Human Monkeypox: A Comprehensive Overview of Epidemiology, Pathogenesis, Diagnosis, Treatment, and Prevention Strategies
Abstract
1. Introduction
2. Public Health Importance
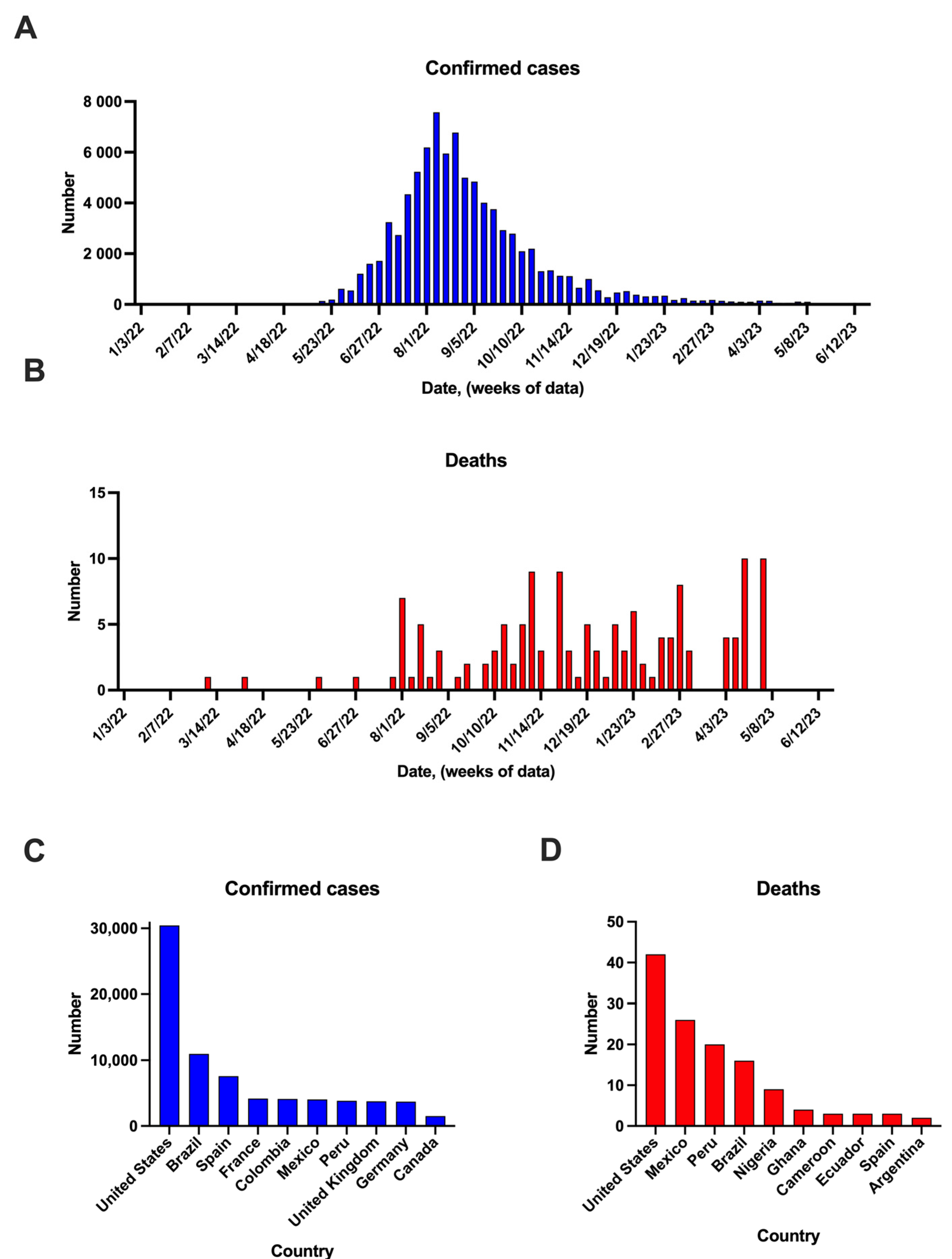
3. Mpox Cases in the Post-COVID-19 Pandemic Period
4. Animal Reservoirs
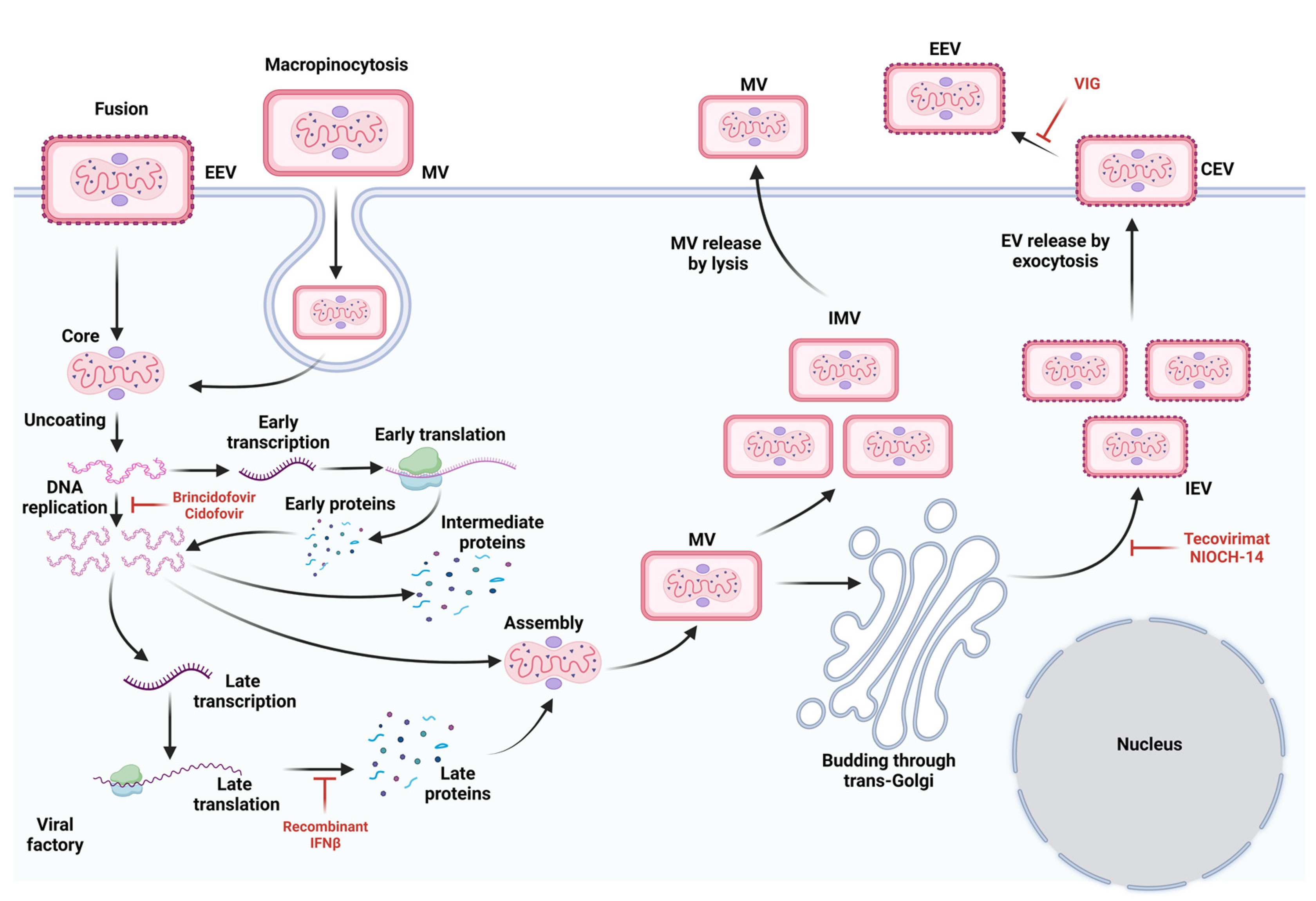
5. Viral Attachment and Pathogenesis
6. Immunopathophysiology
7. Modes of Transmission
8. Laboratory Diagnosis
9. Clinical Features
10. Animal Models Employed in the Study of Mpox Infection
| Animal | MPXV Clade | Inoculation Route | Dosage Used (PFU) | Mortality (%) | Time to Death |
|---|---|---|---|---|---|
| Guinea pigs [105] | WA | Intravenous, intracerebral, intracardiac, intraperitoneal, intranasal, intradermous, oral, and scarified skin | 101–102 | 0 | NA |
| Rabbits | |||||
| Adult rabbit [105] | WA | Intravenous | 107 | 8 | 1-month post-infection |
| Oral | 109 | 0 | NA | ||
| Intradermal | 105 | 0 | NA | ||
| 10–12 days old [105] | WA | Oral | 106 | 85 | 4–14 days |
| Intranasal | 106 | 83 | 4–5 days | ||
| White rats [105] | |||||
| Adult | WA | Intravenous, intranasal, cutaneous | 101–103 | 0 | 0 |
| 1–3-day-old white rats | WA | Intranasal | 101–103 | 100 | 5–6 days |
| White mice [105] | |||||
| 8–15 days old | WA | Intranasal, intraperitoneal | 1.2 × 106 | 100 | Not provided |
| Oral | 1.2 × 106 | 40 | Not provided | ||
| Foot pad | 6 × 102 | 100 | Not provided | ||
| Intradermic | 1.2 × 106 | 50 | Not provided | ||
| 12 days old | WA | Oral | 1.2 × 106 | 14 | Not provided |
| 15 days old | WA | Intranasal | 1.2 × 106 | 100 | Not provided |
| Hamsters [105] | |||||
| WA | Intranasal, oral, intracardiac, scarified skin | 1.5–5.9 × 107 | 0 | NA | |
| Squirrels | |||||
| Ground squirrels [110] | WA [111] | Intraperitoneal Intranasal | 105.1 106.1 | 100 | 9 days |
| Rope squirrels [109] | CB | Intranasal Intradermal | 106 | 75 50 | 13 days 11 days |
| Mouse | |||||
| C57BL/6 lab mice [106] | CB | Intraperitoneal Intranasal | 5 × 104 | 0 | |
| CAST/EiJ [112] | CB | Intranasal | 104–106 | 100 | 5–8 days |
| 103 | 60 | ||||
| 102 | 0 | NA | |||
| WB | Intranasal | 105–106 | 100 | 8 days | |
| 104 | 50 | ||||
| 103 | 12.5 | ||||
| 102 | 0 | NA | |||
| SCID [106] | CB | Intranasal | 275 | 100 | 16.8 days |
| DBA A/Ncr C3HeJ [106] | CB | Intranasal Footpad injection | 5 × 104 | 0 | NA |
| BALB/c IFN-yR−/− [106] | CB | Intranasal | 990 | 0 | NA |
| Type 1 IFNR−/− [106] | CB | FP | 6 × 103 | 0 | NA |
| C57BL/6 stat1−/− [106] | CB | Intranasal | 470 | 90 | 9.3 days |
| 129 stat1−/− [106] | CB | Intranasal | 4700 | 40 | 10 days |
| SCID-BALB/c mice [113] | WA CB | Intraperitoneal | 105 | 100 | 9 days |
| Dormouse (Graphiurus kelleni) [114] | CB WA | Footpad injection | 104 | 92 | 7–10 days |
| CB | Intranasal | 2000–200 | 100 | 7.9–8.7 days | |
| Gambian pouched rat | |||||
| [115] | WA CB | Scarification | 104 | 25 0 | 13 days NA |
| [108] | CB | Intradermal Intranasal | 106 | 0 | NA |
| Prairie dogs (Cynomys ludovicianus) | |||||
| [116] | CB | Intranasal | 104.5 | 25 | 13 days |
| CB | Intradermic | 104.5 | 50 | 11–12 days | |
| WA | Intranasal Intradermic | 104.5 | 0 | NA | |
| [117] | WA | Intraperitoneal | 105.1 | 100 | 8–11 (IP route) |
| Intranasal | 105.1 | 0 | NA | ||
| [118] | WA | Intranasal | 104 | 33 | 14 days |
| [119] | CB | Intranasal | 106 | 0 | NA |
11. Cross-Immunity and Vaccines
12. Treatment and Prevention
| Model | MPXV Clade | Therapeutic Agent | Inoculation Route | Dosage Used (PFU) | Mortality (%) | Results |
|---|---|---|---|---|---|---|
| Animal models | ||||||
| Prairie dogs [140] | CB | IMVAMUNE® ACAM2000® | Intranasal | 104 106 | 75 100 | Vaccination provided some level of protection to the animals against a challenge of 2 × LD50, but it did not protect them against a challenge of 170 × LD5 |
