Evidence of West Nile Virus Circulation in Horses and Dogs in Libya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- (1)
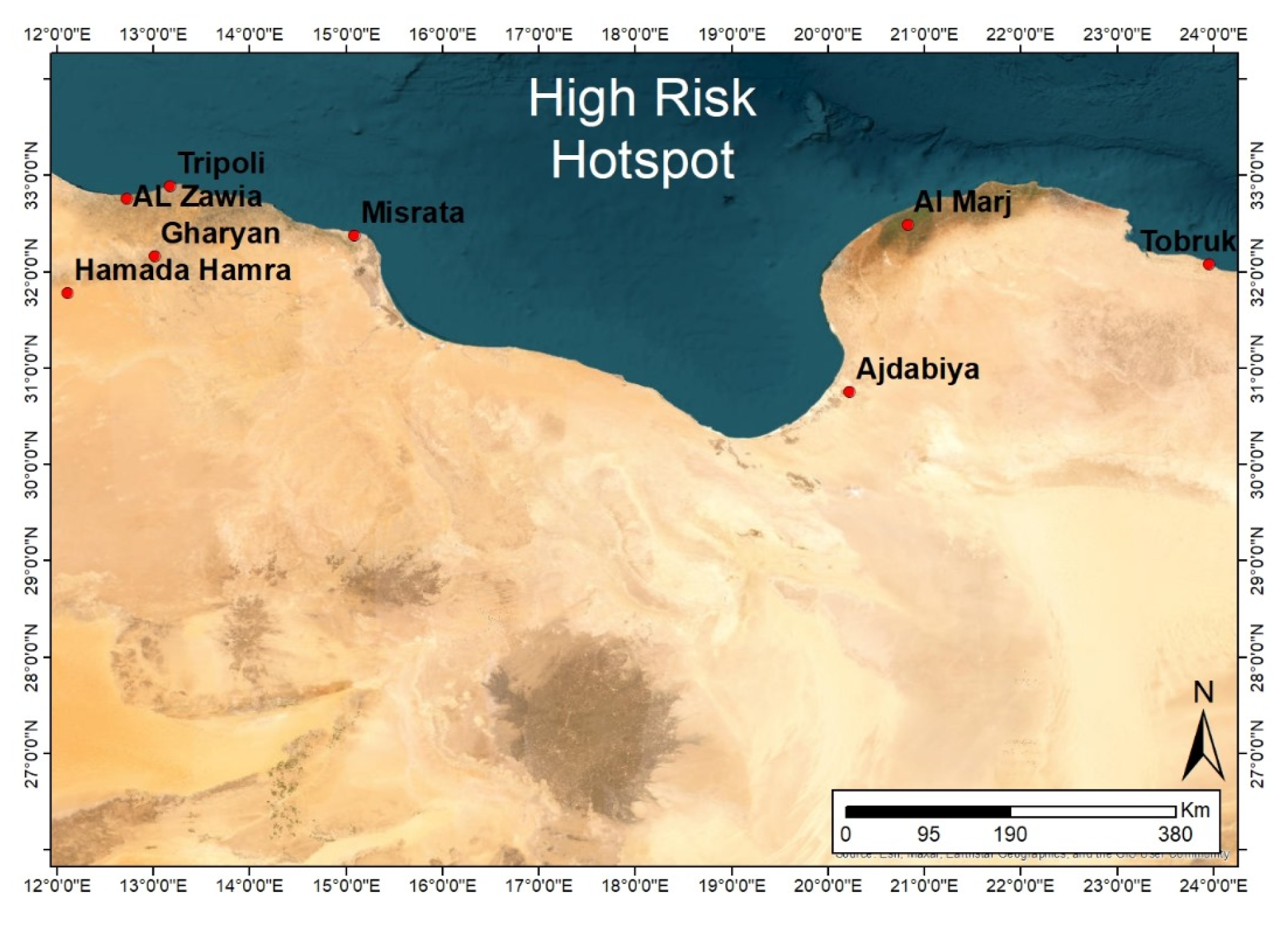
- Suitable environment for vectors based on rainfall and green vegetation areas (FAO’s RVF Early Warning/Decision Support Tool).
- (2)
- High animal density and frequent movement based on animal movement survey and information available at the National Center for Animal Health (NCAH) in Libya.
