Preliminary Results of Feasibility and Acceptability of Self-Collection for Cervical Screening in Italian Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Samples Collection and Processing
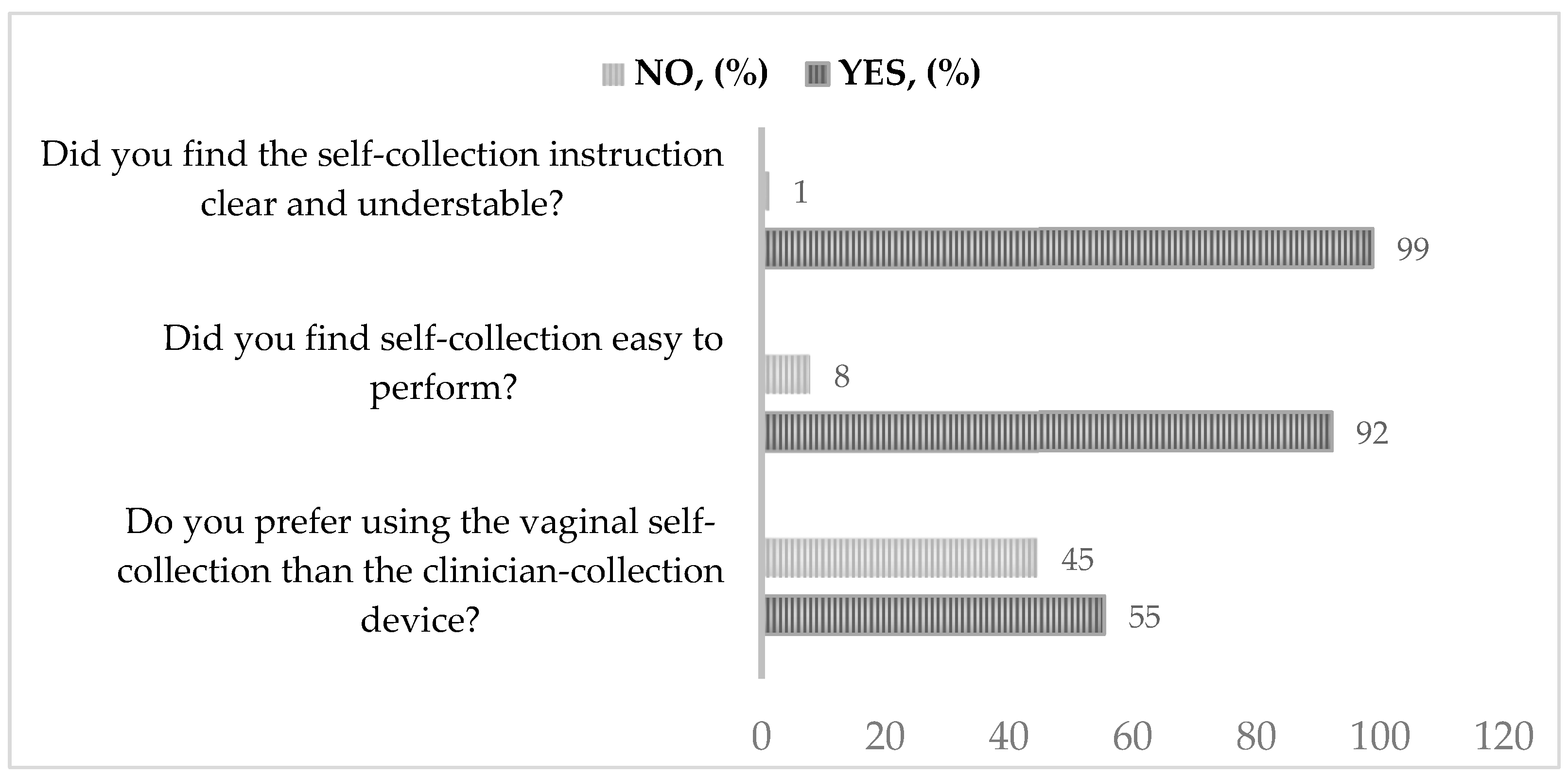
2.3. Questionnaire for Epidemiological Data Collection and Acceptance of Self-Sampling
- ▪
- Did you find the self-collection instruction clear and understandable?
