 164–174. [Google Scholar] [CrossRef]
- Haggerty, C.L. Evidence for a role of Mycoplasma genitalium in pelvic inflammatory disease. Curr. Opin. Infect. Dis. 2008, 21, 65–69. [Google Scholar] [CrossRef]
- Ruiter, M.; Wentholt, H.M.M. A pleuropneumonia-like organism in primary fusospirochetal gangrene of the penis. J. Investig. Dermatol. 1950, 15, 301–304. [Google Scholar] [CrossRef]
- Yáñez, A.; Martínez-Ramos, A.; Calixto, T.; González-Matus, F.J.; Rivera-Tapia, J.A.; Giono, S.; Gil, C.; Cedillo, L. Animal model of Mycoplasma fermentans respiratory infection. BMC Res. Notes 2013, 6, 9. [Google Scholar] [CrossRef]
- Coutlée, F.; Saint-Louis, G.; Voyer, H.; Daloze, P.; Ghadirian, P. Mycoplasma fermentans DNA is infrequently detected in urine specimens from renal transplant recipients. Mol. Cell Probes. 1998, 12, 201–206. [Google Scholar] [CrossRef]
- Wang, R.Y.; Hu, W.S.; Dawson, M.S.; Shih, J.W.; Lo, S.C. Selective detection of Mycoplasma fermentans by polymerase chain reaction and by using a nucleotide sequence within the insertion sequence-like element. J. Clin. Microbiol. 1992, 30, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Grau, O.; Kovacic, R.; Griffais, R.; Launay, V.; Montagnier, L. Development of PCR-based assays for the detection of two human mollicute species, Mycoplasma penetrans and M. hominis. Mol. Cell. Probes. 1994, 8, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; James, G.; Ma, Z.; Gordon, S.; Bin, W.; Gilbert, G.L. Phylogenetic analysis of Ureaplasma urealyticum—Support for the establishment of a new species, Ureaplasma parvum. Int. J. Syst. Bacteriol. 1999, 49 Pt 4, 1879–1889. [Google Scholar] [CrossRef]
- Rivera, A.; Cedillo, L.; Giono, S.; Rodriguez, N. Biofilm formation by Mycoplasma fermentans on intrauterine devices. Int. J. Med. Sci. 2009, 1, 267–271. [Google Scholar]
- Baumann, L.; Cina, M.; Egli-Gany, D.; Goutaki, M.; Halbeisen, F.S.; Lohrer, G.-R.; Ali, H.; Scott, P.; Low, N. Prevalence of Mycoplasma genitalium in different population groups: Systematic review and meta-analysis. Sex. Transm. Infect. 2018, 94, 255–262. [Google Scholar] [CrossRef]
- Mahlangu, M.P.; Müller, E.E.; Venter, J.M.E.; Maseko, D.V.; Kularatne, R.S. The prevalence of Mycoplasma genitalium and association with human immunodeficiency virus infection in symptomatic patients, Johannesburg, South Africa, 2007–2014. Sex Transm. Dis. 2019, 46, 395–399. [Google Scholar] [CrossRef]
- Abiodun-Ojo, O.A.; Guerra-Infante, F.M.; Taylor, B.D. Identification of Mycoplasma genitalium among Mexican women using the Seeplex STD6 ACE Detection kit: Are results accurate? Int. J. STD AIDS 2019, 30, 1450–1451. [Google Scholar] [CrossRef]
- Casillas-Vega, N.; Morfín-Otero, R.; García, S.; Llaca-Díaz, J.; Rodríguez-Noriega, E.; Camacho-Ortiz, A.; Ayala-Castellanos, M.d.l.M.; Mendoza-Olazarán, S.M.-O.; Flores-Treviño, S.; Petersen-Morfín, S.; et al. Sexually transmitted pathogens, coinfections and risk factors in patients attending obstetrics and gynecology clinics in Jalisco, Mexico. Salud Publica Mex. 2016, 58, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Contreras, M.; Contreras-Paredes, A.; Chavez-Blanco, A.; Lizano, M.; De la Cruz-Hernandez, Y.; De la Cruz-Hernandez, E. Prevalence of sexually transmitted pathogens associated with HPV infection in cervical samples in a Mexican population: Prevalence of STIs in Cervical Samples. J. Med. Virol. 2015, 87, 2098–2105. [Google Scholar] [CrossRef]
- Oliphant, J.; Azariah, S. Pelvic inflammatory disease associated with Chlamydia trachomatis but not Mycoplasma genitalium in New Zealand. Sex. Health 2016, 13, 43. [Google Scholar] [CrossRef]