2.2. Targeted Animals and Sampling Strategy
2.3. Sample Data
2.4. Collection and Processing of Blood Samples
2.5. Serological Tests
2.5.1. Competitive Enzyme-Linked Immunosorbent Assay (c-ELISA)
2.5.2. Virus Neutralisation Test (VNT)
2.6. Statistical Analysis for Horses’ Samples
3. Results
3.1. Seroprevalence of WNV in Horses
3.2. Seroprevalence of WNV in Dogs
3.3. Risk Factor Analysis (ELISA IgG for Horses)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–492. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front. Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Haussig, J.M. West Nile virus keeps on moving up in Europe. Euro. Surveill. 2020, 25, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Negl. Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Nyamwaya, D.; Wang’ondu, V.; Amimo, J.; Michuki, G.; Ogugo, M.; Ontiri, E.; Sang, R.; Lindahl, J.; Grace, D.; Bett, B. Detection of West Nile virus in wild birds in Tana River and Garissa Counties, Kenya. BMC Infect. Dis. 2016, 16, 696. [Google Scholar] [CrossRef]
- Vogels, C.B.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017, 6, e96. [Google Scholar] [CrossRef]
- Iyer, A.V.; Kousoulas, K.G. A review of vaccine approaches for West Nile virus. Int. J. Environ. Res. Public Health 2013, 10, 4200–4223. [Google Scholar] [CrossRef]
- Selim, A.; Megahed, A.; Kandeel, S.; Alouffi, A.; Almutairi, M.M. West Nile virus seroprevalence and associated risk factors among horses in Egypt. Sci. Rep. 2021, 11, 20932. [Google Scholar] [CrossRef]
- Petruccelli, A.; Zottola, T.; Ferrara, G.; Iovane, V.; Di Russo, C.; Pagnini, U.; Montagnaro, S. West Nile Virus and Related Flavivirus in European Wild Boar (Sus scrofa), Latium Region, Italy: A Retrospective Study. Animals 2020, 10, 494. [Google Scholar] [CrossRef]
- Currenti, L.; Tasca, P.; Díaz, M.D.P.; Contigiani, M.; Spinsanti, L. Serological survey for Saint Louis encephalitis virus and West Nile virus in domestic mammals in Córdoba, Argentina: Are our pets potential sentinels? Arch. Virol. 2020, 165, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Teehee, M.L.; Bunning, M.L.; Stevens, S.; Bowen, R.A. Experimental infection of pigs with West Nile virus. Arch. Virol. 2005, 150, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Gyure, K.A. West Nile virus infections. J. Neuropathol. Exp. Neurol. 2009, 68, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.Y.; Guzman, H.; Zhang, H.; Travassos da Rosa, A.P.; Tesh, R.B. West Nile virus infection in the golden hamster (Mesocricetus auratus): A model for West Nile encephalitis. Emerg. Infect. Dis. 2001, 7, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Granwehr, B.P.; Lillibridge, K.M.; Higgs, S.; Mason, P.W.; Aronson, J.F.; Campbell, G.A.; Barrett, A.D. West Nile virus: Where are we now? Lancet. Infect. Dis. 2004, 4, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Emig, M.; Apple, D.J. Severe West Nile virus disease in healthy adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 38, 289–292. [Google Scholar] [CrossRef]
- Pepperell, C.; Rau, N.; Krajden, S.; Kern, R.; Humar, A.; Mederski, B.; Simor, A.; Low, D.E.; McGeer, A.; Mazzulli, T.; et al. West Nile virus infection in 2002: Morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ 2003, 168, 1399–1405. [Google Scholar]
- Guthrie, A.J.; Howell, P.G.; Gardner, I.A.; Swanepoel, R.E.; Nurton, J.P.; Harper, C.K.; Pardini, A.; Groenewald, D.; Visage, C.W.; Hedges, J.F.; et al. West Nile virus infection of Thoroughbred horses in South Africa (2000–2001). Equine Vet. J. 2003, 35, 601–605. [Google Scholar] [CrossRef]