- ▪
- Did you find self-collection easy to perform?
- ▪
- Do you prefer using the vaginal self-collection than the clinician-collection device?
2.4. Statistical Analyses
3. Results
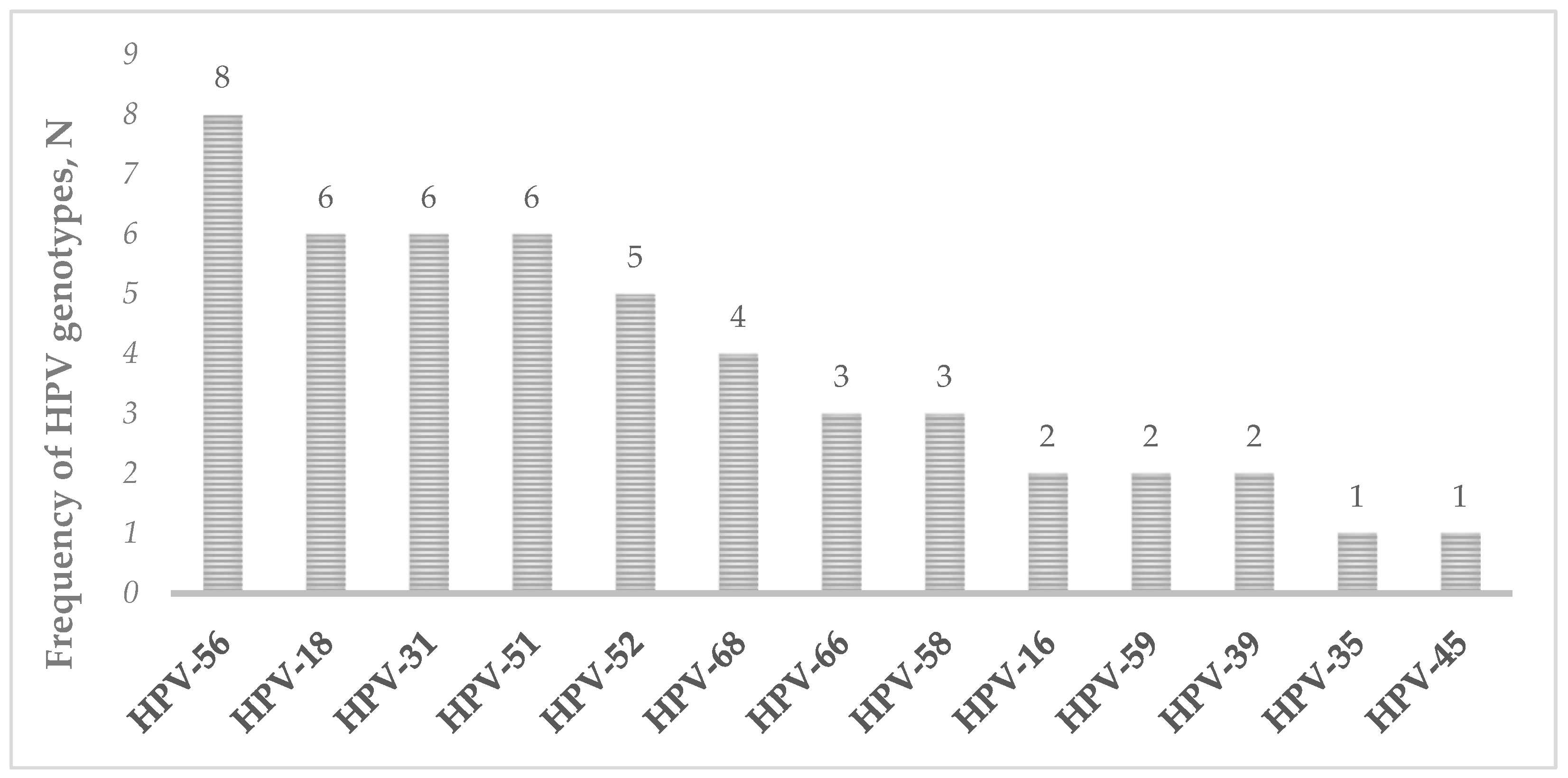
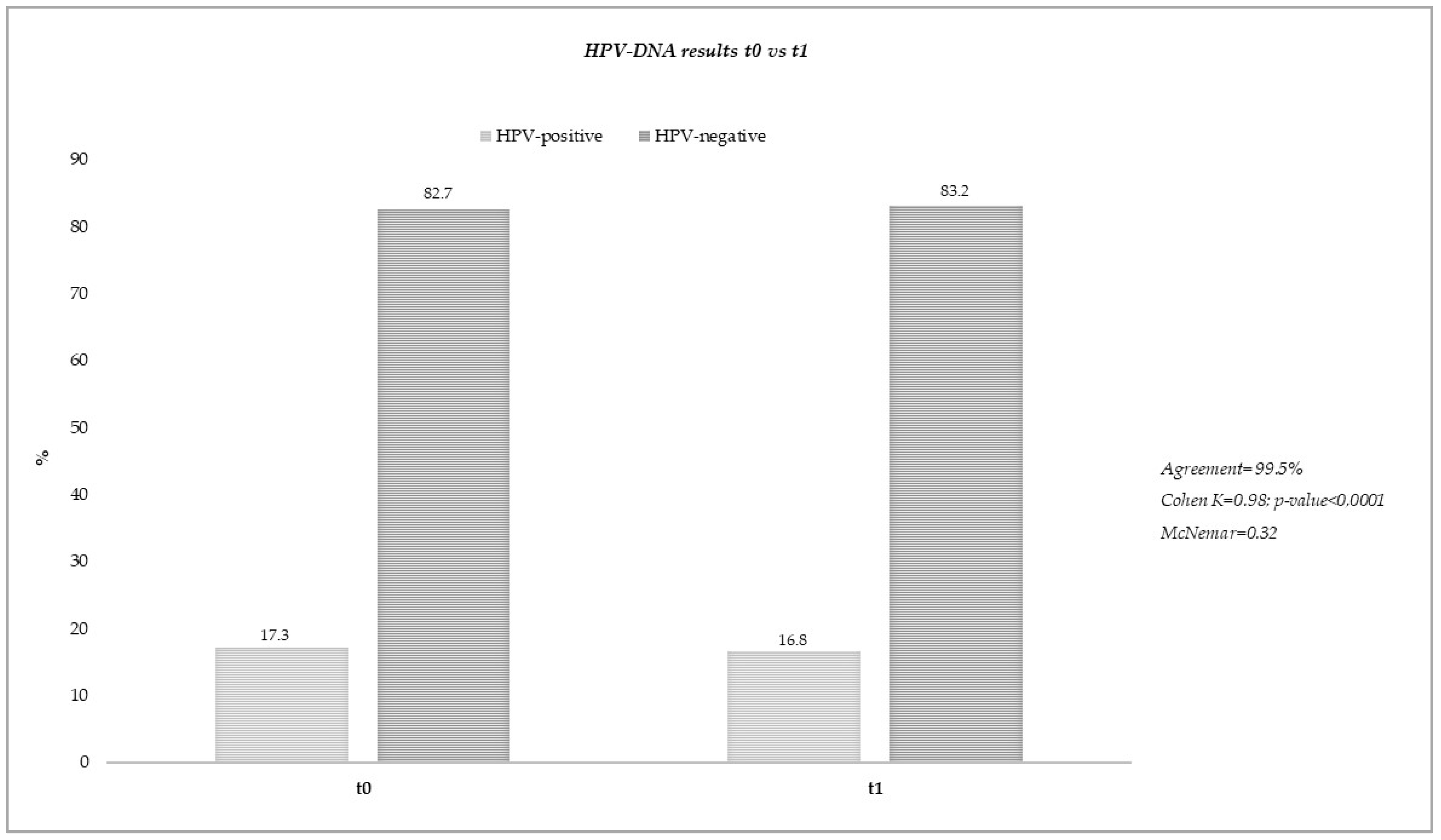
3.1. Stability of Vaginal Self-Collected Samples Eluted in eNat® Medium
3.2. Results of Acceptability Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory-International Agency for Research on Cancer. Available online: https://gco.iarc.fr/ (accessed on 30 March 2021).
- Centers for Diseases Control and Prevention. Available online: https://www.cdc.gov/cancer/cervical/index.htm (accessed on 10 October 2022).
