Female Sex Hormones Upregulate the Replication Activity of HIV-1 Sub-Subtype A6 and CRF02_AG but Not HIV-1 Subtype B
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Ethical Aspects
2.3. Infection with Different HIV Subtypes
2.4. Hormone Concentrations
2.5. Monitoring of Virus Replication
2.6. Flow Cytometry Analysis of CCR5 and CXCR4 Co-Receptors
2.7. Analysis of Toll-Like Receptor (TLR) Gene Expression
2.8. Statistical Analysis
3. Results
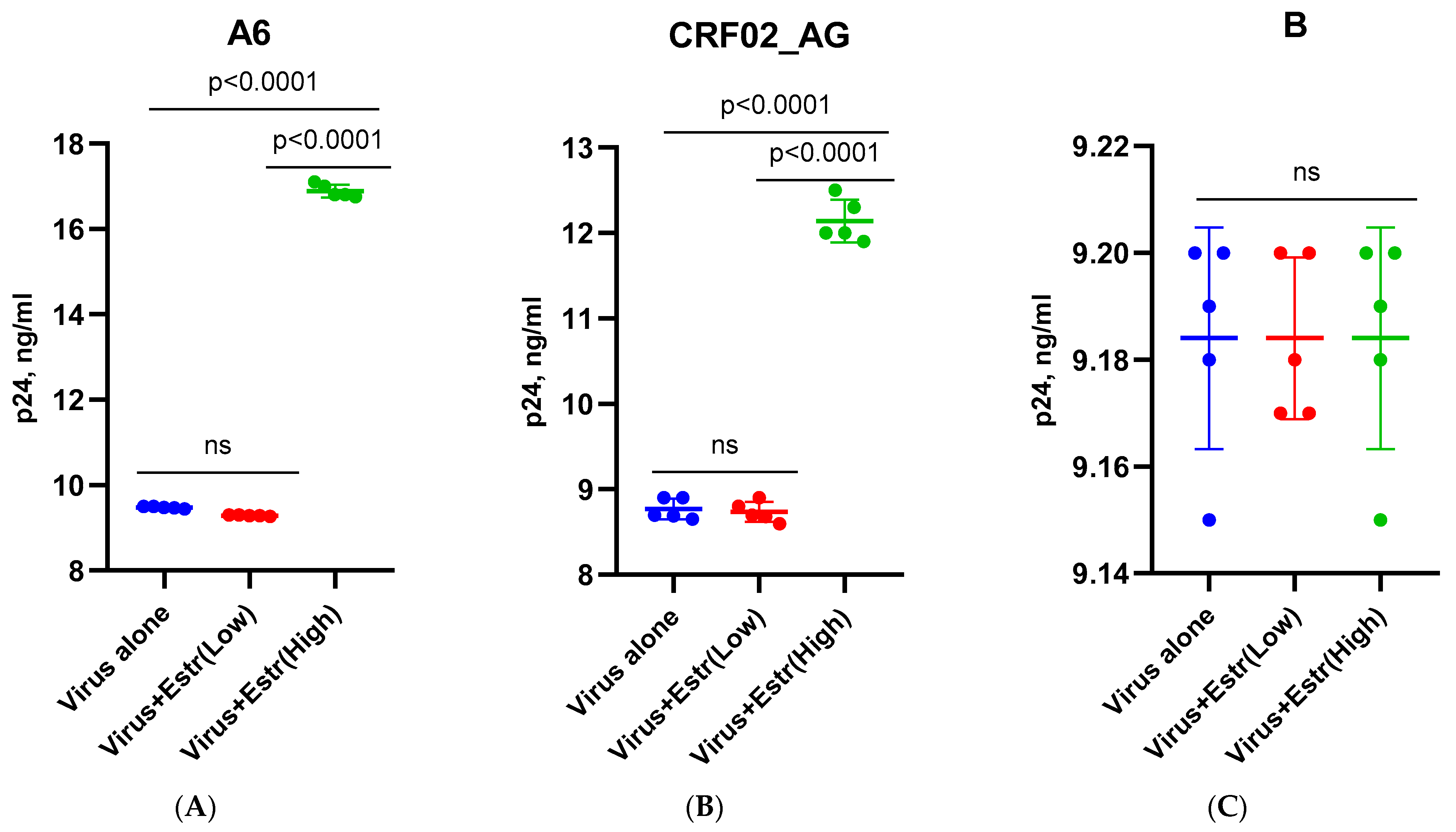
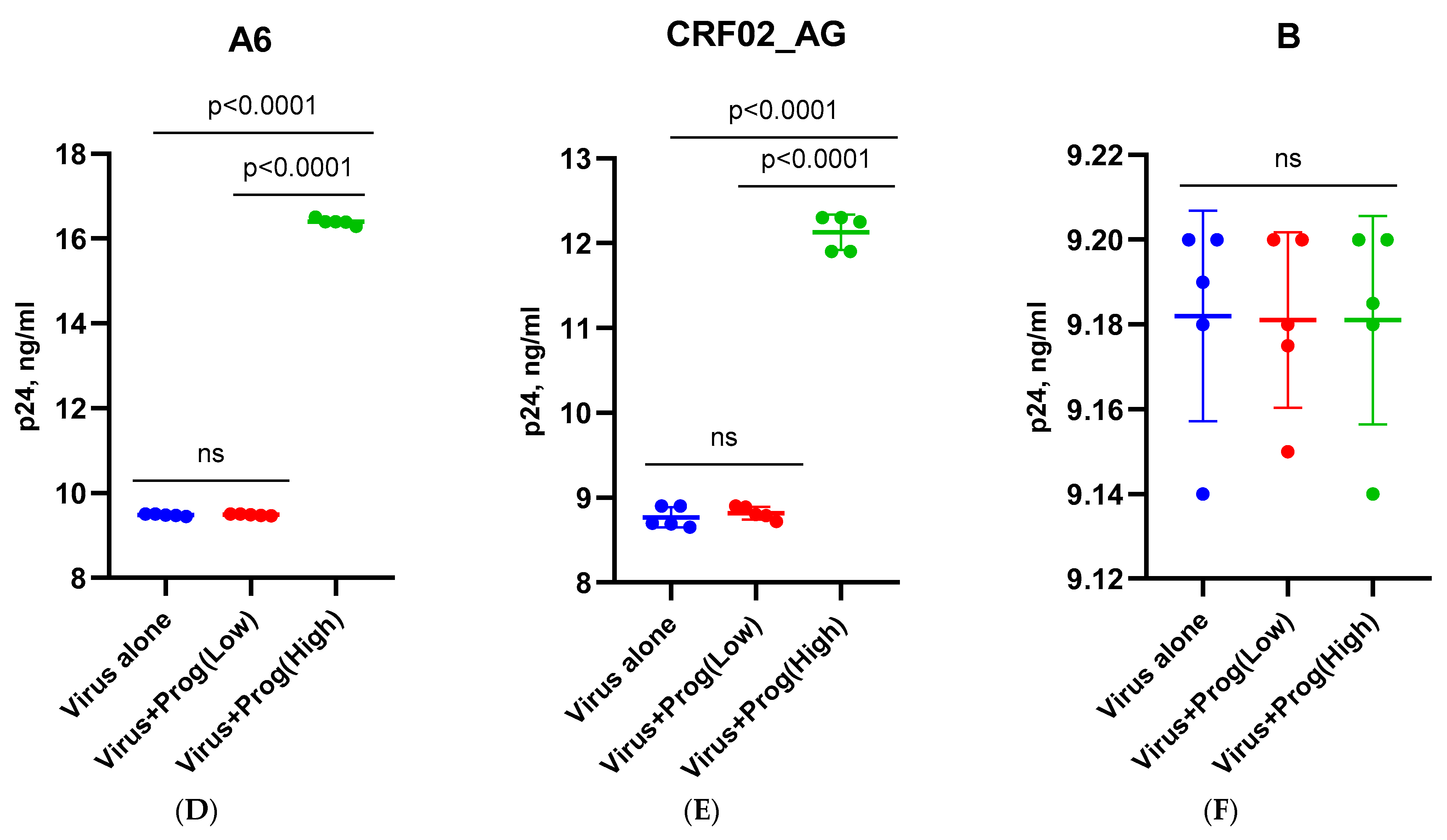