- Kile, J.C.; Panella, N.A.; Komar, N.; Chow, C.C.; MacNeil, A.; Robbins, B.; Bunning, M.L. Serologic survey of cats and dogs during an epidemic of West Nile virus infection in humans. J. Am. Vet. Med. Assoc. 2005, 226, 1349–1353. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Marfin, A.A. Manifestations of West Nile neuroinvasive disease. Rev. Med. Virol. 2006, 16, 209–224. [Google Scholar] [CrossRef]
- Bertram, F.M.; Thompson, P.N.; Venter, M. Epidemiology and Clinical Presentation of West Nile Virus Infection in Horses in South Africa, 2016–2017. Pathogens 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Savic, V.; Klobucar, A.; Ferenc, T.; Ilic, M.; Bogdanic, M.; Tabain, I.; Stevanovic, V.; Santini, M.; Curman Posavec, M.; et al. Emerging Trends in the West Nile Virus Epidemiology in Croatia in the ‘One Health’ Context, 2011–2020. Trop. Med. Infect. Dis. 2021, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.; Traub-Dargatz, J.L.; Morley, P.S.; Wilmot, D.D.; Steffen, D.J.; Cunningham, W.E.; Salman, M.D. Outcome of equids with clinical signs of West Nile virus infection and factors associated with death. J. Am. Vet. Med. Assoc. 2004, 225, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Durand, B.; Haskouri, H.; Lowenski, S.; Vachiery, N.; Beck, C.; Lecollinet, S. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: Dog as an alternative WNV sentinel species? Epidemiol. Infect. 2016, 144, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Shaibi, T.; Saadawi, W.K.; Aghila, H.; Annajar, B.B. Prevalence of IgG antibodies for the West Nile virus in human population in Tripoli, Libya. J. Vector Borne Dis. 2017, 54, 183–186. [Google Scholar] [PubMed]
- Eybpoosh, S.; Fazlalipour, M.; Baniasadi, V.; Pouriayevali, M.H.; Sadeghi, F.; Ahmadi Vasmehjani, A.; Karbalaie Niya, M.H.; Hewson, R.; Salehi-Vaziri, M. Epidemiology of West Nile Virus in the Eastern Mediterranean region: A systematic review. PLoS Negl. Trop. Dis. 2019, 13, e0007081. [Google Scholar] [CrossRef]
- Benjelloun, A.; El Harrak, M.; Belkadi, B. West Nile Disease Epidemiology in North-West Africa: Bibliographical Review. Transbound. Emerg. Dis. 2016, 63, e153–e159. [Google Scholar] [CrossRef]
- M’ghirbi, Y.; Mousson, L.; Moutailler, S.; Lecollinet, S.; Amaral, R.; Beck, C.; Aounallah, H.; Amara, M.; Chabchoub, A.; Rhim, A.; et al. West Nile, Sindbis and Usutu Viruses: Evidence of Circulation in Mosquitoes and Horses in Tunisia. Pathogens 2023, 12, 360. [Google Scholar] [CrossRef]
- Ben Hassine, T.; Conte, A.; Calistri, P.; Candeloro, L.; Ippoliti, C.; De Massis, F.; Danzetta, M.L.; Bejaoui, M.; Hammami, S. Identification of Suitable Areas for West Nile Virus Circulation in Tunisia. Transbound. Emerg. Dis. 2017, 64, 449–458. [Google Scholar] [CrossRef]
- Lafri, I.; Hachid, A.; Bitam, I. West Nile virus in Algeria: A comprehensive overview. New Microbes New Infect. 2018, 27, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.W.; Meek, A.H. Veterinary Epidemiology: Principles and Methods. In Sampling Methods; Willeberg, P., Ed.; Iowa State University Press: Ames, IA, USA, 1987; pp. 22–47. [Google Scholar]
- Di Gennaro, A.; Lorusso, A.; Casaccia, C.; Conte, A.; Monaco, F.; Savini, G. Serum neutralization assay can efficiently replace plaque reduction neutralization test for detection and quantitation of West Nile virus antibodies in human and animal serum samples. Clin. Vaccine Immunol. 2014, 21, 1460–1462. [Google Scholar] [CrossRef] [PubMed]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Euro. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Olivares, J.; Wood, J. West Nile virus infection of horses. Vet. Res. 2004, 35, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Lauth, A.M.; Bowen, R.A. West Nile Virus: Veterinary Health and Vaccine Development. J. Med. Entomol. 2019, 56, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Ndione, M.; Rosà, R.; Marini, G.; Diagne, C.T.; Diagne, M.M.; Fall, G.; Faye, O.; Diallo, M.; Faye, O.; et al. Epidemiology of West Nile virus in Africa: An underestimated threat. PLoS Negl. Trop. Dis. 2022, 16, e0010075. [Google Scholar] [CrossRef]
