Latent Tuberculosis Infection and Associated Risk Factors among People Living with HIV and HIV-Uninfected Individuals in Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Subjects
- (1)
- Hospitalized or consulting for any reason except HIV in Vilnius University Hospital Infectious Diseases Centre;
- (2)
- Consulting for any reason except HIV in the outpatient clinic of the Republican Siauliai County Hospital Infectious Diseases Department;
- (3)
- Consulting for any reason except HIV in the outpatient clinic of Klaipeda University Hospital Infectious Diseases Department.
- -
- Screening for infectious and parasitic diseases (Z11), 50.8% of patients;
- -
- Viral hepatitis (B15–B19), 20.8% of patients;
- -
- Viral infections of the central nervous system (A80–A89), 7.5% of patients;
- -
- Lyme disease (A69.2), 3.5% of patients;
- -
- Fever of unknown origin (R50.9), 3% of patients.
- -
- With active TB or past history of TB;
- -
- Pregnant or breastfeeding women;
- -
- Persons who could not give informed consent owing to a mental disorder.
2.2. Ethical Statement
2.3. Data Collection and Assessment of LTBI
2.4. Primary and Secondary Outcomes
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Prevalence of LTBI
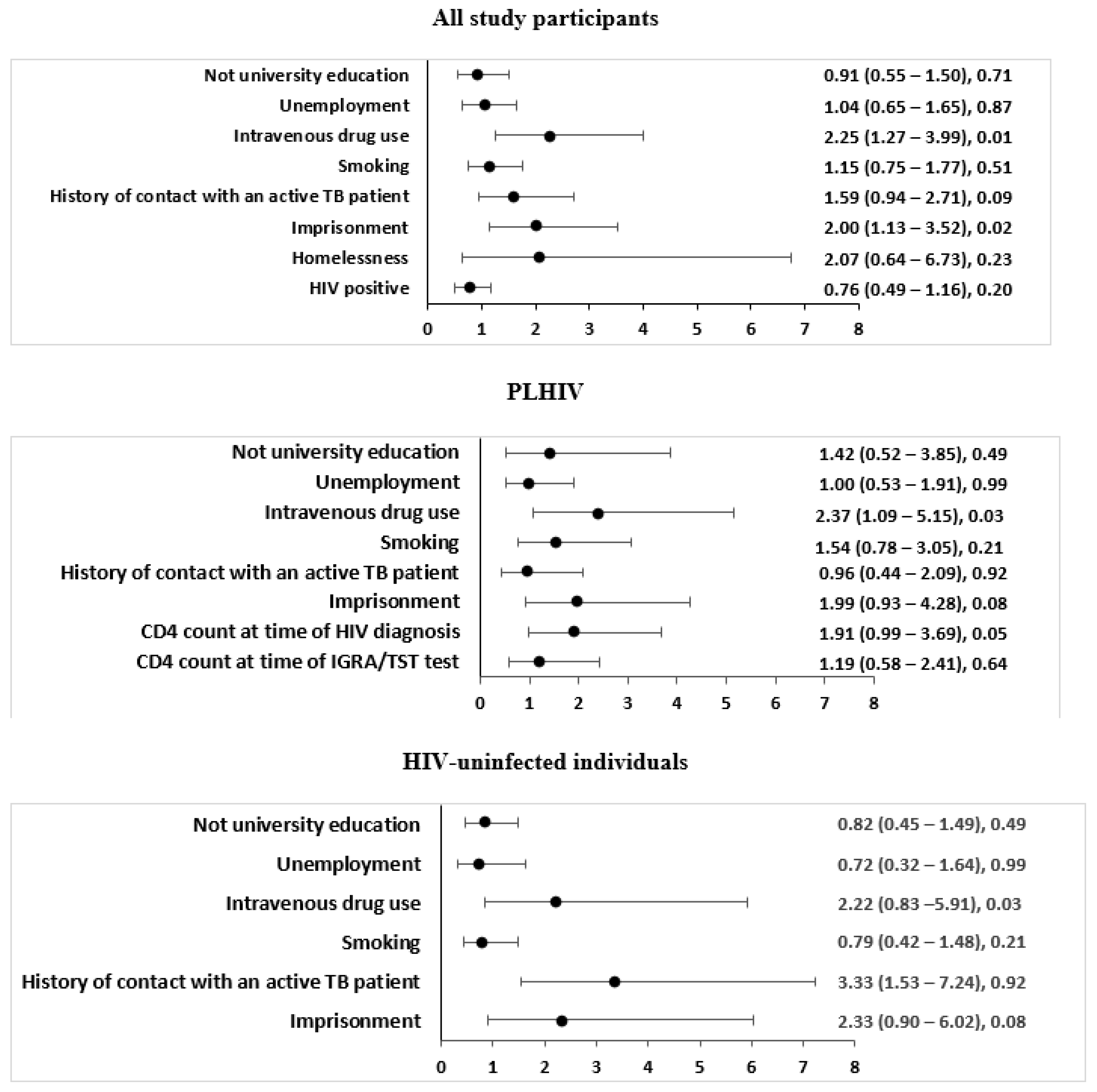
3.3. Factors Associated with LTBI in All Study Participants
3.4. Factors Associated with LTBI among PLHIV
3.5. Factors Associated with LTBI among HIV-Uninfected Individuals
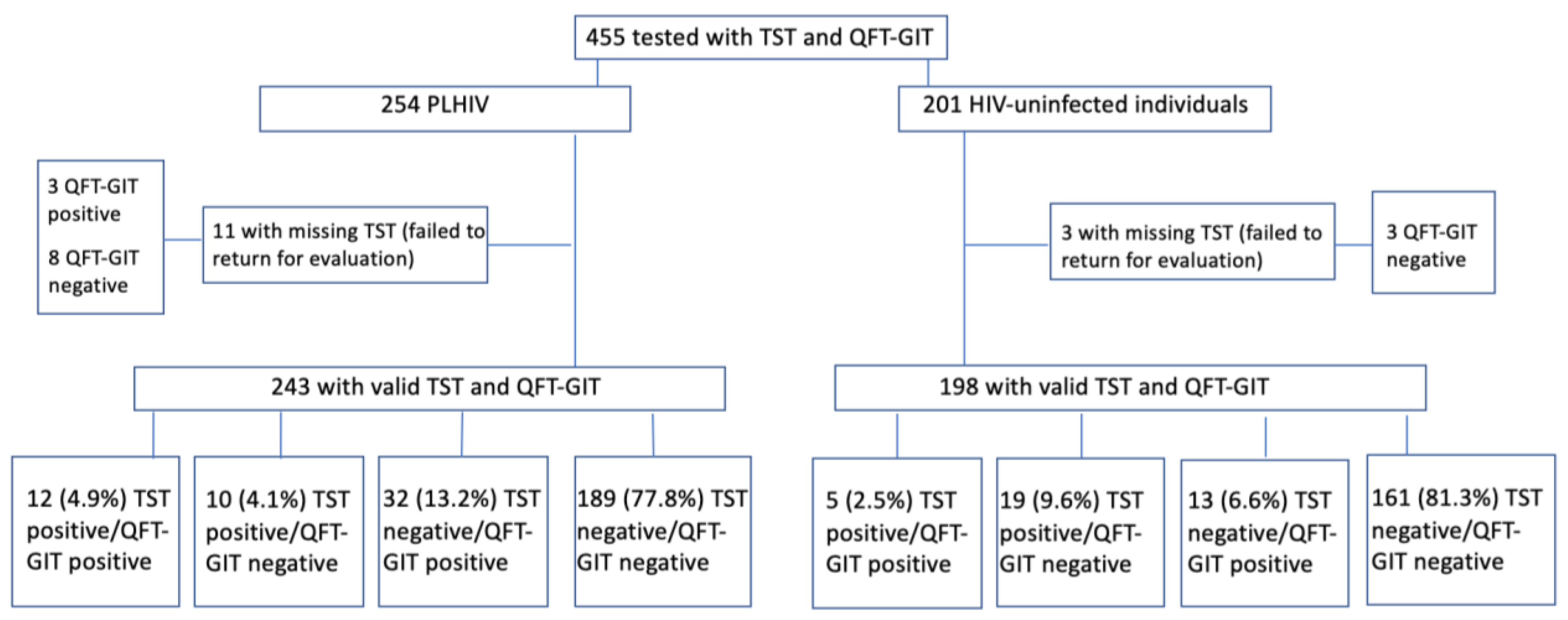
3.6. Agreement between IGRA and TST