- Peipert, J.F.; Zhao, Q.; Schreiber, C.A.; Teal, S.; Turok, D.K.; Natavio, M.; Cordon, S.; Daggy, J. Intrauterine device use, sexually transmitted infections, and fertility: A prospective cohort study. Am. J. Obstet. Gynecol. 2021, 225, 157.e1–157.e9. [Google Scholar] [CrossRef] [PubMed]
- Idahl, A.; Jurstrand, M.; Olofsson, J.I.; Fredlund, H. Mycoplasma genitalium serum antibodies in infertile couples and fertile women. Sex. Transm. Infect. 2015, 91, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, F.J.; Herrera-Mendoza, A.P.; Giono-Cerezo, S.; Guerra-Infante, F.M. Mycoplasma hominis attaches to and locates intracellularly in human spermatozoa. Hum. Reprod. 2006, 21, 1591–1598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xianchun, F.; Jun, F.; Zhijun, D.; Mingyun, H. Effects of Ureaplasma urealyticum infection on semen quality and sperm morphology. Front. Endocrinol. 2023, 14, 1113130. [Google Scholar] [CrossRef]
- Svenstrup, H.F.; Fedder, J.; Abraham-Peskir, J.; Birkelund, S.; Christiansen, G. Mycoplasma genitalium attaches to human spermatozoa. Hum. Reprod. 2003, 18, 2103–2109. [Google Scholar] [CrossRef]
- Peuchant, O.; Le Roy, C.; Desveaux, C.; Paris, A.; Asselineau, J.; Maldonado, C.; Chêne, G.; Horovitz, J.; Dallay, D.; de Barbeyrac, B.; et al. Screening for Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium should it be integrated into routine pregnancy care in French young pregnant women? Diagn. Microbiol. Infect. Dis. 2015, 82, 14–19. [Google Scholar] [CrossRef]
- Lu, G.C.; Schwebke, J.R.; Duffy, L.B.; Cassell, G.H.; Hauth, J.C.; Andrews, W.W.; Goldenberg, R.L. Midtrimester vaginal Mycoplasma genitalium in women with subsequent spontaneous preterm birth. Am. J. Obstet. Gynecol. 2001, 185, 163–165. [Google Scholar] [CrossRef]
- Smullin, C.P.; Green, H.; Peters, R.; Nyemba, D.; Qayiya, Y.; Myer, L.; Klausner, J.; Davey, D.J. Prevalence and incidence of Mycoplasma genitalium in a cohort of HIV-infected and HIV-uninfected pregnant women in Cape Town, South Africa. Sex. Transm. Infect. 2020, 96, 501–508. [Google Scholar] [CrossRef]
- Perin, J.; Coleman, J.S.; Ronda, J.; Neibaur, E.; Gaydos, C.A.; Trent, M. Maternal and fetal outcomes in an observational cohort of women with Mycoplasma genitalium infections. Sex. Transm. Dis. 2021, 48, 991–996. [Google Scholar] [CrossRef]
- Frenzer, C.; Egli-Gany, D.; Vallely, L.M.; Vallely, A.J.; Low, N. Adverse pregnancy and perinatal outcomes associated with Mycoplasma genitalium: Systematic review and meta-analysis. Sex. Transm. Infect. 2022, 98, 222–227. [Google Scholar] [CrossRef]
- Lis, R.; Rowhani-Rahbar, A.; Manhart, L.E. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin. Infect. Dis. 2015, 61, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Labbé, A.-C.; Frost, E.; Deslandes, S.; Mendonça, A.P.; Alves, A.C.; Pépin, J. Mycoplasma genitalium is not associated with adverse outcomes of pregnancy in Guinea-Bissau. Sex. Transm. Infect. 2002, 78, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Averbach, S.H.; Hacker, M.R.; Yiu, T.; Modest, A.M.; Dimitrakoff, J.; Ricciotti, H.A. Mycoplasma genitalium and preterm delivery at an urban community health center. Int. J. Gynaecol. Obstet. 2013, 123, 54–57. [Google Scholar] [CrossRef]
- Viscardi, R.M. Ureaplasma species: Role in diseases of prematurity. Clin. Perinatol. 2010, 37, 393–409. [Google Scholar] [CrossRef]