- Jupp, P.G. The ecology of West Nile virus in South Africa and the occurrence of outbreaks in humans. Ann. N. Y. Acad. Sci. 2001, 951, 143–152. [Google Scholar] [CrossRef]
- Venter, M.; Steyl, J.; Human, S.; Weyer, J.; Zaayman, D.; Blumberg, L.; Leman, P.A.; Paweska, J.; Swanepoel, R. Transmission of West Nile virus during horse autopsy. Emerg. Infect. Dis. 2010, 16, 573–575. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Barros, S.C.; Ramos, F.; Fagulha, T.; Duarte, M.; Henriques, A.M.; Waap, H.; Luís, T.; Costa, T.; Amador, R.; Quintans, S.; et al. West Nile virus in horses during the summer and autumn seasons of 2015 and 2016, Portugal. Vet. Microbiol. 2017, 212, 75–79. [Google Scholar] [CrossRef]
- Monastiri, A.; Mechri, B.; Vázquez-González, A.; Ar Gouilh, M.; Chakroun, M.; Loussaief, C.; Mastouri, M.; Dimassi, N.; Boughzala, L.; Aouni, M.; et al. A four-year survey (2011–2014) of West Nile virus infection in humans, mosquitoes and birds, including the 2012 meningoencephalitis outbreak in Tunisia. Emerg. Microbes Infect. 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, W.; Wang, Y.; Sui, H.; Liu, B.; Li, F.; He, Y.; Li, Z.; Fu, S.; Wang, L.; et al. Surveillance of West Nile virus infection in Kashgar Region, Xinjiang, China, 2013–2016. Sci. Rep. 2021, 11, 14010. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Petrovic, T.; Savic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Klobucar, A.; Mrzljak, A.; Ilic, M.; Bogdanic, M.; et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B.; Diallo, M.; Boye, C.S.; Sall, A.A. Usutu virus in Africa. Vector Borne Zoonotic Dis. 2011, 11, 1417–1423. [Google Scholar] [CrossRef]
- Guis, H.; Raveloarijaona, B.N.; Rasamoelina, V.M.; Rakotoharinome, V.M.; Rabarisoa, R.; Raveloson, B.; Razafindralambo, J.R.; Ravaomanana, J.; Cetre-Sossah, C.; Kantorovitch, V.; et al. In Proceedings of the Abstract Book of the 15th International Symposium of Veterinary Epidemiology and Economics (ISVEE 15). Chiang Mai, Thailand, 12–16 November 2018; p. 457. [Google Scholar]
- García-Bocanegra, I.; Arenas-Montes, A.; Napp, S.; Jaén-Téllez, J.A.; Fernández-Morente, M.; Fernández-Molera, V.; Arenas, A. Seroprevalence and risk factors associated to West Nile virus in horses from Andalusia, Southern Spain. Vet. Microbiol. 2012, 160, 341–346. [Google Scholar] [CrossRef]
- Rios, L.M.; Sheu, J.J.; Day, J.F.; Maruniak, J.E.; Seino, K.; Zaretsky, H.; Long, M.T. Environmental risk factors associated with West Nile virus clinical disease in Florida horses. Med. Vet. Entomol. 2009, 23, 357–366. [Google Scholar] [CrossRef]

| Total Number of Samples | Age (Months) | Sex | Animal Breed | Area |
|---|---|---|---|---|
| Horses (n = 574) | 2–240 | 49.3% male (n = 283) 50.7% female (n = 291) | Arabian (n = 145, 25.3%) | Western Libya |
| Local thoroughbred (n = 202, 35.2%) | ||||
| Imported thoroughbred (n = 93, 16.2%) | ||||
| Local Libyan (n = 35, 6.1%) | ||||
| Mixed (n = 99, 17.2%) | Eastern Libya | |||
| Dogs (n = 63) | 3–72 | 49.2% male (n = 31) 50.8% female (n = 32) | Many breeds | Tripoli |
| Total Samples | Positive Samples | VNT Titre Range | |
|---|---|---|---|
| c-ELISA | VNT | ||
| Horses (n = 574) | 13.2% (n = 76/574) | Of positive ELISA: n = 62/80 (77.5%) Of total samples: n = 62/574 (10.8%) | 1:10–1:640 |
| Dogs (n = 63) | 30.2% (n = 19/63) | Of positive ELISA: n = 17/19 (89.5%) Of total samples: n = 17/63 (27%) | 1:10–1:320 |
| Breed | ELISA IgG | Total | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Arabian | Count | 116 | 29 | 145 |