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2021–2020 Data; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Kaef, C.; Benzon, A.; Panteleev, A.; Skrahina, A.; Bolokadze, N.; Tetradov, S.; Podlasin, R.; Karpov, I.; Borodulina, E.; Denisova, E.; et al. Delayed diagnosis of tuberculosis in persons living with HIV in Eastern Europe: Associated factors and effect on mortality—A multicentre prospective cohort study. BMC Infect. Dis. 2021, 21, 1038. [Google Scholar]
- WHO. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2018.
- Dye, C.; Scheele, S.; Dolin, P.; Pathania, V.; Raviglione, M.C. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. J. Am. Med. Assoc. 1999, 282, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines on the Management of Latent Tuberculosis Infection; World Health Organization: Geneva, Switzerland, 2022.
- Acuña-Villaorduña, C.; Jones-López, E.C.; Fregona, G.; Marques-Rodrigues, P.; Gaeddert, M.; Geadas, C.; Hadad, D.J.; White, L.F.; Molina, L.P.D.; Vinhas, S.; et al. Intensity of exposure to pulmonary tuberculosis determines risk of tuberculosis infection and disease. Eur. Respir. J. 2018, 51, 1701578. [Google Scholar] [CrossRef]
- Saunders, M.J.; Wingfield, T.; Tovar, M.A.; Baldwin, M.R.; Datta, S.; Zevallos, K.; Montoya, R.; Valencia, T.R.; Friedland, J.S.; Moulton, L.H.; et al. A score to predict and stratify risk of tuberculosis in adult contacts of tuberculosis index cases: A prospective derivation and external validation cohort study. Lancet Infect. Dis. 2017, 17, 1190–1199. [Google Scholar] [CrossRef]