| Prairie dogs (Cynomys ludovicianus) [141] | CB | ST-246 Tecovirimat | Intranasal | 105 | 0 | All animals that were administered ST-246 survived the challenge, and those that received treatment prior to the onset of remained mainly without symptoms |
| Cynomolgus monkeys (Macaca fascicularis) [142] | WA | ST-246 Tecovirimat | Intravenous | 5 × 107 | 0 | Administering an oral dose of around 3 mg/kg/day (36 mg/m2) to nonfasted NHPs for 14 days, starting at 3 dpi, resulted in complete protection against mortality |
| Dormouse (Graphiurus kelleni) [114] | CB | Cidofovir | Intranasal | 75. 4 × 103 5 × 103 | 0 | Dormice that received a single dose of cidofovir 4 h after being exposed to MPXV showed significant protection against mortality, whereas the group treated with the control presented uniform mortality |
| CB | Dryvax vaccine | Intranasal | 2 × 104 | 19 | Animals vaccinated with the Dryvax vaccine 4 weeks prior to challenge presented solid protection from mortality when challenged with 2 × 104 PFU of MPXV-ZAI-79 | |
| Rhesus macaques (Macaca mulatta) [136] | CB | VIG | Intravenous | 5 × 107 | 0 | VIG showed promising cross-neutralizing activity sufficient to protect rhesus macaques from a lethal mpox infection |
| C57BL/6 stat1−/− mice [106] | CB | Dryvax vaccine | Intranasal | 4.2 × 104 | 90 | Mice that received a single vaccination of MVA on day-56 or a double vaccination of MVA with a booster on both day-56 and day-28 had comparable survival rates of approximately 90% |
| CB | CMX001 | Intranasal | 5000 | 0 | After being challenged with MPXV, all mice survived, experienced minimal weight loss, and seroconverted. However, when the mice were rechallenged at day 38 after the initial infection, 20% of them died by 8 days post-rechallenge | |
| Marmot model [143] | CB | NIOCH-14 | Intranasal | 3.4 log10 | 60 | The mechanism of the antiviral action of these compounds is focused on the inhibition of the formation of different enveloped forms of the virus (intracellular, cell-associated, and extracellular) |
| Rabbits (Oryctolagus cuniculus) [128] | RPXV | 4pox vaccine | Intramuscular Electroporation | 1 × 105 | 0 | The 4pox DNA vaccine protected NHPs, mice, and, in this study, rabbits against fatal infection by MPXV, VACV, and RPXV, respectively |
| In vitro | ||||||
| HeLa and BSC-40 cells [138] | WA | PAV-164 | In vitro | Not provided | NA | At non-cytotoxic concentrations, the compounds demonstrated strong virucidal activity and inhibited infection with VACV, monkeypox, cowpox, and Akhmeta virus when administered before, during, or after viral adsorption |
| HeLa and human foreskin fibroblasts (HFFs) [139] | WA CB | Resveratrol | In vitro | 2 × 109 [144] | NA | Resveratrol reduced VACV and mpox replication. The suppression appears to affect the viral DNA synthesis step |
| Vero 76, Vero E6 [145] | CB | Ribavirin | In vitro | 1 × 105 | NA | Ribavirin showed an antiviral function in Vero cells under mpox infection measured by a neutral red uptake assay |
| HeLa cells, VA (R645), VA-9, and VN36 cell lines [146] | CB | Recombinant IFN-β | In vitro | 3.42 × 106 8.37 × 106 3.53 × 107 [147] | NA | The induction of the antiviral protein MxA was observed in infected cells treated with IFN-β, and it was demonstrated that constitutive expression of MxA inhibits mpox infection |
13. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiecco, G.; Degli Antoni, M.; Storti, S.; Tomasoni, L.R.; Castelli, F.; Quiros-Roldan, E. Monkeypox, a Literature Review: What Is New and Where Does This Concerning Virus Come From? Viruses 2022, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-like Disease in Cynomolgus Monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Multi-Country Monkeypox Outbreak: Situation Update. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 (accessed on 22 April 2023).