- Licence: CC BY-NC-SA 3.0 IGO; Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. World Health Organization: Geneva, Switzerland, 2020.
- Arbyn, M.; Gultekin, M.; Morice, P.; Nieminen, P.; Cruickshank, M.; Poortmans, P.; Kelly, D.; Poljak, M.; Bergeron, C.; Ritchie, D.; et al. The European response to the WHO call to eliminate cervical cancer as a public health problem. Int. J. Cancer 2021, 148, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health 2022, 10, e1115–e1127. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità—Sorveglianza Passi. 2021. Available online: https://www.epicentro.iss.it/passi/dati/screeningcervicale (accessed on 26 August 2023).
- Preti, M.; Selk, A.; Stockdale, C.; Bevilacqua, F.; Vieira-Baptista, P.; Borella, F.; Gallio, N.; Cosma, S.; Melo, C.; Micheletti, L.; et al. Knowledge of Vulvar Anatomy and Self-examination in a Sample of Italian Women. J. Low. Genit. Tract Dis. 2021, 25, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Rosso, S.; Micheletti, L.; Libero, C.; Sobrato, I.; Giordano, L.; Busso, P.; Gallio, N.; Cosma, S.; Bevilacqua, F.; et al. Risk of HPV-related extra-cervical cancers in women treated for cervical intraepithelial neoplasia. BMC Cancer 2020, 20, 972. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Verdoodt, F.; Snijders, P.J.F.; Verhoef, V.M.J.; Suonio, E.; Dillner, L.; Minozzi, S.; Bellisario, C.; Banzi, R.; Zhao, F.-H.; et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis. Lancet Oncol. 2014, 15, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [PubMed]
- Sechi, I.; Cocuzza, C.E.; Martinelli, M.; Muresu, N.; Castriciano, S.; Sotgiu, G.; Piana, A. Comparison of Different Self-Sampling Devices for Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs): A Pilot Study. Healthcare 2022, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-W.; Hong, J.H.; Min, K.J.; Ouh, Y.-T.; Seong, S.J.; Moon, J.H.; Cho, S.H.; Lee, J.K. Performance and Diagnostic Accuracy of Human Papillomavirus Testing on Self-Collected Urine and Vaginal Samples in a Referral Population. Cancer Res. Treat. 2021, 53, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Coleman, J.S. The HPV self-collection paradox: Boosting cervical cancer screening, struggling with follow-up care. Lancet Public Health 2023, 8, e394–e395. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Latsuzbaia, A.; Castle, P.E.; Sahasrabuddhe, V.V.; Broeck, D.V. HPV testing of self-samples: Influence of collection and sample handling procedures on clinical accuracy to detect cervical precancer. Lancet Reg. Health-Eur. 2022, 14, 100332. [Google Scholar] [CrossRef] [PubMed]
- Presser, B.E.; Katz, M.L.; Shoben, A.B.; Moore, D.; Ruffin, M.T.; Paskett, E.D.; Reiter, P.L. Effects of an Education Intervention about HPV Self-Testing for Healthcare Providers and Staff. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2018, 33, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Seegene AdvanSure™ E3 SYSTEM—Automated Liquid Handling Workstations for Nucleic Acid Extraction. Available online: https://seegenetech.com/seegene-advansure (accessed on 10 October 2022).
- Anyplex™ II HPV HR Detection. Available online: https://www.seegene.com/assays/anyplex2_hpv_hr_detection# (accessed on 10 October 2022).
- Martinelli, M.; Giubbi, C.; Sechi, I.; Bottari, F.; Iacobone, A.D.; Musumeci, R.; Perdoni, F.; Muresu, N.; Piana, A.; Fruscio, R.; et al. Evaluation of BD Onclarity™ HPV Assay on Self-Collected Vaginal and First-Void Urine Samples as Compared to Clinician-Collected Cervical Samples: A Pilot Study. Diagnostics 2022, 12, 3075. [Google Scholar] [CrossRef] [PubMed]
- Connor, L.; Cuschieri, K.; Sargent, A. The self-sampling journey: Technical considerations and challenges. Available online: www.HPVWorld.com (accessed on 26 August 2023).