- Ruhwald, M.; Diel, R. Escaping the Plato’s cave of latent tuberculosis testing: A path for developers of predictive tests for risk of tuberculosis. Eur. Respir. J. 2018, 52, 1801616. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2020—2018 Data; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2021—2019 Data; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2022—2021 Data; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2023—2021 Data; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- The State Information System of Tuberculosis Home Page. Available online: https://2014.esinvesticijos.lt/lt/dokumentai//2014-2020-metu-europos-sajungos-fondu-investiciju-veiksmu-programos-8-prioriteto-socialines-itraukties-didinimas-ir-kova-su-skurdu-priemones-nr-08-4-2-esfa-r-615-priemoniu-gerinanciu-ambulatoriniu-sveikatos-prieziuros-paslaugu-prieinamuma-tuberkulioz/tuberkuliozes-valstybes-informacines-sistemos-registro-duomenys (accessed on 15 July 2022).
- The Centre for Communicable Diseases and AIDS Home Page. Available online: https://nvsc.lrv.lt (accessed on 17 July 2022).
- Matulyte, E.; Davidaviciene, E.; Kancauskiene, Z.; Diktanas, S.; Kausas, A.; Velyvyte, D.; Urboniene, J.; Lipnickiene, V.; Laurencikaite, M.; Danila, E.; et al. The socio-demographic, clinical characteristics and outcomes of tuberculosis among HIV infected adults in Lithuania: A thirteen-year analysis. PLoS ONE 2023, 18, e0282046. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2020–2019 Data; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Recommendations for Investigating Contacts of Persons with Infectious Tuberculosis in Low- and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2012.