- Rosa, R.B.; Ferreira de Castro, E.; Vieira da Silva, M.; Paiva Ferreira, D.C.; Jardim, A.C.G.; Santos, I.A.; Marinho, M.D.S.; Ferreira França, F.B.; Pena, L.J. In Vitro and In Vivo Models for Monkeypox. Iscience 2023, 26, 105702. [Google Scholar] [CrossRef] [PubMed]
- Happi, C.; Adetifa, I.; Mbala, P.; Njouom, R.; Nakoune, E.; Happi, A.; Ndodo, N.; Ayansola, O.; Mboowa, G.; Bedford, T.; et al. Urgent Need for a Non-Discriminatory and Non-Stigmatizing Nomenclature for Monkeypox Virus. PLoS Biol. 2022, 20, e3001769. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Salang Mamada, S.; Salampe, M.; Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S.; et al. Monkeypox: A Comprehensive Review. Viruses 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Nguyen, L.-B.L.; Breban, R. Modelling Human-to-Human Transmission of Monkeypox. Bull. World Health Organ. 2020, 98, 638–640. [Google Scholar] [CrossRef]
- WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 12 January 2023).
- WHO Recommends New Name for Monkeypox Disease. Available online: https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease (accessed on 12 January 2023).
- WHO Declares End of Mpox Emergency, Calls for Sustained Efforts for Long-Term Management of the Disease. PAHO/WHO | Pan American Health Organization. Available online: https://www.paho.org/en/news/11-5-2023-who-declares-end-mpox-emergency-calls-sustained-efforts-long-term-management-disease (accessed on 30 May 2023).
- Adnan, N.; ul Haq, Z.; Malik, A.; Mehmood, A.; Ishaq, U.; Faraz, M.; Malik, J.; Mehmoodi, A. Human Monkeypox Virus: An Updated Review. Medicine 2022, 101, e30406. [Google Scholar] [CrossRef]
- Diaz, J.H. The Disease Ecology, Epidemiology, Clinical Manifestations, Management, Prevention, and Control of Increasing Human Infections with Animal Orthopoxviruses. Wilderness Environ. Med. 2021, 32, 528–536. [Google Scholar] [CrossRef]
- Onchonga, D. Monkeypox Viral Disease Outbreak in Non-Endemic Countries in 2022: What Clinicians and Healthcare Professionals Need to Know. Saudi Pharm. J. 2022, 30, 1679–1681. [Google Scholar] [CrossRef]
- Daskalakis, D.; McClung, R.P.; Mena, L.; Mermin, J. Monkeypox: Avoiding the Mistakes of Past Infectious Disease Epidemics. Ann. Intern. Med. 2022, 175, 1177–1178. [Google Scholar] [CrossRef]
- Cardeti, G.; Gruber, C.E.M.; Eleni, C.; Carletti, F.; Castilletti, C.; Manna, G.; Rosone, F.; Giombini, E.; Selleri, M.; Lapa, D.; et al. Fatal Outbreak in Tonkean Macaques Caused by Possibly Novel Orthopoxvirus, Italy, January 2015. Emerg. Infect. Dis. 2017, 23, 1941–1949. [Google Scholar] [CrossRef]
- Vora, N.M.; Li, Y.; Geleishvili, M.; Emerson, G.L.; Khmaladze, E.; Maghlakelidze, G.; Navdarashvili, A.; Zakhashvili, K.; Kokhreidze, M.; Endeladze, M.; et al. Human Infection with a Zoonotic Orthopoxvirus in the Country of Georgia. N. Engl. J. Med. 2015, 372, 1223–1230. [Google Scholar] [CrossRef]
- Gigante, C.M.; Gao, J.; Tang, S.; McCollum, A.M.; Wilkins, K.; Reynolds, M.G.; Davidson, W.; McLaughlin, J.; Olson, V.A.; Li, Y. Genome of Alaskapox Virus, a Novel Orthopoxvirus Isolated from Alaska. Viruses 2019, 11, 708. [Google Scholar] [CrossRef]
- Shchelkunov, S.N. An Increasing Danger of Zoonotic Orthopoxvirus Infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 Outbreak and the Pathobiology of the Monkeypox Virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- CDC Mpox in the U.S. Available online: https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html (accessed on 22 April 2023).