- Chang-tai, Z.; Zhong-yi, H.; Chun-lei, D.; Chang-song, Z.; Mei-zhen, W.; Yang, L. Investigation of Ureaplasma urealyticum biovars and their relationship with antimicrobial resistance. Indian J. Med. Microbiol. 2011, 29, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Kokkayil, P.; Dhawan, B. Ureaplasma: Current perspectives. Indian J. Med. Microbiol. 2015, 33, 205–214. [Google Scholar] [CrossRef]
- Sprong, K.E.; Mabenge, M.; Wright, C.A.; Govender, S. Ureaplasma species and preterm birth: Current perspectives. Crit. Rev. Microbiol. 2020, 46, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Jonduo, M.E.; Vallely, L.M.; Wand, H.; Sweeney, E.L.; Egli-Gany, D.; Kaldor, J.; Vallely, A.J.; Low, N. Adverse pregnancy and birth outcomes associated with Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum: A systematic review and meta-analysis. BMJ Open 2022, 12, e062990. [Google Scholar] [CrossRef]
- Nundlall, N.; Ngobese, B.; Singh, R.; Tinarwo, P.; Abbai, N. Mycoplasma hominis increases the risk for Ureaplasma parvum infection in Human immunodeficiency virus infected pregnant women. J. Infect. Dev. Ctries. 2024, 18, 258–265. [Google Scholar] [CrossRef]
- Coudray, M.S.; Madhivanan, P. Bacterial vaginosis-A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef]
- Nava-Memije, K.; Hernández-Cortez, C.; Ruiz-González, V.; Saldaña-Juárez, C.A.; Medina-Islas, Y.; Dueñas-Domínguez, R.A.; Aguilera-Arreola, M.G. Bacterial Vaginosis and Sexually Transmitted Infections in an HIV-Positive Cohort. Front. Reprod. Health 2021, 3, 660672. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, E.K.; Contreras-Paredes, A.; Martinez-Abundis, E.; Garcia-Chan, D.; Lizano, M.; de la Cruz-Hernandez, E. Molecular epidemiology of bacterial vaginosis and its association with genital micro-organisms in asymptomatic women. J. Med. Microbiol. 2019, 68, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- González-Mustri, K.V.; Guerra-Infante, F.M.; Villeda-Rangel, G.; López-Hurtado, M. Frequency and molecular detection of Gardnerella vaginalis in a third level institute. Perinatol. Reprod. Hum. 2023, 37, 91–98. [Google Scholar] [CrossRef]
- Keane, F.E.; Thomas, B.J.; Gilroy, C.B.; Renton, A.; Taylor-Robinson, D. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: Observations on heterosexual women and their male partners. Int. J. STD AIDS 2000, 11, 356–360. [Google Scholar] [CrossRef]
- Lendamba, R.W.; Mbeang Nguema, P.P.; Onanga, R.; Mombo, L.E. Determination of the prevalence of Mycoplasma hominis and Ureaplasma species in Bacterial vaginosis patients in association with antibiotic resistance profile in Franceville, Gabon. Microb. Pathog. 2022, 166, 105528. [Google Scholar] [CrossRef]
- Austin, M.N.; Beigi, R.H.; Meyn, L.A.; Hillier, S.L. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J. Clin. Microbiol. 2005, 43, 4492–4497. [Google Scholar] [CrossRef]
- Abbe, C.; Mitchell, C.M. Bacterial vaginosis: A review of approaches to treatment and prevention. Front. Reprod. Health 2023, 5, 1100029. [Google Scholar] [CrossRef]
- Gustin, A.T.; Thurman, A.R.; Chandra, N.; Schifanella, L.; Alcaide, M.; Fichorova, R.; Doncel, G.F.; Gale, M.; Klatt, N.R. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am. J. Obstet. Gynecol. 2022, 226, 225.e1–225.e15. [Google Scholar] [CrossRef] [PubMed]
- Zwittink, R.D.; van den Munckhof, E.H.A.; Leverstein-van Hall, M.A.; Boers, K.; Molijn, A.; Knetsch, C.W.; Kuijper, E.J. The vaginal microbiota in the course of bacterial vaginosis treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 651–656. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Pereira, S.A.; Cerca, N. Fighting polymicrobial biofilms in bacterial vaginosis. Microb. Biotechnol. 2023, 16, 1423–1437. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).