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Cohen, A.; Mathiasen, V.D.; Schön, T.; Wejse, C. The global prevalence of latent tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2019, 54, 1900655. [Google Scholar] [CrossRef]
- Lin, W.C.; Lin, H.H.; Lee, S.S.; Sy, C.L.; Wu, K.S.; Chen, J.K.; Tsai, H.C.; Chen, Y.S. Prevalence of latent tuberculosis infection in persons with and without human immunodeficiency virus infection using two interferon-gamma release assays and tuberculin skin test in a low human immunodeficiency virus prevalence, intermediate tuberculosis-burden country. J. Microbiol. Immunol. Infect. 2016, 49, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Latorre, I.; Martínez-Lacasa, X.; Font, R.; Lacoma, A.; Puig, J.; Tural, C.; Lite, J.; Prat, C.; Cuchi, E.; Ausina, V.; et al. IFN-γ response on T-cell based assays in HIV-infected patients for detection of tuberculosis infection. BMC Infect. Dis. 2010, 10, 348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cattamanchi, A.; Smith, R.; Steingart, K.R.; Metcalfe, J.Z.; Date, A.; Coleman, C.; Marston, B.J.; Huang, L.M.; Hopewell, P.C.; Pai, M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: A systematic review and meta-analysis. J. Acquir. Immune. Defic. Syndr. 2011, 56, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Tabarsi, P.; Mohammadtaheri, Z.; Chitsaz, E.; Farokhzad, B.; Hadavand, F.; Gachkar, L.; Nemati, K.; Masjedi, M.R. Performance of QuantiFERON-TB Gold test compared to tuberculin skin test in detecting latent tuberculosis infection in HIV-positive individuals in Iran. Ann. Thorac. Med. 2010, 5, 43–46. [Google Scholar]
- Chkhartishvili, N.; Kempker, R.R.; Dvali, N.; Abashidze, L.; Sharavdze, L.; Gabunia, P.; Blumberg, H.M.; del Rio, C.; Tsertsvadze, T. Poor agreement between interferon-gamma release assays and the tuberculin skin test among HIV-infected individuals in the country of Georgia. BMC Infect. Dis. 2013, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Schölvinck, E.; Wilkinson, K.A.; Whelan, A.O.; Martineau, A.R.; Levin, M.; Wilkinson, R.J. Gamma interferon-based immunodiagnosis of tuberculosis: Comparison between whole-blood and enzyme-linked immunospot methods. J. Clin. Microbiol. 2004, 42, 829–831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, N.S.; Leung, C.C.; Chan, K.C.W.; Chan, W.K.; Lin, A.W.C.; Lee, S.S. A longitudinal study on latent TB infection screening and its association with TB incidence in HIV patients. Sci. Rep. 2019, 9, 10093. [Google Scholar] [CrossRef]
- Lin, A.W.; Lau, S.K.; Woo, P.C. Screening and treatment of latent tuberculosis infection among HIV-infected patients in resource-rich settings. Expert. Rev. Anti. Infect. Ther. 2016, 14, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Sester, M.; van Leth, F.; Bruchfeld, J.; Bumbacea, D.; Cirillo, D.M.; Dilektasli, A.G.; Domínguez, J.; Duarte, R.; Ernst, M.; Eyuboglu, F.O.; et al. Risk assessment of tuberculosis in immunocompromised patients. TBNET Study Am. J. Respir. Crit. Care Med. 2014, 190, 1168–1176. [Google Scholar] [CrossRef]