- Allam, Z.; Bibri, S.E.; Sharpe, S.A. The Rising Impacts of the COVID-19 Pandemic and the Russia–Ukraine War: Energy Transition, Climate Justice, Global Inequality, and Supply Chain Disruption. Resources 2022, 11, 99. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.-Y.; Wang, F.-S. Monkeypox Outbreak: A Novel Threat after COVID-19? Mil. Med. Res. 2022, 9, 29. [Google Scholar] [CrossRef]
- Choudhary, O.P.; Fahrni, M.L.; Saied, A.A.; Chopra, H. Ring Vaccination for Monkeypox Containment: Strategic Implementation and Challenges. Int. J. Surg. Lond. Engl. 2022, 105, 106873. [Google Scholar] [CrossRef]
- Quarleri, J.; Delpino, M.V.; Galvan, V. Monkeypox: Considerations for the Understanding and Containment of the Current Outbreak in Non-Endemic Countries. GeroScience 2022, 44, 2095–2103. [Google Scholar] [CrossRef]
- Kozlov, M. Monkeypox Goes Global: Why Scientists Are on Alert. Nature 2022, 606, 15–16. [Google Scholar] [CrossRef]
- Balin, S.J.; Cascalho, M. The Rate of Mutation of a Single Gene. Nucleic Acids Res. 2010, 38, 1575–1582. [Google Scholar] [CrossRef]
- El-Qushayri, A.E.; Reda, A.; Shah, J. COVID-19 and Monkeypox Co-Infection: A Rapid Systematic Review. Front. Immunol. 2022, 13, 1094346. [Google Scholar] [CrossRef]
- Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses. Viruses 2020, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Radonić, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A.; Mätz-Rensing, K.; Boesch, C.; Leendertz, F.H.; Nitsche, A. Fatal Monkeypox in Wild-Living Sooty Mangabey, Côte d’Ivoire, 2012. Emerg. Infect. Dis. 2014, 20, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Khodakevich, L.; Jezek, Z.; Kinzanzka, K. Isolation of Monkeypox Virus from Wild Squirrel Infected in Nature. Lancet Lond. Engl. 1986, 1, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Dutta, P.; Rashid, R.; Jaffery, S.S.; Islam, A.; Farag, E.; Zughaier, S.M.; Bansal, D.; Hassan, M.M. Pathogenicity and Virulence of Monkeypox at the Human-Animal-Ecology Interface. Virulence 2023, 14, 2186357. [Google Scholar] [CrossRef]
- Moore, M.J.; Rathish, B.; Zahra, F. Mpox (Monkeypox). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Orthopoxvirus~Viral Zone. Available online: https://viralzone.expasy.org/149?outline=all_by_species (accessed on 3 May 2023).
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The Virology of Human Monkeypox Virus (HMPXV): A Brief Overview. Virus Res. 2022, 322, 198932. [Google Scholar] [CrossRef]
- Hughes, L.J.; Goldstein, J.; Pohl, J.; Hooper, J.W.; Lee Pitts, R.; Townsend, M.B.; Bagarozzi, D.; Damon, I.K.; Karem, K.L. A Highly Specific Monoclonal Antibody against Monkeypox Virus Detects the Heparin Binding Domain of A27. Virology 2014, 464, 264–273. [Google Scholar] [CrossRef]
- Moss, B. Membrane Fusion during Poxvirus Entry. Semin. Cell Dev. Biol. 2016, 60, 89–96. [Google Scholar] [CrossRef]
- Peng, Q.; Xie, Y.; Kuai, L.; Wang, H.; Qi, J.; Gao, G.F.; Shi, Y. Structure of Monkeypox Virus DNA Polymerase Holoenzyme. Science 2023, 379, 100–105. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox Virus: A Re-Emergent Threat to Humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef]
- Townsley, A.C.; Weisberg, A.S.; Wagenaar, T.R.; Moss, B. Vaccinia Virus Entry into Cells via a Low-PH-Dependent Endosomal Pathway. J. Virol. 2006, 80, 8899–8908. [Google Scholar] [CrossRef]
- Chiu, W.-L.; Lin, C.-L.; Yang, M.-H.; Tzou, D.-L.M.; Chang, W. Vaccinia Virus 4c (A26L) Protein on Intracellular Mature Virus Binds to the Extracellular Cellular Matrix Laminin. J. Virol. 2007, 81, 2149–2157. [Google Scholar] [CrossRef]
- Matho, M.H.; Schlossman, A.; Gilchuk, I.M.; Miller, G.; Mikulski, Z.; Hupfer, M.; Wang, J.; Bitra, A.; Meng, X.; Xiang, Y.; et al. Structure–Function Characterization of Three Human Antibodies Targeting the Vaccinia Virus Adhesion Molecule D8. J. Biol. Chem. 2018, 293, 390–401. [Google Scholar] [CrossRef]
- Schin, A.M.; Diesterbeck, U.S.; Moss, B. Insights into the Organization of the Poxvirus Multicomponent Entry-Fusion Complex from Proximity Analyses in Living Infected Cells. J. Virol. 2021, 95, e00852-21. [Google Scholar] [CrossRef]
- Liszewski, M.K.; Leung, M.K.; Hauhart, R.; Buller, R.M.L.; Bertram, P.; Wang, X.; Rosengard, A.M.; Kotwal, G.J.; Atkinson, J.P. Structure and Regulatory Profile of the Monkeypox Inhibitor of Complement: Comparison to Homologs in Vaccinia and Variola and Evidence for Dimer Formation. J. Immunol. 2006, 176, 3725–3734. [Google Scholar] [CrossRef]
- Realegeno, S.; Puschnik, A.S.; Kumar, A.; Goldsmith, C.; Burgado, J.; Sambhara, S.; Olson, V.A.; Carroll, D.; Damon, I.; Hirata, T.; et al. Monkeypox Virus Host Factor Screen Using Haploid Cells Identifies Essential Role of GARP Complex in Extracellular Virus Formation. J. Virol. 2017, 91, e00011-17. [Google Scholar] [CrossRef]
- Orassay, A.; Berdigaliyev, A.; Sadvokassova, D.; Diassova, A.; Amin, A.; Cao, W.; Xie, Y. Recent Advances on Human Mpox. New Microbes New Infect. 2022, 51, 101066. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus Cell Entry: How Many Proteins Does It Take? Viruses 2012, 4, 688–707. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Rezaei, N. Poxviruses and the Immune System: Implications for Monkeypox Virus. Int. Immunopharmacol. 2022, 113, 109364. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. Orthopoxvirus Genes That Mediate Disease Virulence and Host Tropism. Adv. Virol. 2012, 2012, 524743. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease Epidemiology, Host Immunity and Clinical Interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Nagata, N.; Saijo, M.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Sato, Y.; Iwata-Yoshikawa, N.; Ogata, M.; Kurane, I.; Morikawa, S.; et al. Pathogenesis of Fulminant Monkeypox with Bacterial Sepsis after Experimental Infection with West African Monkeypox Virus in a Cynomolgus Monkey. Int. J. Clin. Exp. Pathol. 2014, 7, 4359–4370. [Google Scholar]
- Smith, S.A.; Kotwal, G.J. Virokines: Novel Immunomodulatory Agents. Expert Opin. Biol. Ther. 2001, 1, 343–357. [Google Scholar] [CrossRef]