- Giubbi, C.; Martinelli, M.; Vallini, I.; Paganoni, S.; Dafa’Alla, T.; Perdoni, F.; Musumeci, R.; Wu, W.; Castriciano, S.; Romano, P.; et al. Human papillomavirus (HPV) detection in vaginal self-samples: Evaluation of eNat® as an alternative suspension medium to ThinPrep®PreservCyt® for vaginal swabs. Open Res. Eur. 2022, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Richard-Greenblatt, M.; Comar, C.E.; Flevaud, L.; Berti, M.; Harris, R.M.; Weiss, S.R.; Glaser, L. Copan eNAT Transport System To Address Challenges in COVID-19 Diagnostics in Regions with Limited Testing Access. J. Clin. Microbiol. 2021, 59, e00110-21. [Google Scholar] [CrossRef] [PubMed]
- Taku, O.; Meiring, T.L.; Gustavsson, I.; Phohlo, K.; Garcia-Jardon, M.; Mbulawa, Z.Z.A.; Businge, C.B.; Gyllensten, U.; Williamson, A.-L. Acceptability of self- collection for human papillomavirus detection in the Eastern Cape, South Africa. PLoS ONE 2020, 15, e0241781. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, V.; Petignat, P.; Burton-Jeangros, C. To what extent will women accept HPV self-sampling for cervical cancer screening? A qualitative study conducted in Switzerland. Int. J. Women’s Health 2015, 7, 883–888. [Google Scholar] [CrossRef]
- Aitken, C.A.; Inturrisi, F.; Kaljouw, S.; Nieboer, D.; Siebers, A.G.; Melchers, W.J.G.; Brule, A.J.C.v.D.; Molijn, A.; Hinrichs, J.W.J.; Niesters, H.G.M.; et al. Sociodemographic Characteristics and Screening Outcomes of Women Preferring Self-Sampling in the Dutch Cervical Cancer Screening Programme: A Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2022, 32, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kirubarajan, A.; Leung, S.; Li, X.; Yau, M.; Sobel, M. Barriers and facilitators for cervical cancer screening among adolescents and young people: A systematic review. BMC Women’s Health 2021, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Italy. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 16 September 2023).
- Muresu, N.; Sotgiu, G.; Marras, S.; Gentili, D.; Sechi, I.; Cossu, A.; Dettori, A.; Pietri, R.E.; Paoni, L.; Ghi, M.E.; et al. Cervical Screening in North Sardinia (Italy): Genotype Distribution and Prevalence of HPV among Women with ASC-US Cytology. Int. J. Environ. Res. Public Health 2022, 19, 693. [Google Scholar] [CrossRef] [PubMed]
- Muresu, N.; Sotgiu, G.; Saderi, L.; Sechi, I.; Cossu, A.; Marras, V.; Meloni, M.; Martinelli, M.; Cocuzza, C.; Tanda, F.; et al. Italian observational study on HPV infection, E6, and p16 expression in men with penile cancer. Virol. J. 2020, 17, 161. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cohort Study (n = 185) | |
|---|---|---|
| Median (IQR) age, years | 41 (34–51) | |
| Education level, n (%) | Elementary school | 0 (0.0) |
| Middle school | 43 (23.4) | |
| High school | 69 (37.5) | |
| Degree | 72 (39.1) | |
| Civil Status, n (%) | Single | 51 (29.7) |
| Widow | 2 (1.2) | |
| Married | 103 (59.9) | |
| Divorced | 16 (9.3) | |
| Smoking status, n (%) | 51 (37.2) | |
| BMI Median (IQR) | 23.0 (20.8–26.2) | |
| Menopause, n (%) | 44 (24.4) | |
| N° pregnancy, Median (IQR) | 1 (0–2) | |
| Use of contraceptives, n (%) | 146 (96.1) | |