- Ho, C.S.; Feng, P.I.; Narita, M.; Stout, J.E.; Chen, M.; Pascopella, L.; Garfein, R.; Reves, R.; Katz, D.J. Tuberculosis Epidemiologic Studies Consortium. Comparison of three tests for latent tuberculosis infection in high-risk people in the USA: An observational cohort study. Lancet Infect. Dis. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Petruccioli, E.; Chiacchio, T.; Navarra, A.; Vanini, V.; Cuzzi, G.; Cimaglia, C.; Codecasa, L.R.; Pinnetti, C.; Riccardi, N.; Palmieri, F.; et al. Effect of HIV-infection on QuantiFERON-plus accuracy in patients with active tuberculosis and latent infection. J. Infect. 2020, 80, 536–546. [Google Scholar] [CrossRef]
- Yang, C.H.; Chan, P.C.; Liao, S.T.; Cheng, S.H.; Wong, W.W.; Huang, L.M.; Hsueh, P.R.; Chiou, H.Y. Strategy to better select HIV-infected individuals for latent TB treatment in BCG-vaccinated population. PLoS ONE 2013, 8, e73069. [Google Scholar] [CrossRef]
- Slovis, B.S.; Plitman, J.D.; Haas, D.W. The case against anergy testing as a routine adjunct to tuberculin skin testing. J. Am. Med. Assoc. 2000, 283, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. BCG vaccine: WHO position paper, February 2018—Recommendations. Vaccine 2018, 36, 3408–3410. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, J.D.; Mody, R.M.; Olsen, C.H.; Harrison, L.H.; Santosham, M.; Aronson, N.E. The Long-term Effect of Bacille Calmette-Guérin Vaccination on Tuberculin Skin Testing: A 55-Year Follow-Up Study. Chest 2017, 152, 282–294. [Google Scholar] [CrossRef]
- Pai, M.; Gokhale, K.; Joshi, R.; Dogra, S.; Kalantri, S.; Mendiratta, D.K.; Narang, P.; Daley, C.L.; Granich, R.M.; Mazurek, G.H.; et al. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole-blood interferon gamma assay with tuberculin skin testing. J. Am. Med. Assoc. 2005, 293, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- James, P.M.; Ganaie, F.A.; Kadahalli, R.L. The performance of quantiferon-TB gold in-tube (QFT-IT) test compared to tuberculin skin test (TST) in detecting latent tuberculosis infection (LTBI) in the presence of HIV coinfection in a high TB-burden area with BCG-vaccinated population. J. Int. Assoc. Provid. AIDS Care 2014, 13, 47–55. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vashishtha, R.; Chauhan, L.S.; Sreenivas, V.; Seth, D. Comparison of TST and IGRA in Diagnosis of Latent Tuberculosis Infection in a High TB-Burden Setting. PLoS ONE 2017, 12, e0169539. [Google Scholar] [CrossRef]
- Ayubi, E.; Doosti-Irani, A.; Sanjari Moghaddam, A.; Sani, M.; Nazarzadeh, M.; Mostafavi, E. The Clinical Usefulness of Tuberculin Skin Test versus Interferon-Gamma Release Assays for Diagnosis of Latent Tuberculosis in HIV Patients: A Meta-Analysis. PLoS ONE 2016, 11, e0161983, Erratum in PLoS ONE 2016, 11, e0165143. [Google Scholar] [CrossRef] [PubMed]