- Johnston, J.B.; McFadden, G. Poxvirus Immunomodulatory Strategies: Current Perspectives. J. Virol. 2003, 77, 6093–6100. [Google Scholar] [CrossRef]
- Walzer, T.; Galibert, L.; De Smedt, T. Poxvirus Semaphorin A39R Inhibits Phagocytosis by Dendritic Cells and Neutrophils. Eur. J. Immunol. 2005, 35, 391–398. [Google Scholar] [CrossRef]
- Li, H.; Huang, Q.-Z.; Zhang, H.; Liu, Z.-X.; Chen, X.-H.; Ye, L.-L.; Luo, Y. The Land-Scape of Immune Response to Monkeypox Virus. eBioMedicine 2023, 87, 104424. [Google Scholar] [CrossRef]
- Arndt, W.D.; Cotsmire, S.; Trainor, K.; Harrington, H.; Hauns, K.; Kibler, K.V.; Huynh, T.P.; Jacobs, B.L. Evasion of the Innate Immune Type I Interferon System by Monkeypox Virus. J. Virol. 2015, 89, 10489–10499. [Google Scholar] [CrossRef]
- Crouse, J.; Kalinke, U.; Oxenius, A. Regulation of Antiviral T Cell Responses by Type I Interferons. Nat. Rev. Immunol. 2015, 15, 231–242. [Google Scholar] [CrossRef]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox Virus Evades Antiviral CD4+ and CD8+ T Cell Responses by Suppressing Cognate T Cell Activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef]
- Campbell, J.A.; Trossman, D.S.; Yokoyama, W.M.; Carayannopoulos, L.N. Zoonotic Orthopoxviruses Encode a High-Affinity Antagonist of NKG2D. J. Exp. Med. 2007, 204, 1311–1317. [Google Scholar] [CrossRef]
- Campbell, J.A.; Davis, R.S.; Lilly, L.M.; Fremont, D.H.; French, A.R.; Carayannopoulos, L.N. FCRL5 on Innate B Cells Is Targeted by a Poxvirus MHC Class I-like Immunoevasin. J. Immunol. 2010, 185, 28–32. [Google Scholar] [CrossRef]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef]
- Johnston, S.C.; Johnson, J.C.; Stonier, S.W.; Lin, K.L.; Kisalu, N.K.; Hensley, L.E.; Rimoin, A.W. Cytokine Modulation Correlates with Severity of Monkeypox Disease in Humans. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2015, 63, 42–45. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Mohamed, M.G.; Dabou, E.A.; Abuijlan, I.; Chandran, D.; El-Shall, N.A.; Chopra, H.; Dhama, K. Monkeypox (Mpox) in Immunosuppressed Patients. F1000Research 2023, 12, 127. [Google Scholar] [CrossRef]
- Saeed, S.; Shabbir, H.; Basit, J.; Ur Rehman, M.E. Monkeypox: Potentially Another Pandemic or a Mere Hoax? Ann. Med. Surg. 2022, 81, 104364. [Google Scholar] [CrossRef]
- Vivancos-Gallego, M.J.; Sánchez-Conde, M.; Rodríguez-Domínguez, M.; Fernandez-Gonzalez, P.; Martínez-García, L.; Garcia-Mouronte, E.; Martínez-Sanz, J.; Moreno-Zamora, A.M.; Casado, J.L.; Ron, R.; et al. Human Monkeypox in People With HIV: Transmission, Clinical Features, and Outcome. Open Forum Infect. Dis. 2022, 9, ofac557. [Google Scholar] [CrossRef]
- Guarner, J.; Del Rio, C.; Malani, P.N. Monkeypox in 2022-What Clinicians Need to Know. JAMA 2022, 328, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Boesecke, C.; Monin, M.B.; van Bremen, K.; Schlabe, S.; Hoffmann, C. Severe Monkeypox-Virus Infection in Undiagnosed Advanced HIV Infection. Infection 2022, 50, 1633–1634. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-López, P.; Borras-Bermejo, B.; López Pérez, L.; Antón, A.; Piñana, M.; García-Pérez, J.; Descalzo, V.; Monforte, A.; Martínez-Gómez, X.; Falcó, V.; et al. Suspected Case of Monkeypox Reinfection versus Reactivation in a Immunocompetent Patient, Barcelona, 2022. Int. J. STD AIDS 2023. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.Q.; Fonsi, M.; Tancredi, M.V.; Ramos de Alencar, H.D.; Abbud, A.; da Silva, M.H. Mpox in a Couple Living with HIV: Relapse or Reinfection? AIDS Res. Hum. Retroviruses 2023. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Jessen, H.; Wyen, C.; Grunwald, S.; Noe, S.; Teichmann, J.; Krauss, A.-S.; Kolarikal, H.; Scholten, S.; Schuler, C.; et al. Clinical Characteristics of Monkeypox Virus Infections among Men with and without HIV: A Large Outbreak Cohort in Germany. HIV Med. 2023, 24, 389–397. [Google Scholar] [CrossRef]
- Viguier, C.; de Kermel, T.; Boumaza, X.; Benmedjahed, N.S.; Izopet, J.; Pasquier, C.; Delobel, P.; Mansuy, J.-M.; Martin-Blondel, G. A Severe Monkeypox Infection in a Patient with an Advanced HIV Infection Treated with Tecovirimat: Clinical and Virological Outcome. Int. J. Infect. Dis. 2022, 125, 135–137. [Google Scholar] [CrossRef]
- Chapman, J.L.; Nichols, D.K.; Martinez, M.J.; Raymond, J.W. Animal Models of Orthopoxvirus Infection. Vet. Pathol. 2010, 47, 852–870. [Google Scholar] [CrossRef]
- Domán, M.; Fehér, E.; Varga-Kugler, R.; Jakab, F.; Bányai, K. Animal Models Used in Monkeypox Research. Microorganisms 2022, 10, 2192. [Google Scholar] [CrossRef]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.-J.T. Maternal and Fetal Outcomes Among Pregnant Women with Human Monkeypox Infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef]
- Monkeypox: Public Health Advice for Gay, Bisexual and Other Men Who Have Sex with Men. Available online: https://www.who.int/news/item/25-05-2022-monkeypox--public-health-advice-for-gay--bisexual-and-other-men-who-have-sex-with-men (accessed on 28 May 2023).
- Kozlov, M. Monkeypox Outbreaks: 4 Key Questions Researchers Have. Nature 2022, 606, 238–239. [Google Scholar] [CrossRef]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Eurosurveillance 2022, 27, 2200421. [Google Scholar] [CrossRef]
- Davido, B.; D’Anglejan, E.; Baudoin, R.; Dahmane, L.; Chaud, A.; Cortier, M.; Vauloup-Fellous, C.; De Truchis, P.; Ghosn, J. Monkeypox Outbreak 2022: An Unusual Case of Peritonsillar Abscess in a Person Previously Vaccinated against Smallpox. J. Travel Med. 2022, 29, taac082. [Google Scholar] [CrossRef]
- Branda, F.; Pierini, M.; Mazzoli, S. Monkeypox: Early Estimation of Basic Reproduction Number R0 in Europe. J. Med. Virol. 2023, 95, e28270. [Google Scholar] [CrossRef]
- Rossi, L.; Tiecco, G.; Venturini, M.; Castelli, F.; Quiros-Roldan, E. Human Orf with Immune-Mediated Reactions: A Systematic Review. Microorganisms 2023, 11, 1138. [Google Scholar] [CrossRef]
- Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 20 January 2023).