| % within breed | 80% | 20% | 100.0% | |
| % of total | 24.4% | 6.1% | 30.5% | |
| Local Thoroughbred | Count | 172 | 30 | 202 |
| % within breed | 85.1% | 14.9% | 100.0% | |
| % of total | 36.2% | 6.3% | 42.5% | |
| Imported Thoroughbred | Count | 80 | 13 | 93 |
| % within breed | 86.0% | 14.0% | 100.0% | |
| % of total | 16.8% | 2.7% | 19.6% | |
| Libyan | Count | 31 | 4 | 35 |
| % within breed | 88.6% | 11.4% | 100.0% | |
| % of total | 6.5% | 0.8% | 7.4% | |
| Total * | Count | 399 | 76 | 475 |
| % within breed | 84% | 16% | 100.0% | |
| % of total | 84% | 16% | 100.0% | |
| Animal | Animal Origin (City) | c-ELISA (IgG) Results | |
|---|---|---|---|
| Negative | Positive | ||
| Horses | Al-Marj a (n = 99) | n = 99 (100%) | n = 0 (0%) |
| Gasr Ben Ghashir b (n = 140) | n = 119 (85%) | n = 21 (15%) | |
| Al-Swani b (n = 68) | n = 54 (79.4%) | n = 14 (20.6%) | |
| Zawarah b (n = 56) | n = 51 (91.1%) | n = 5 (8.9%) | |
| Tripoli b (n = 157) | n = 135 (86%) | n = 22 (14%) | |
| Al-Zawia b (n = 26) | n = 15 (57.7%) | n = 11 (42.3%) | |
| Surman b (n = 28) | n = 25 (89.3%) | n = 3 (10.7%) | |
| Total (574) | n = 498 (86.8%) | n = 76 (13.2%) | |
| Dogs | Tripoli b (n = 63) | n = 44 (69.8%) | n = 19 (30.2%) |
| Age Group | ELISA IgG | VNT | Total | ||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Doubtful | Negative | Positive | |||
| <6 months | Count | 16 | 0 | 0 | 0 | 0 | 16 |
| % within age group | 100.0% | 0.0% | 0.0% | N/D | N/D | 100.0% | |
| 7–18 months | Count | 115 | 5 | 2 | 7 | 0 | 122 |
| % within age group | 94.2% | 4.1% | 1.6% | 100% | 0.0% | 100.0% | |
| 19–48 months | Count | 303 | 51 | 1 | 7 | 45 | 355 |
| % within age group | 85.4% | 14.4% | 0.2% | 13.5% | 86.5% | 100.0% | |
| 49–72 months | Count | 39 | 10 | 1 | 1 | 10 | 50 |
| % within age group | 78% | 20% | 2% | 9.1% | 90.9% | 100.0% | |
| >72 months | Count | 21 | 10 | 0 | 3 | 7 | 31 |
| % within age group | 67.7% | 32.3% | 0.0% | 30% | 70% | 100.0% | |
| Total | Count | 494 | 76 | 4 | 18 | 62 | 574 |
| % within age group | 86.1% | 13.2% | 0.7% | 22.5% | 77.5% | 100.0% | |
| Variables in Equation | |||||||
|---|---|---|---|---|---|---|---|
| B | SE | Wald | df | p-Value | Exp(B) | ||
| Step 1 a | Age group | 0.698 | 0.150 | 21.610 | 1 | 0.0001 | 2.010 |
| Constant | −4.017 | 0.501 | 64.399 | 1 | 0.0001 | 0.018 | |
| Step 2 b | Age group | 0.661 | 0.157 | 17.760 | 1 | 0.0001 | 1.936 |
| Sex | 0.610 | 0.265 | 5.298 | 1 | 0.021 | 1.841 | |
| Constant | −4.874 | 0.655 | 55.443 | 1 | 0.0001 | 0.008 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Mostafa, K.K.; Savini, G.; Di Gennaro, A.; Teodori, L.; Leone, A.; Monaco, F.; Alaoqib, M.M.A.; Rayes, A.A.; Dayhum, A.; Eldaghayes, I. Evidence of West Nile Virus Circulation in Horses and Dogs in Libya. Pathogens 2024, 13, 41. https://doi.org/10.3390/pathogens13010041
Ben-Mostafa KK, Savini G, Di Gennaro A, Teodori L, Leone A, Monaco F, Alaoqib MMA, Rayes AA, Dayhum A, Eldaghayes I. Evidence of West Nile Virus Circulation in Horses and Dogs in Libya. Pathogens. 2024; 13(1):41. https://doi.org/10.3390/pathogens13010041
Chicago/Turabian StyleBen-Mostafa, Kholoud Khalid, Giovanni Savini, Annapia Di Gennaro, Liana Teodori, Alessandra Leone, Federica Monaco, Mohammed Masoud A. Alaoqib, Abdunnabi A. Rayes, Abdunaser Dayhum, and Ibrahim Eldaghayes. 2024. "Evidence of West Nile Virus Circulation in Horses and Dogs in Libya" Pathogens 13, no. 1: 41. https://doi.org/10.3390/pathogens13010041
APA StyleBen-Mostafa, K. K., Savini, G., Di Gennaro, A., Teodori, L., Leone, A., Monaco, F., Alaoqib, M. M. A., Rayes, A. A., Dayhum, A., & Eldaghayes, I. (2024). Evidence of West Nile Virus Circulation in Horses and Dogs in Libya. Pathogens, 13(1), 41. https://doi.org/10.3390/pathogens13010041