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’Ambrosio, L.; Centis, R.; Goletti, D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe 2021, 17, 210079. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.; Shibabaw, A.; Kiflie, A.; Bewket, G.; Abate, E.; Gelaw, B. Latent tuberculosis infection and associated risk factors among people living with HIV and apparently healthy blood donors at the University of Gondar referral hospital, Northwest Ethiopia. BMC Res. Notes 2019, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, T.; Wang, Z.; Peng, H.; Kong, W.; Zhou, Y.; Shao, Y.; Zhu, L.; Lu, W. High Latent TB Infection Rate and Associated Risk Factors in the Eastern China of Low TB Incidence. PLoS ONE 2015, 10, e0141511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rüütel, K.; Karnite, A.; Talu, A.; Abel-Ollo, K.; Kirvelaite, G.; Kliiman, K.; Loit, H.-M.; Uusküla, A. Prevalence of IGRA-positivity and risk factors for tuberculosis among injecting drug users in Estonia and Latvia. Int. J. Drug Policy 2014, 25, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, J.P.; Sotgiu, G.; Corradi, M.; Durando, P. The diagnosis of latent tuberculosis infection (LTBI): Currently available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med. Lav. 2020, 111, 170–183. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, J.; Jiang, Y.; Li, P.; Li, J.; Lu, L.; Zhao, Y.; Tang, L.; Zhang, T.; Wu, Z.; et al. Prevalence of latent tuberculosis infection and incidence of active tuberculosis in school close contacts in Shanghai, China: Baseline and follow-up results of a prospective cohort study. Front. Cell Infect. Microbiol. 2022, 12, 1000663. [Google Scholar] [CrossRef]
- Meijerink, H.; Wisaksana, R.; Lestari, M.; Meilana, I.; Chaidir, L.; van der Ven, A.J.; Alisjahbana, B.; van Crevel, R. Active and latent tuberculosis among HIV-positive injecting drug users in Indonesia. J. Int. AIDS Soc. 2015, 18, 19317. [Google Scholar] [CrossRef]
- Kraef, C.; Bentzon, A.; Skrahina, A.; Mocroft, A.; Peters, L.; Lundgren, J.D.; Chkhartishvili, N.; Podlekareva, D.; Kirk, O. Improving healthcare for patients with HIV, tuberculosis and hepatitis C in eastern Europe: A review of current challenges and important next steps. HIV Med. 2022, 23, 48–59. [Google Scholar] [CrossRef]
| Characteristic | PLHIV (n = 391) | HIV-Uninfected Individuals (n = 443) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LTBI (n = 80), n (%) | No LTBI (n = 311), n (%) | Crude OR (95%CI) | p-Value | LTBI (n = 68), n (%) | No LTBI (n = 375), n (%) | Crude OR (95%CI) | p-Value | ||
| Gender | Male | 58 (72.5) | 215 (69.1) | 1.18 (0.68–2.03) | 0.559 | 46 (67.7) | 244 (65.1) | 1.12 (0.65–1.95) | 0.681 |
| Female | 22 (27.5) | 96 (30.9) | 22 (32.3) | 131 (34.9) | |||||
| Age | ≤40 years | 43 (53.8) | 147 (47.3) | 1.30 (0.79–2.12) | 0.301 | 31 (45.6) | 151 (40.3) | 1.24 (0.74–2.09) | 0.412 |
| >40 years | 37 (46.2) | 164 (52.7) | 37 (54.4) | 224 (59.7) | |||||