- Lim, C.K.; Roberts, J.; Moso, M.; Liew, K.C.; Taouk, M.L.; Williams, E.; Tran, T.; Steinig, E.; Caly, L.; Williamson, D.A. Mpox Diagnostics: Review of Current and Emerging Technologies. J. Med. Virol. 2023, 95, e28429. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A Tale of Two Clades: Monkeypox Viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Uhteg, K.; Mostafa, H.H. Validation and Implementation of an Orthopoxvirus Qualitative Real-Time PCR for the Diagnosis of Monkeypox in the Clinical Laboratory. J. Clin. Virol. 2023, 158, 105327. [Google Scholar] [CrossRef]
- Li, Y.; Olson, V.A.; Laue, T.; Laker, M.T.; Damon, I.K. Detection of Monkeypox Virus with Real-Time PCR Assays. J. Clin. Virol. 2006, 36, 194–203. [Google Scholar] [CrossRef]
- de Oliveira Thomasi, R.M.; Silva Correa, T.D.; do Carmo, D.S.; Rodrigues, D.F.; da Silva Correa, L.V.; Xavier, S.R.; Silva, L.S.; Oliveira da Silva, J.; dos Santos, M.; da Silva Dantas, A.; et al. Molecular Methods for Diagnosis of Monkeypox: A Mini-Review. medRxiv 2022. medRxiv: 2022.12.04.22283083. [Google Scholar] [CrossRef]
- Kuo, S.-C.; Wang, Y.-M. Identification of Pan-Orthopoxvirus, Monkeypox-Specific and Smallpox-Specific DNAs by Real-Time PCR Assay. J. Med. Sci. Taiwan 2013, 33, 293–303. [Google Scholar] [CrossRef]
- Wawina-Bokalanga, T.; Sklenovska, N.; Vanmechelen, B.; Bloemen, M.; Vergote, V.; Laenen, L.; André, E.; Van Ranst, M.; Muyembe-Tamfum, J.-J.; Maes, P. An Accurate and Rapid Real-Time PCR Approach for Human Monkeypox Virus Diagnosis. medRxiv 2022. medRxiv: 2022.06.23.22276033. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. Viral Loads in Clinical Samples of Men with Monkeypox Virus Infection: A French Case Series. Lancet Infect. Dis. 2023, 23, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.B.; MacNeil, A.; Reynolds, M.G.; Hughes, C.M.; Olson, V.A.; Damon, I.K.; Karem, K.L. Evaluation of the Tetracore Orthopox BioThreat® Antigen Detection Assay Using Laboratory Grown Orthopoxviruses and Rash Illness Clinical Specimens. J. Virol. Methods 2013, 187, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.; Olson, V.A.; Smith, S.K.; Pietraszczyk, M.; Miller, L.; Miethe, P.; Dorner, B.G.; Nitsche, A. Rapid and Sensitive Point-of-Care Detection of Orthopoxviruses by ABICAP Immunofiltration. Virol. J. 2016, 13, 207. [Google Scholar] [CrossRef]
- Pittman, P.R.; Martin, J.W.; Kingebeni, P.M.; Tamfum, J.-J.M.; Wan, Q.; Reynolds, M.G.; Quinn, X.; Norris, S.; Townsend, M.B.; Satheshkumar, P.S.; et al. Clinical Characterization of Human Monkeypox Infections in the Democratic Republic of the Congo. medRxiv 2022. medRxiv: 2022.05.26.22273379. [Google Scholar]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 1742–1751. [Google Scholar] [CrossRef]
- Miura, F.; van Ewijk, C.E.; Backer, J.A.; Xiridou, M.; Franz, E.; Op de Coul, E.; Brandwagt, D.; van Cleef, B.; van Rijckevorsel, G.; Swaan, C.; et al. Estimated Incubation Period for Monkeypox Cases Confirmed in the Netherlands, May 2022. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2022, 27, 2200448. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Charniga, K.; Masters, N.B.; Asher, J.; Fahrenwald, L.; Still, W.; Chen, J.; Kipperman, N.; Bui, D.; Shea, M.; et al. Serial Interval and Incubation Period Estimates of Monkeypox Virus Infection in 12 U.S. Jurisdictions, May–August 2022. medRxiv 2022. medRxiv: 2022.10.26.22281516. [Google Scholar]
- Sukhdeo, S.; Mishra, S.; Walmsley, S. Human Monkeypox: A Comparison of the Characteristics of the New Epidemic to the Endemic Disease. BMC Infect. Dis. 2022, 22, 928. [Google Scholar] [CrossRef]
- Brown, K.; Leggat, P.A. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop. Med. Infect. Dis. 2016, 1, 8. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical Manifestations of Human Monkeypox Influenced by Route of Infection. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C.; et al. Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model. mSphere 2021, 6, e00927-20. [Google Scholar] [CrossRef]
- Hutson, C.L.; Damon, I.K. Monkeypox Virus Infections in Small Animal Models for Evaluation of Anti-Poxvirus Agents. Viruses 2010, 2, 2763–2776. [Google Scholar] [CrossRef]
- Shchelukhina, E.M.; Marennikova, S.S. Generalized monkeypox in orally infected rabbits and white mice. Vopr. Virusol. 1975, 6, 703–705. [Google Scholar]
- Marennikova, S.S.; Šeluhina, E.M. Susceptibility of Some Rodent Species to Monkeypox Virus, and Course of the Infection. Bull. World Health Organ. 1976, 53, 13–20. [Google Scholar]
- Stabenow, J.; Buller, R.M.; Schriewer, J.; West, C.; Sagartz, J.E.; Parker, S. A Mouse Model of Lethal Infection for Evaluating Prophylactics and Therapeutics against Monkeypox Virus. J. Virol. 2010, 84, 3909–3920. [Google Scholar] [CrossRef]
- Hutson, C.L.; Gallardo-Romero, N.; Carroll, D.S.; Clemmons, C.; Salzer, J.S.; Nagy, T.; Hughes, C.M.; Olson, V.A.; Karem, K.L.; Damon, I.K. Transmissibility of the Monkeypox Virus Clades via Respiratory Transmission: Investigation Using the Prairie Dog-Monkeypox Virus Challenge System. PLoS ONE 2013, 8, e55488. [Google Scholar] [CrossRef]