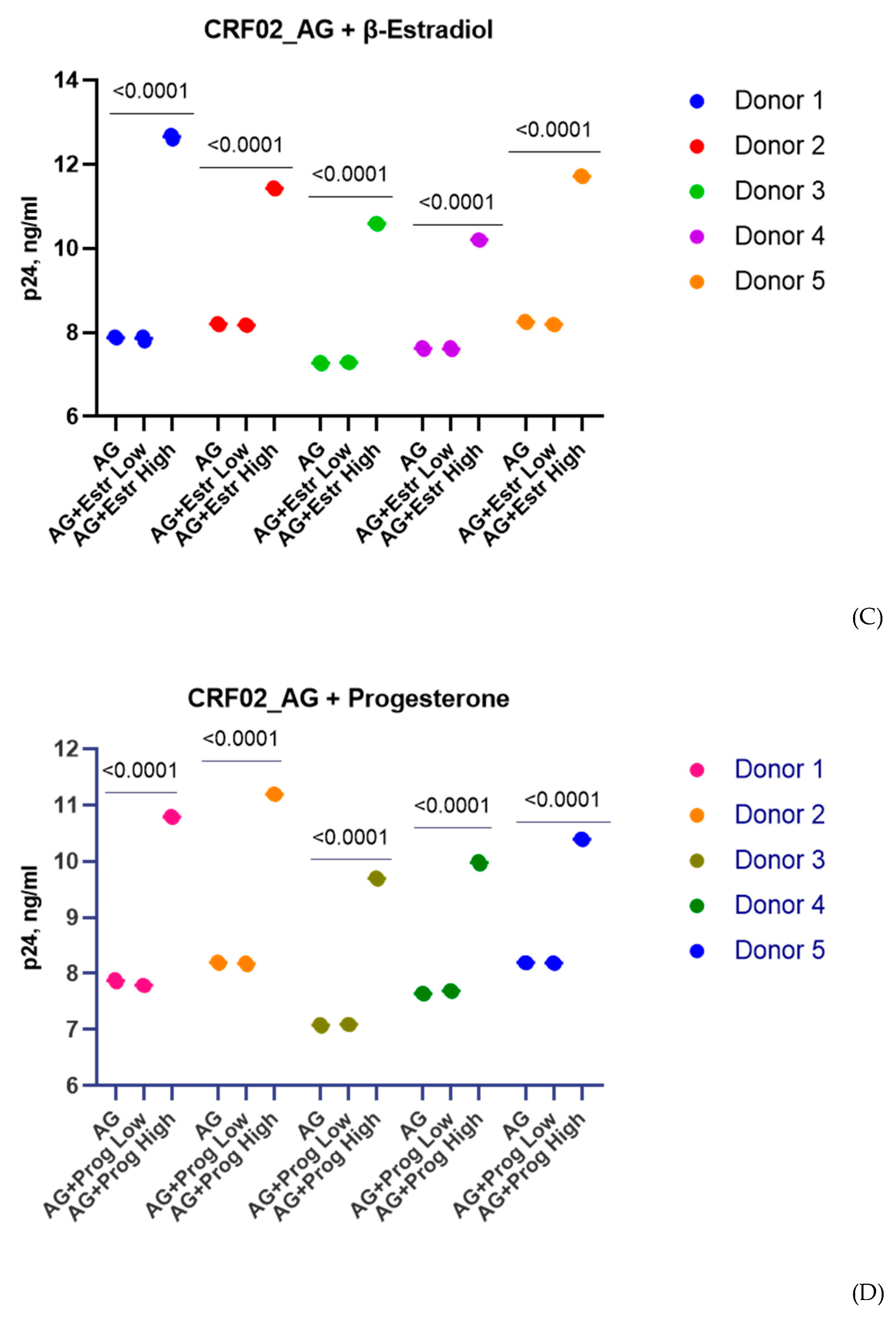
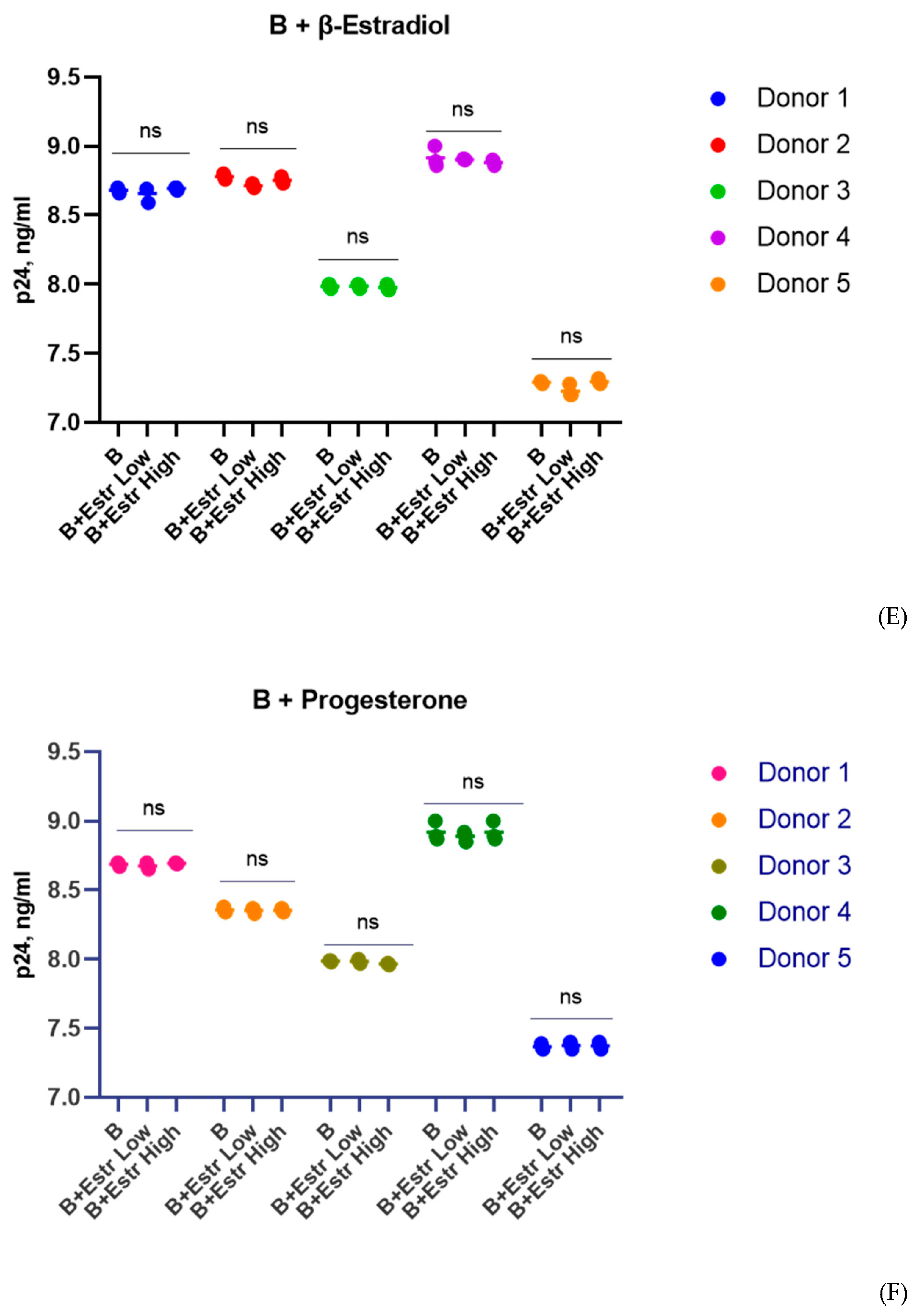
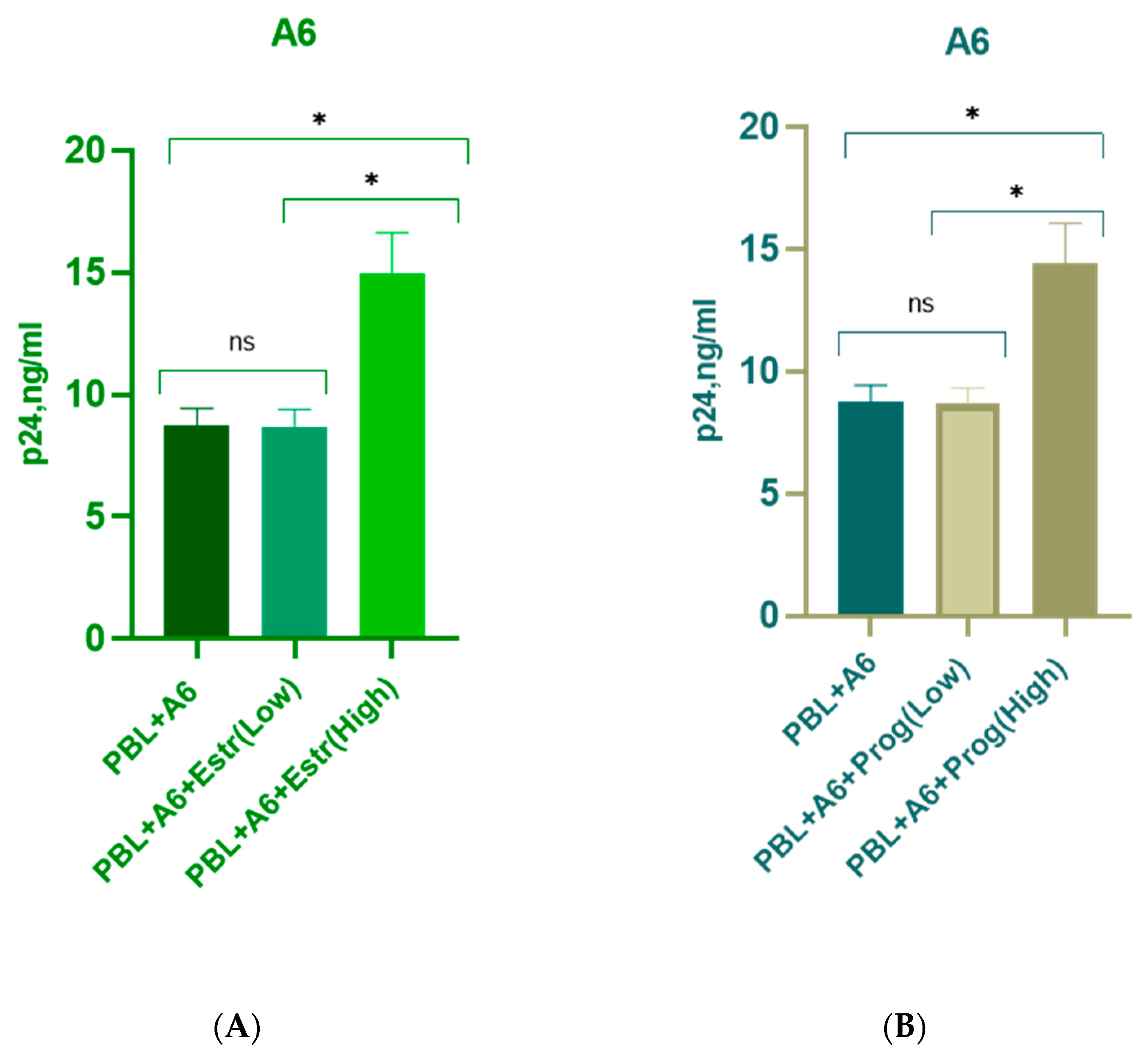
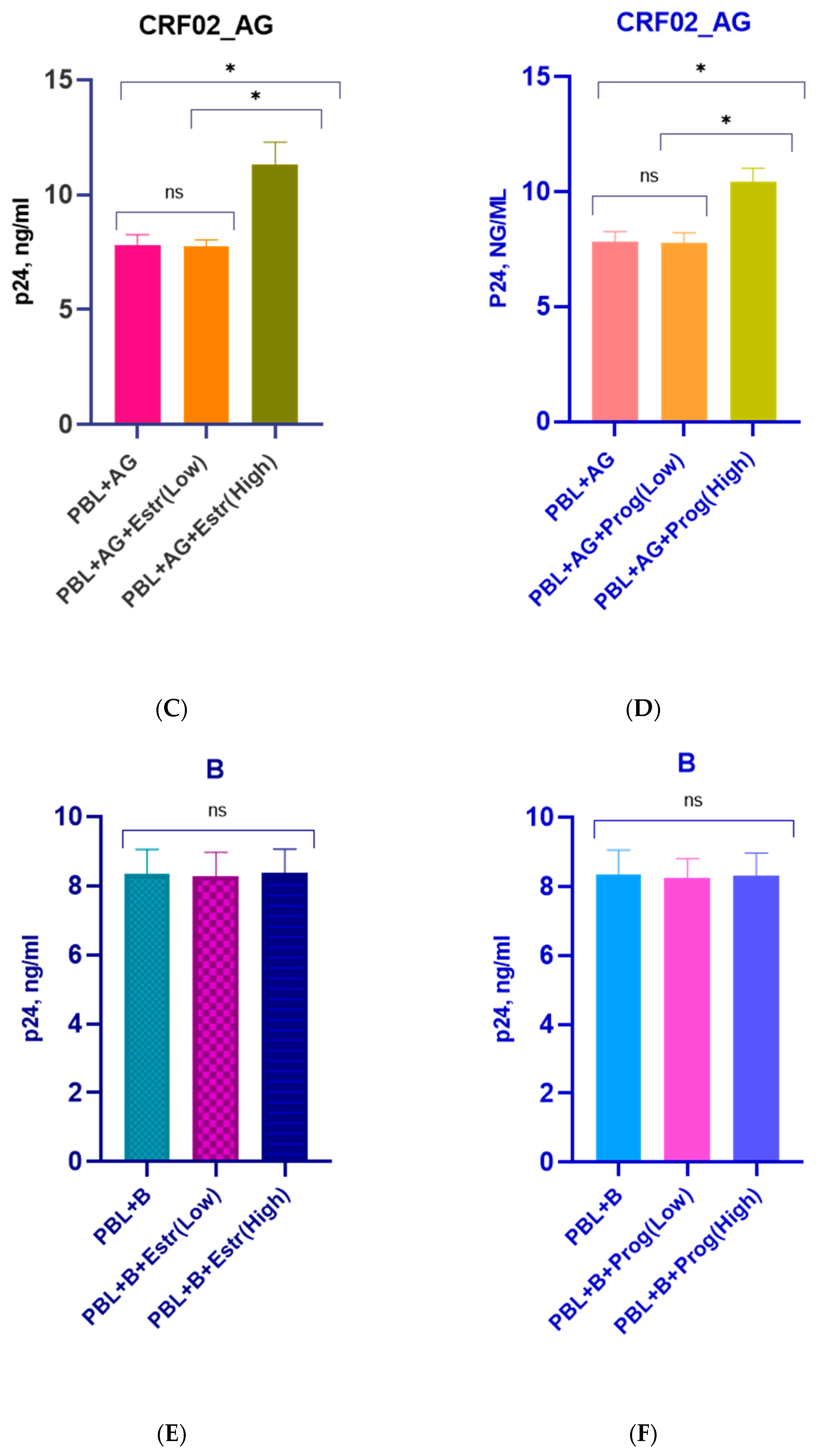