| Educational status | Not University | 74 (92.5) | 235 (75.6) | 3.99 (1.67–9.53) | 0.002 | 45 (66.2) | 253 (67.5) | 0.94 (0.55–1.63) | 0.835 |
| University | 6 (7.5) | 76 (24.4) | 23 (33.8) | 122 (32.5) | |||||
| Unemployment | Yes | 46 (57.5) | 103 (33.1) | 2.73 (1.65–4.51) | <0.001 | 9 (13.2) | 52 (13.9) | 0.95 (0.44–2.03) | 0.889 |
| No | 34 (42.5) | 208 (66.9) | 59 (86.8) | 323 (86.1) | |||||
| History of contact with an active TB patient | Yes | 12 (15) | 43 (13.8) | 1.10 (0.55–2.20) | 0.788 | 12 (17.6) | 23 (6.1) | 3.28 (1.54–6.96) | 0.002 |
| No/unknown | 68 (85) | 268 (86.2) | 56 (82.4) | 352 (93.9) | |||||
| Imprisonment history | Yes | 51 (63.8) | 91 (29.3) | 4.25 (2.53–7.13) | <0.001 | 13 (19.1) | 30 (8) | 2.72 (1.34–5.53) | 0.006 |
| No | 29 (36.2) | 220 (70.7) | 55 (80.9) | 345 (92) | |||||
| BCG-vaccinated | Yes | 73 (91.3) | 279 (89.7) | 1.20 (0.51–2.82) | 0.682 | 64 (94.1) | 356 (95.2) | 0.81 (0.27–2.47) | 0.710 |
| No | 7 (8.7) | 32 (10.3) | 4 (5.9) | 18 (4.8) | |||||
| Anti-HCV positive (n = 556) | Yes | 55 (79.7) | 96 (37.6) | 6.01 (3.22–11.23) | <0.001 | 22 (50) | 86 (45.7) | 1.19 (0.61–2.29) | 0.611 |
| No | 14 (20.3) | 159 (62.4) | 22 (50) | 102 (54.3) | |||||
| HBsAg positive (n = 487) | Yes | 3 (4.6) | 9 (3.8) | 1.22 (0.32–4.62) | 0.775 | 2 (7.1) | 27 (16.9) | 0.38 (0.08–1.68) | 0.200 |
| No | 62 (95.4) | 226 (96.2) | 26 (92.9) | 132 (83.1) | |||||
| Smoking | Yes | 65 (81.2) | 179 (57.6) | 3.20 (1.75–5.85) | <0.001 | 24 (35.3) | 125 (33.3) | 1.09 (0.63–1.88) | 0.753 |
| No | 15 (18.8) | 132 (42.4) | 44 (64.7) | 250 (66.7) | |||||
| Intravenous drug use | Ever | 55 (68.8) | 100 (32.2) | 4.71 (2.77–8.00) | <0.001 | 11 (16.2) | 27 (7.2) | 2.49 (1.17–5.29) | 0.018 |
| Never | 25 (31.2) | 212 (67.8) | 57 (83.8) | ||||||
| Alcohol abuse | Yes | 6 (7.5) | 25 (8) | 0.93 (0.37–2.34) | 0.874 | 3 (6.1) | 15 (3.8) | 1.61 (0.51–5.05) | 0.413 |
| No | 74 (92.5) | 286 (92) | 46 (93.9) | 379 (96.2) | |||||
| BMI, kg/m2 | <18.5 | 2 (2.5) | 11 (3.5) | 0.70 (0.15–3.22) | 0.646 | 1 (2.0) | 7 (1.8) | 1.86 (0.37–9.43) | 0.452 |
| ≥18.5 | 78 (97.5) | 300 (96.5) | 48 (98.0) | 387 (98.2) | |||||
| CD4 count at time of HIV diagnosis, cells/mm3 (n = 366) | ≤350 | 28 (39.4) | 181 (61.4) | - | - | - | - | ||
| >350 | 43 (60.6) | 114 (38.6) | 2.44 (1.43–4.14) | 0.001 | |||||
| CD4 count at time of IGRA/TST test, cells/mm3 (n = 385) | ≤350 | 20 (25) | 114 (37.4) | - | - | - | - | ||
| >350 | 60 (75) | 191 (62.6) | 1.79 (1.03–3.12) | 0.040 | |||||
| HIV RNA at time of HIV diagnosis, copies/mL (n = 338) | <200 | 8 (12.1) | 24 (8.8) | - | - | - | - | ||
| ≥200 | 58 (87.9) | 248 (91.2) | 0.70 (0.30–1.64) | 0.414 | |||||
| HIV RNA at time of IGRA/TST test, copies/mL (n = 380) | <200 | 46 (57.5) | 183 (61) | ||||||
| ≥200 | 34 (42.5) | 117 (39) | 1.16 (0.70–1.91) | 0.570 | - | - | - | - | |
| On ART (n = 381) | Yes | 49 (61.3) | 201 (66.8) | 0.79 (0.47–1.31) | 0.356 | - | - | - | - |
| No | 31 (38.7) | 100 (33.2) | |||||||
| Centre | Vilnius | Siauliai | Klaipeda | p-Value |