- Falendysz, E.A.; Lopera, J.G.; Lorenzsonn, F.; Salzer, J.S.; Hutson, C.L.; Doty, J.; Gallardo-Romero, N.; Carroll, D.S.; Osorio, J.E.; Rocke, T.E. Further Assessment of Monkeypox Virus Infection in Gambian Pouched Rats (Cricetomys gambianus) Using In Vivo Bioluminescent Imaging. PLoS Negl. Trop. Dis. 2015, 9, e0004130. [Google Scholar] [CrossRef]
- Falendysz, E.A.; Lopera, J.G.; Doty, J.B.; Nakazawa, Y.; Crill, C.; Lorenzsonn, F.; Kalemba, L.N.; Ronderos, M.D.; Mejia, A.; Malekani, J.M.; et al. Characterization of Monkeypox Virus Infection in African Rope Squirrels (Funisciurus Sp.). PLoS Negl. Trop. Dis. 2017, 11, e0005809. [Google Scholar] [CrossRef]
- Tesh, R.B.; Watts, D.M.; Sbrana, E.; Siirin, M.; Popov, V.L.; Xiao, S.-Y. Experimental Infection of Ground Squirrels (Spermophilus Tridecemlineatus) with Monkeypox Virus. Emerg. Infect. Dis. 2004, 10, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Americo, J.L.; Moss, B.; Earl, P.L. Identification of Wild-Derived Inbred Mouse Strains Highly Susceptible to Monkeypox Virus Infection for Use as Small Animal Models. J. Virol. 2010, 84, 8172–8180. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Iams, K.P.; Meteyer, C.U.; Rocke, T.E. Comparison of Monkeypox Viruses Pathogenesis in Mice by in Vivo Imaging. PLoS ONE 2009, 4, e6592. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.A.; Sagartz, J.E.; Huso, D.L.; Buller, R.M.L. Experimental Infection of an African Dormouse (Graphiurus kelleni) with Monkeypox Virus. Virology 2009, 383, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Nakazawa, Y.J.; Self, J.; Olson, V.A.; Regnery, R.L.; Braden, Z.; Weiss, S.; Malekani, J.; Jackson, E.; Tate, M.; et al. Laboratory Investigations of African Pouched Rats (Cricetomys gambianus) as a Potential Reservoir Host Species for Monkeypox Virus. PLoS Negl. Trop. Dis. 2015, 9, e0004013. [Google Scholar] [CrossRef]
- Hutson, C.L.; Olson, V.A.; Carroll, D.S.; Abel, J.A.; Hughes, C.M.; Braden, Z.H.; Weiss, S.; Self, J.; Osorio, J.E.; Hudson, P.N.; et al. A Prairie Dog Animal Model of Systemic Orthopoxvirus Disease Using West African and Congo Basin Strains of Monkeypox Virus. J. Gen. Virol. 2009, 90, 323–333. [Google Scholar] [CrossRef]
- Xiao, S.-Y.; Sbrana, E.; Watts, D.M.; Siirin, M.; da Rosa, A.P.A.T.; Tesh, R.B. Experimental Infection of Prairie Dogs with Monkeypox Virus. Emerg. Infect. Dis. 2005, 11, 539–545. [Google Scholar] [CrossRef]
- Falendysz, E.A.; Londoño-Navas, A.M.; Meteyer, C.U.; Pussini, N.; Lopera, J.G.; Osorio, J.E.; Rocke, T.E. Evaluation of monkeypox virus infection of black-tailed prairie dogs (Cynomys ludovicianus) using in vivo bioluminescent imaging. J. Wildl. Dis. 2014, 50, 524–536. [Google Scholar] [CrossRef]
- Weiner, Z.P.; Salzer, J.S.; LeMasters, E.; Ellison, J.A.; Kondas, A.V.; Morgan, C.N.; Doty, J.B.; Martin, B.E.; Satheshkumar, P.S.; Olson, V.A.; et al. Characterization of Monkeypox Virus Dissemination in the Black-Tailed Prairie Dog (Cynomys ludovicianus) through in Vivo Bioluminescent Imaging. PLoS ONE 2019, 14, e0222612. [Google Scholar] [CrossRef]
- Titanji, B.K.; Tegomoh, B.; Nematollahi, S.; Konomos, M.; Kulkarni, P.A. Monkeypox: A Contemporary Review for Healthcare Professionals. Open Forum Infect. Dis. 2022, 9, ofac310. [Google Scholar] [CrossRef]
- Farahat, R.A.; Abdelaal, A.; Shah, J.; Ghozy, S.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; McHugh, T.D.; Leblebicioglu, H. Monkeypox Outbreaks during COVID-19 Pandemic: Are We Looking at an Independent Phenomenon or an Overlapping Pandemic? Ann. Clin. Microbiol. Antimicrob. 2022, 21, 26. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Update: Multistate Outbreak of Monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 589–590. [Google Scholar]
- de la Calle-Prieto, F.; Estébanez Muñoz, M.; Ramírez, G.; Díaz-Menéndez, M.; Velasco, M.; Azkune Galparsoro, H.; Salavert Lletí, M.; Mata Forte, T.; Blanco, J.L.; Mora-Rillo, M.; et al. Treatment and Prevention of Monkeypox. Enfermedades Infecc. Microbiol. Clínica Engl. Ed. 2023, in press. [Google Scholar] [CrossRef]
- Sanz-Muñoz, I.; Sánchez-dePrada, L.; Sánchez-Martínez, J.; Rojo-Rello, S.; Domínguez-Gil, M.; Hernán-García, C.; Fernández-Espinilla, V.; de Lejarazu-Leonardo, R.O.; Castrodeza-Sanz, J.; Eiros, J.M. Possible Mpox Protection from Smallpox Vaccine–Generated Antibodies among Older Adults. Emerg. Infect. Dis. 2023, 29, 656–658. [Google Scholar] [CrossRef]
- Pugh, C.; Keasey, S.; Korman, L.; Pittman, P.R.; Ulrich, R.G. Human Antibody Responses to the Polyclonal Dryvax Vaccine for Smallpox Prevention Can Be Distinguished from Responses to the Monoclonal Replacement Vaccine ACAM2000. Clin. Vaccine Immunol. CVI 2014, 21, 877–885. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Vaccines | Smallpox | CDC. Available online: https://www.cdc.gov/smallpox/clinicians/vaccines.html (accessed on 5 March 2023).
- Mucker, E.M.; Golden, J.W.; Hammerbeck, C.D.; Kishimori, J.M.; Royals, M.; Joselyn, M.D.; Ballantyne, J.; Nalca, A.; Hooper, J.W. A Nucleic Acid-Based Orthopoxvirus Vaccine Targeting the Vaccinia Virus L1, A27, B5, and A33 Proteins Protects Rabbits against Lethal Rabbitpox Virus Aerosol Challenge. J. Virol 2022, 96, e01504-21. [Google Scholar] [CrossRef]
- Immunomedicine Group: Databases: EPIMHC. Available online: http://imed.med.ucm.es/epipox/ (accessed on 13 February 2023).