3.1. Effect of β-Estradiol and Progesterone on HIV-1 Replication in T-Cell Lines and PBMCs
3.2. Effect of β-Estradiol and Progesterone on CCR5 and CXCR4 Levels
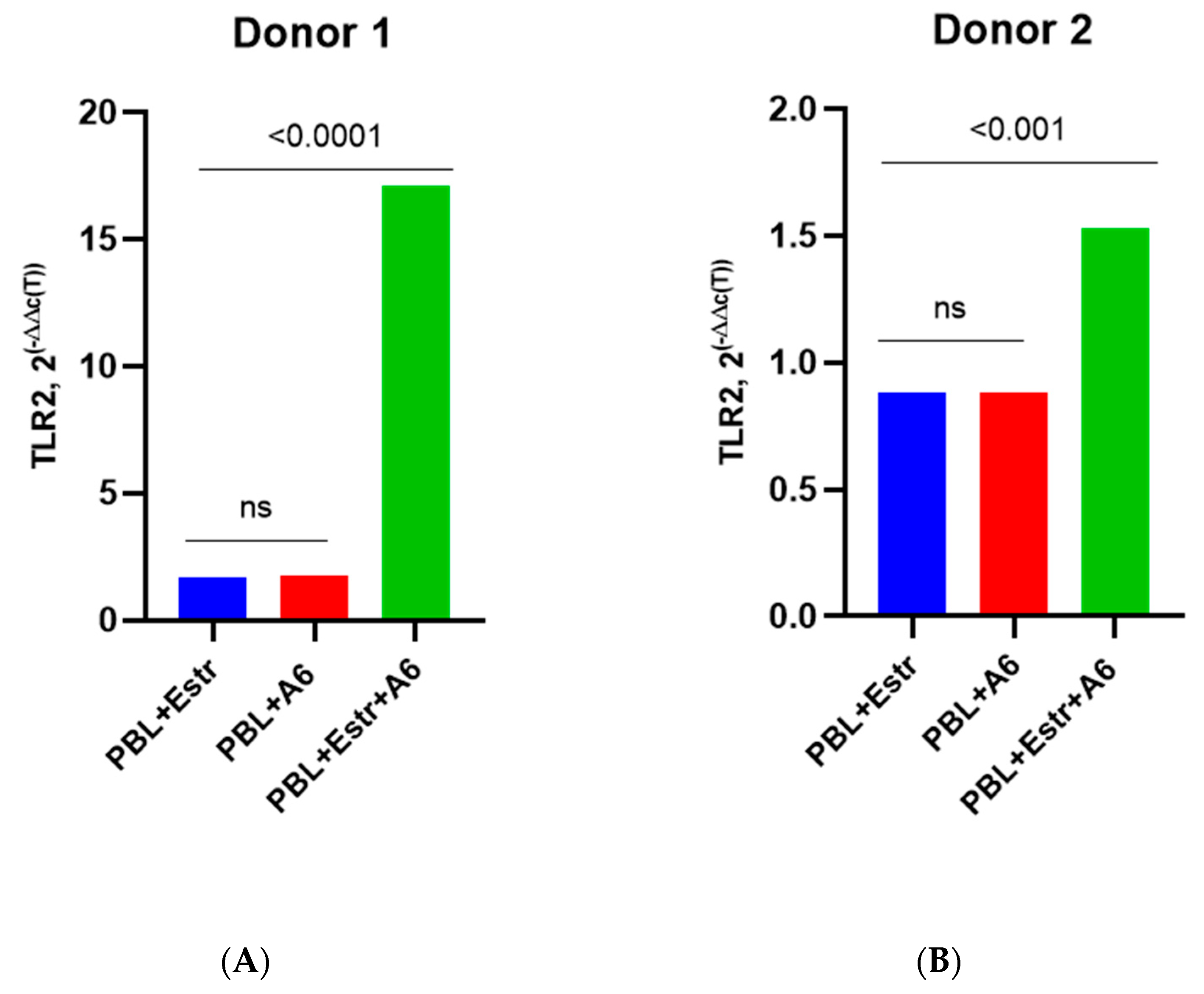
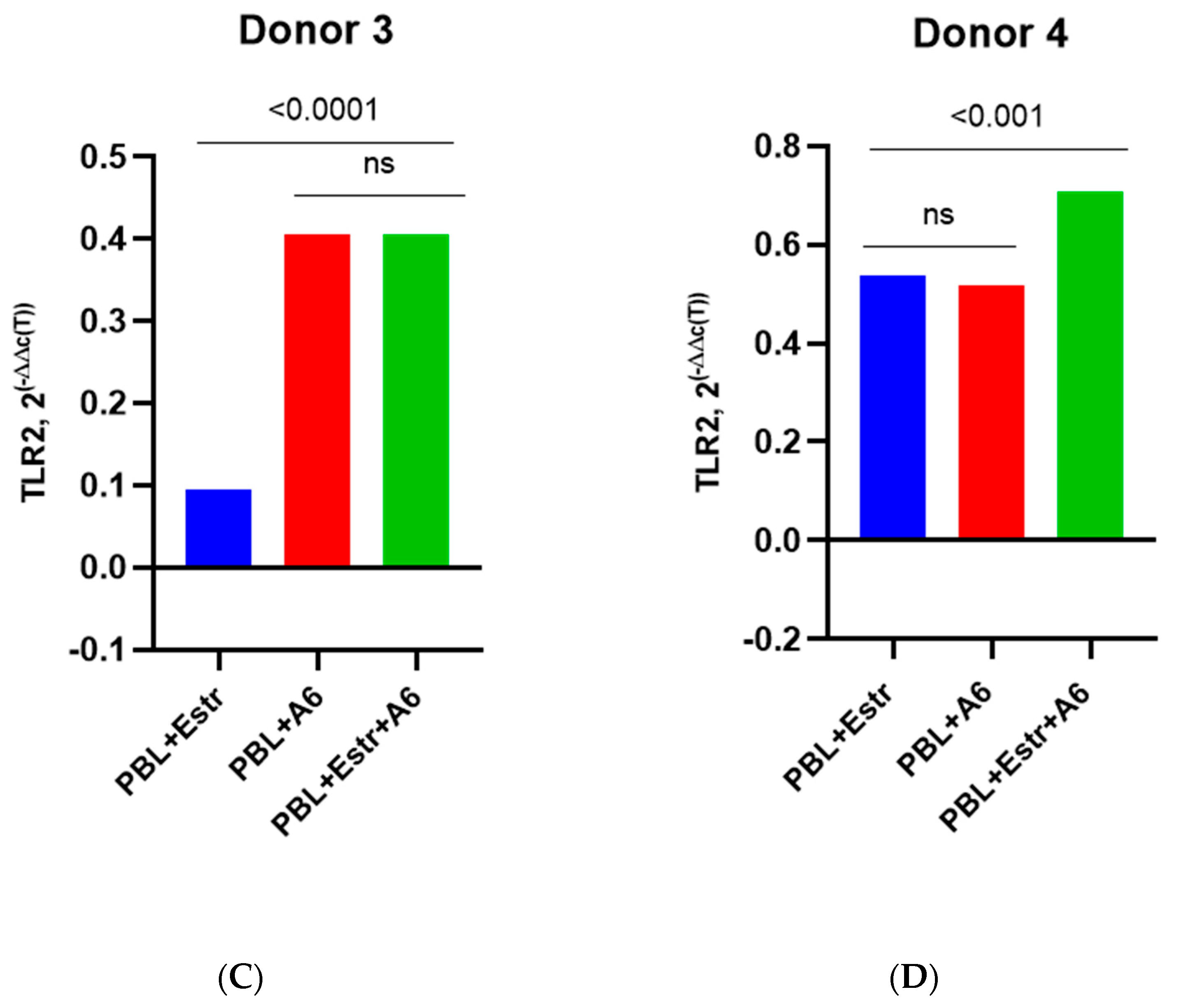
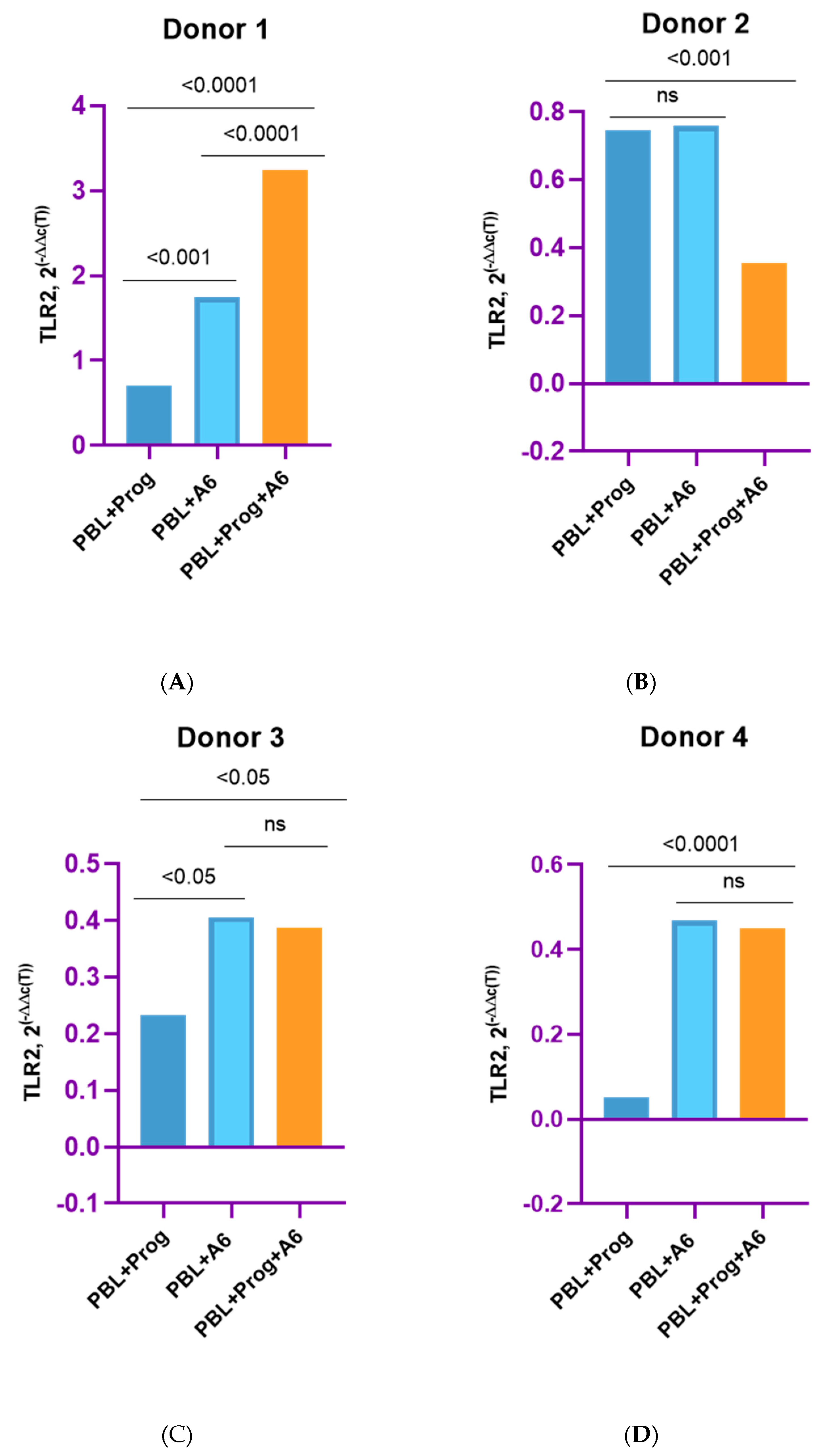