| Use of condom, n (%) | Never | 38 (22.8) |
| Always | 82 (49.1) | |
| Occasionally | 22 (13.2) | |
| In the past | 25 (15.0) | |
| History of STI, n (%) | 14 (7.9) | |
| Sample ID | HPV-DNA t0 * | HPV-DNA t1 * |
|---|---|---|
| SS014 | HPV-51(+++) | HPV-51(+++) |
| SS016 | HPV-56(++) | HPV-56(+) |
| NU026 | HPV-18(++); HPV-39(+); HPV-56(++) | HPV-18(++); HPV-39(+); HPV-56(++) |
| NU046 | HPV-31(+) | HPV-31(+) |
| NU048 | HPV-51(+); HPV-58(++) | HPV-51(++); HPV-58(+) |
| NU050 | HPV-52(+); HPV-56(+) | HPV-52(+); HPV-56(+) |
| NU051 | HPV-18(+) | Negative |
| NU056 | HPV-58(+++) | HPV-58(+++) |
| NU069 | HPV-51(+) | HPV-51(+) |
| NU070 | HPV-56(+); HPV-66(++); HPV-68(++) | HPV-56(++); HPV-66(++); HPV-68(+) |
| NU071 | HPV-31(++); HPV-51(++) | HPV-31(++); HPV-51(++) |
| SS021 | HPV-39 (+) | HPV-39 (++) |
| CA067 | HPV-31(+) | HPV-31(+) |
| CA069 | HPV-52(++) | HPV-52(++) |
| CA070 | HPV-68(+) | HPV-68(+) |
| CA071 | HPV-52(++) | HPV-52(++) |
| CA073 | HPV-56(++) | HPV-56(++) |
| CA076 | HPV-56(+) | HPV-56(+) |
| CA078 | HPV-52(+) | HPV-52(+) |
| CA079 | HPV-56(++); HPV-68(++) | HPV-56(++); HPV-68(++) |
| CA082 | HPV-16(++) | HPV-16(++) |
| CA089 | HPV-51(+); HPV-56(++) | HPV-51(+); HPV-56(++) |
| CA095 | HPV-18(++); HPV-58(+) | HPV-18(++); HPV-58(+) |
| NU076 | HPV-18(+); HPV-31(++) | HPV-18(++); HPV-31(++) |
| NU090 | HPV-31(++); HPV-35(+); HPV-45(++) | HPV-31(++); HPV-35(+); HPV-45(++) |
| NU094 | HPV-18(++) | HPV-18(++) |
| NU095 | HPV-66(+++) | HPV-66(+) |
| NU096 | HPV-51(+) | HPV-51(++) |
| SS053 | HPV-52(++); HPV-59(+) | HPV-52(++); HPV-59(+) |
| SS057 | HPV-31(+); HPV-66(+) | HPV-31(+); HPV-66(+) |
| SS090 | HPV-18(++) | HPV-18(+) |
| SS095 | HPV-16(++); HPV-68(+++) | HPV-16(++); HPV-68(+++) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sechi, I.; Muresu, N.; Puci, M.V.; Saderi, L.; Del Rio, A.; Cossu, A.; Muroni, M.R.; Castriciano, S.; Martinelli, M.; Cocuzza, C.E.; et al. Preliminary Results of Feasibility and Acceptability of Self-Collection for Cervical Screening in Italian Women. Pathogens 2023, 12, 1169. https://doi.org/10.3390/pathogens12091169
Sechi I, Muresu N, Puci MV, Saderi L, Del Rio A, Cossu A, Muroni MR, Castriciano S, Martinelli M, Cocuzza CE, et al. Preliminary Results of Feasibility and Acceptability of Self-Collection for Cervical Screening in Italian Women. Pathogens. 2023; 12(9):1169. https://doi.org/10.3390/pathogens12091169
Chicago/Turabian StyleSechi, Illari, Narcisa Muresu, Mariangela V. Puci, Laura Saderi, Arcadia Del Rio, Andrea Cossu, Maria R. Muroni, Santina Castriciano, Marianna Martinelli, Clementina E. Cocuzza, and et al. 2023. "Preliminary Results of Feasibility and Acceptability of Self-Collection for Cervical Screening in Italian Women" Pathogens 12, no. 9: 1169. https://doi.org/10.3390/pathogens12091169
APA StyleSechi, I., Muresu, N., Puci, M. V., Saderi, L., Del Rio, A., Cossu, A., Muroni, M. R., Castriciano, S., Martinelli, M., Cocuzza, C. E., Sotgiu, G., & Piana, A. (2023). Preliminary Results of Feasibility and Acceptability of Self-Collection for Cervical Screening in Italian Women. Pathogens, 12(9), 1169. https://doi.org/10.3390/pathogens12091169