- Pascual-Iglesias, A.; Canton, J.; Ortega-Prieto, A.M.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A. An Overview of Vaccines against SARS-CoV-2 in the COVID-19 Pandemic Era. Pathog. Basel Switz. 2021, 10, 1030. [Google Scholar] [CrossRef]
- Jain, N.; Lansiaux, E.; Simanis, R. The New Face of Monkeypox Virus: An Emerging Global Emergency. New Microbes New Infect. 2022, 47, 100989. [Google Scholar] [CrossRef]
- Reynolds, M.G.; McCollum, A.M.; Nguete, B.; Shongo Lushima, R.; Petersen, B.W. Improving the Care and Treatment of Monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses 2017, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pevear, D.C.; Davies, M.H.; Collett, M.S.; Bailey, T.; Rippen, S.; Barone, L.; Burns, C.; Rhodes, G.; Tohan, S.; et al. An Orally Bioavailable Antipoxvirus Compound (ST-246) Inhibits Extracellular Virus Formation and Protects Mice from Lethal Orthopoxvirus Challenge. J. Virol. 2005, 79, 13139–13149. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.T.; Grosenbach, D.W.; Chinsangaram, J.; Honeychurch, K.M.; Long, P.G.; Lovejoy, C.; Maiti, B.; Meara, I.; Hruby, D.E. An Overview of Tecovirimat for Smallpox Treatment and Expanded Anti-Orthopoxvirus Applications. Expert Rev. Anti Infect. Ther. 2021, 19, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox Vaccine-Induced Antibodies Are Necessary and Sufficient for Protection against Monkeypox Virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef]
- FDA. Vaccinia Immune Globulin Intravenous (Human); FDA: Silver Spring, MD, USA, 2022. [Google Scholar]
- Priyamvada, L.; Burgado, J.; Baker-Wagner, M.; Kitaygorodskyy, A.; Olson, V.; Lingappa, V.R.; Satheshkumar, P.S. New Methylene Blue Derivatives Suggest Novel Anti-Orthopoxviral Strategies. Antivir. Res. 2021, 191, 105086. [Google Scholar] [CrossRef]
- Cao, S.; Realegeno, S.; Pant, A.; Satheshkumar, P.S.; Yang, Z. Suppression of Poxvirus Replication by Resveratrol. Front. Microbiol. 2017, 8, 2196. [Google Scholar] [CrossRef]
- Keckler, M.S.; Salzer, J.S.; Patel, N.; Townsend, M.B.; Nakazawa, Y.J.; Doty, J.B.; Gallardo-Romero, N.F.; Satheshkumar, P.S.; Carroll, D.S.; Karem, K.L.; et al. IMVAMUNE® and ACAM2000® Provide Different Protection against Disease When Administered Postexposure in an Intranasal Monkeypox Challenge Prairie Dog Model. Vaccines 2020, 8, 396. [Google Scholar] [CrossRef]
- Smith, S.K.; Self, J.; Weiss, S.; Carroll, D.; Braden, Z.; Regnery, R.L.; Davidson, W.; Jordan, R.; Hruby, D.E.; Damon, I.K. Effective Antiviral Treatment of Systemic Orthopoxvirus Disease: ST-246 Treatment of Prairie Dogs Infected with Monkeypox Virus. J. Virol. 2011, 85, 9176–9187. [Google Scholar] [CrossRef]
- Jordan, R.; Goff, A.; Frimm, A.; Corrado, M.L.; Hensley, L.E.; Byrd, C.M.; Mucker, E.; Shamblin, J.; Bolken, T.C.; Wlazlowski, C.; et al. ST-246 Antiviral Efficacy in a Nonhuman Primate Monkeypox Model: Determination of the Minimal Effective Dose and Human Dose Justification. Antimicrob. Agents Chemother. 2009, 53, 1817–1822. [Google Scholar] [CrossRef]
- Mazurkov, O.Y.; Kabanov, A.S.; Shishkina, L.N.; Sergeev, A.A.; Skarnovich, M.O.; Bormotov, N.I.; Skarnovich, M.A.; Ovchinnikova, A.S.; Titova, K.A.; Galahova, D.O.; et al. New Effective Chemically Synthesized Anti-Smallpox Compound NIOCH-14. J. Gen. Virol. 2016, 97, 1229–1239. [Google Scholar] [CrossRef]
- Earl, P.L.; Cooper, N.; Wyatt, L.S.; Moss, B.; Carroll, M.W. Preparation of Cell Cultures and Vaccinia Virus Stocks. Curr. Protoc. Protein Sci. 1998, 13, 5.12.1–5.12.12. [Google Scholar] [CrossRef]
- Baker, R.O.; Bray, M.; Huggins, J.W. Potential Antiviral Therapeutics for Smallpox, Monkeypox and Other Orthopoxvirus Infections. Antivir. Res. 2003, 57, 13–23. [Google Scholar] [CrossRef]
- Johnston, S.C.; Lin, K.L.; Connor, J.H.; Ruthel, G.; Goff, A.; Hensley, L.E. In Vitro Inhibition of Monkeypox Virus Production and Spread by Interferon-β. Virol. J. 2012, 9, 5. [Google Scholar] [CrossRef]
- Goff, A.J.; Chapman, J.; Foster, C.; Wlazlowski, C.; Shamblin, J.; Lin, K.; Kreiselmeier, N.; Mucker, E.; Paragas, J.; Lawler, J.; et al. A Novel Respiratory Model of Infection with Monkeypox Virus in Cynomolgus Macaques. J. Virol. 2011, 85, 4898–4909. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Fernández, D.E.; Fernández-Quezada, D.; Casillas-Muñoz, F.A.G.; Carrillo-Ballesteros, F.J.; Ortega-Prieto, A.M.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A. Human Monkeypox: A Comprehensive Overview of Epidemiology, Pathogenesis, Diagnosis, Treatment, and Prevention Strategies. Pathogens 2023, 12, 947. https://doi.org/10.3390/pathogens12070947
Martínez-Fernández DE, Fernández-Quezada D, Casillas-Muñoz FAG, Carrillo-Ballesteros FJ, Ortega-Prieto AM, Jimenez-Guardeño JM, Regla-Nava JA. Human Monkeypox: A Comprehensive Overview of Epidemiology, Pathogenesis, Diagnosis, Treatment, and Prevention Strategies. Pathogens. 2023; 12(7):947. https://doi.org/10.3390/pathogens12070947
Chicago/Turabian StyleMartínez-Fernández, Diana Emilia, David Fernández-Quezada, Fidel Antonio Guadalupe Casillas-Muñoz, Francisco Josué Carrillo-Ballesteros, Ana Maria Ortega-Prieto, Jose M. Jimenez-Guardeño, and Jose Angel Regla-Nava. 2023. "Human Monkeypox: A Comprehensive Overview of Epidemiology, Pathogenesis, Diagnosis, Treatment, and Prevention Strategies" Pathogens 12, no. 7: 947. https://doi.org/10.3390/pathogens12070947
APA StyleMartínez-Fernández, D. E., Fernández-Quezada, D., Casillas-Muñoz, F. A. G., Carrillo-Ballesteros, F. J., Ortega-Prieto, A. M., Jimenez-Guardeño, J. M., & Regla-Nava, J. A. (2023). Human Monkeypox: A Comprehensive Overview of Epidemiology, Pathogenesis, Diagnosis, Treatment, and Prevention Strategies. Pathogens, 12(7), 947. https://doi.org/10.3390/pathogens12070947