|---|---|---|---|---|
| TB incidence per 100,000 population in the county in 2019–2021, ranges [16] | 17.7–20.6 | 33.5–37.7 | 23.4–38.1 | - |
| Enrolled PLHIV | n = 258 | n = 59 | n = 74 | - |
| 495 (287–674) | 617 (386–799) | 343 (182–561) | <0.001 * |
| Enrolled HIV-uninfected individuals | n = 213 | n = 120 | n = 110 | - |
| Type of IGRA | QuantiFERON-TB Gold In-Tube | QuantiFERON-TB Gold In-Tube | LIOFeron TB/LTBI | - |
| IGRA and/or TST positivity, n (%) | ||||
| 59 (22.9%) | - | - | 0.08 ** |
| 35 (16.4%) | - | - | |
| IGRA positivity, n (%) | ||||
| 49 (19.1%) | 15 (25.4%) | 6 (8.1%) | 0.03 * |
| 16 (8.8%) | 15 (12.5%) | 18 (12.8%) | 0.44 * |
| TST positivity, n (%) | ||||
| 22/243 (9.1%) | - | - | 0.30 ** |
| 24/198 (12.1%) | - | - | |
| Kappa IGRA/TST (95% CI) | ||||
| 0.28 (0.12–0.43) | - | - | - |
| 0.15 (−0.03–0.33) | - | - | - |
| Positivity rate: IGRA vs. TST | ||||
| 19.1% vs. 9.1% | - | - | 0.001 ** |
| 8.8% vs. 12.1% | - | - | 0.21 ** |
| Results | PLHIV | HIV-Uninfected Individuals |
|---|---|---|
| Positive TST and positive QFT-GIT | 12 | 5 |
| Negative TST and negative QFT-GIT | 189 | 161 |
| Negative TST and positive QFT-GIT | 32 | 13 |
| Positive TST and negative QFT-GIT | 10 | 19 |
| Kappa (95% CI) | 0.28 (0.12–0.43) | 0.15 (−0.03–0.33) |
| Total | 243 (11 cases not returned for TST evaluation) | 198 (3 cases not returned for TST evaluation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulyte, E.; Kancauskiene, Z.; Kausas, A.; Urboniene, J.; Lipnickiene, V.; Kopeykiniene, J.; Gudaitis, T.; Raudonis, S.; Danila, E.; Costagliola, D.; et al. Latent Tuberculosis Infection and Associated Risk Factors among People Living with HIV and HIV-Uninfected Individuals in Lithuania. Pathogens 2023, 12, 990. https://doi.org/10.3390/pathogens12080990
Matulyte E, Kancauskiene Z, Kausas A, Urboniene J, Lipnickiene V, Kopeykiniene J, Gudaitis T, Raudonis S, Danila E, Costagliola D, et al. Latent Tuberculosis Infection and Associated Risk Factors among People Living with HIV and HIV-Uninfected Individuals in Lithuania. Pathogens. 2023; 12(8):990. https://doi.org/10.3390/pathogens12080990
Chicago/Turabian StyleMatulyte, Elzbieta, Zavinta Kancauskiene, Aidas Kausas, Jurgita Urboniene, Vilnele Lipnickiene, Jelena Kopeykiniene, Tomas Gudaitis, Sarunas Raudonis, Edvardas Danila, Dominique Costagliola, and et al. 2023. "Latent Tuberculosis Infection and Associated Risk Factors among People Living with HIV and HIV-Uninfected Individuals in Lithuania" Pathogens 12, no. 8: 990. https://doi.org/10.3390/pathogens12080990
APA StyleMatulyte, E., Kancauskiene, Z., Kausas, A., Urboniene, J., Lipnickiene, V., Kopeykiniene, J., Gudaitis, T., Raudonis, S., Danila, E., Costagliola, D., & Matulionyte, R. (2023). Latent Tuberculosis Infection and Associated Risk Factors among People Living with HIV and HIV-Uninfected Individuals in Lithuania. Pathogens, 12(8), 990. https://doi.org/10.3390/pathogens12080990