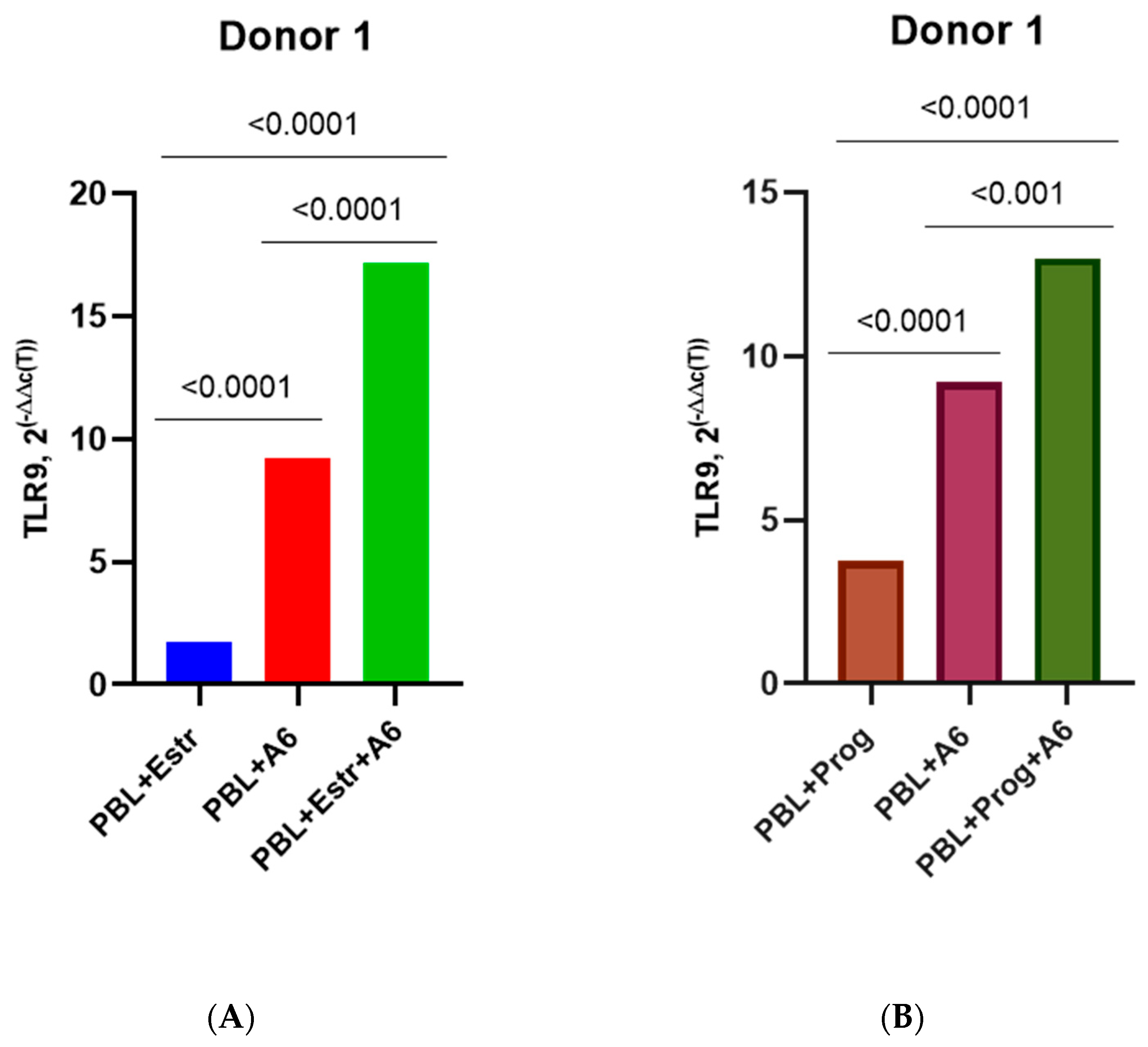
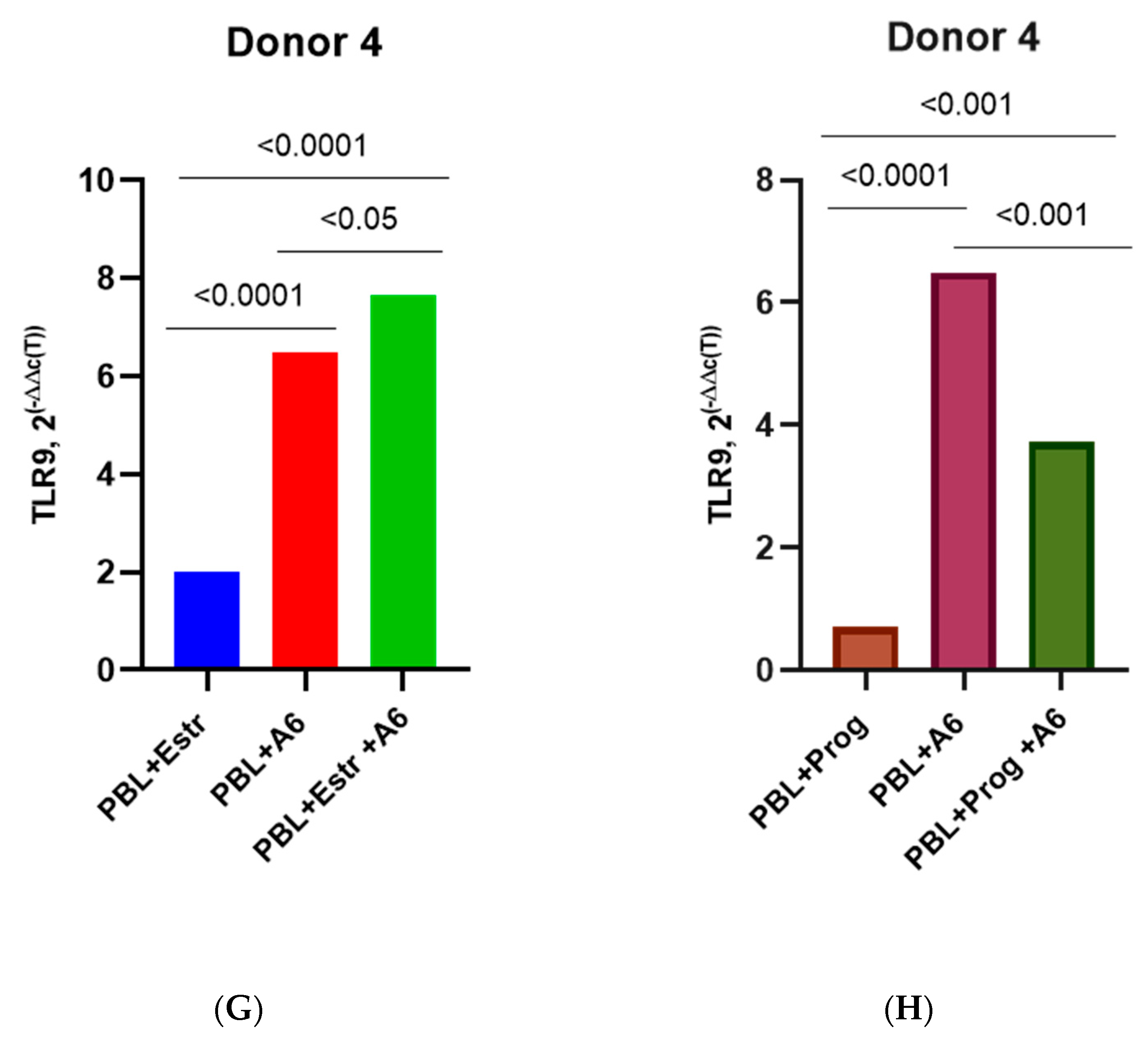
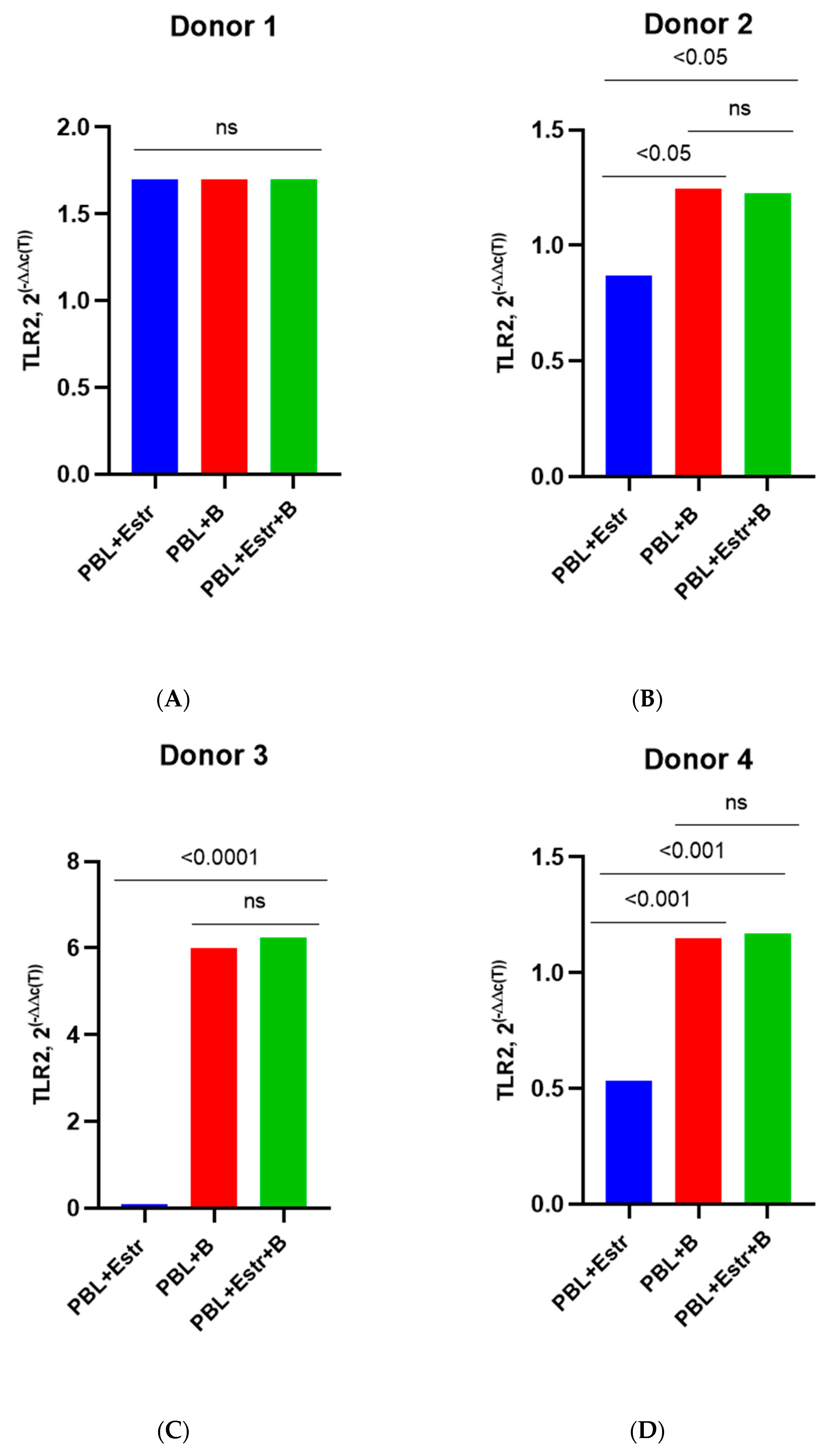
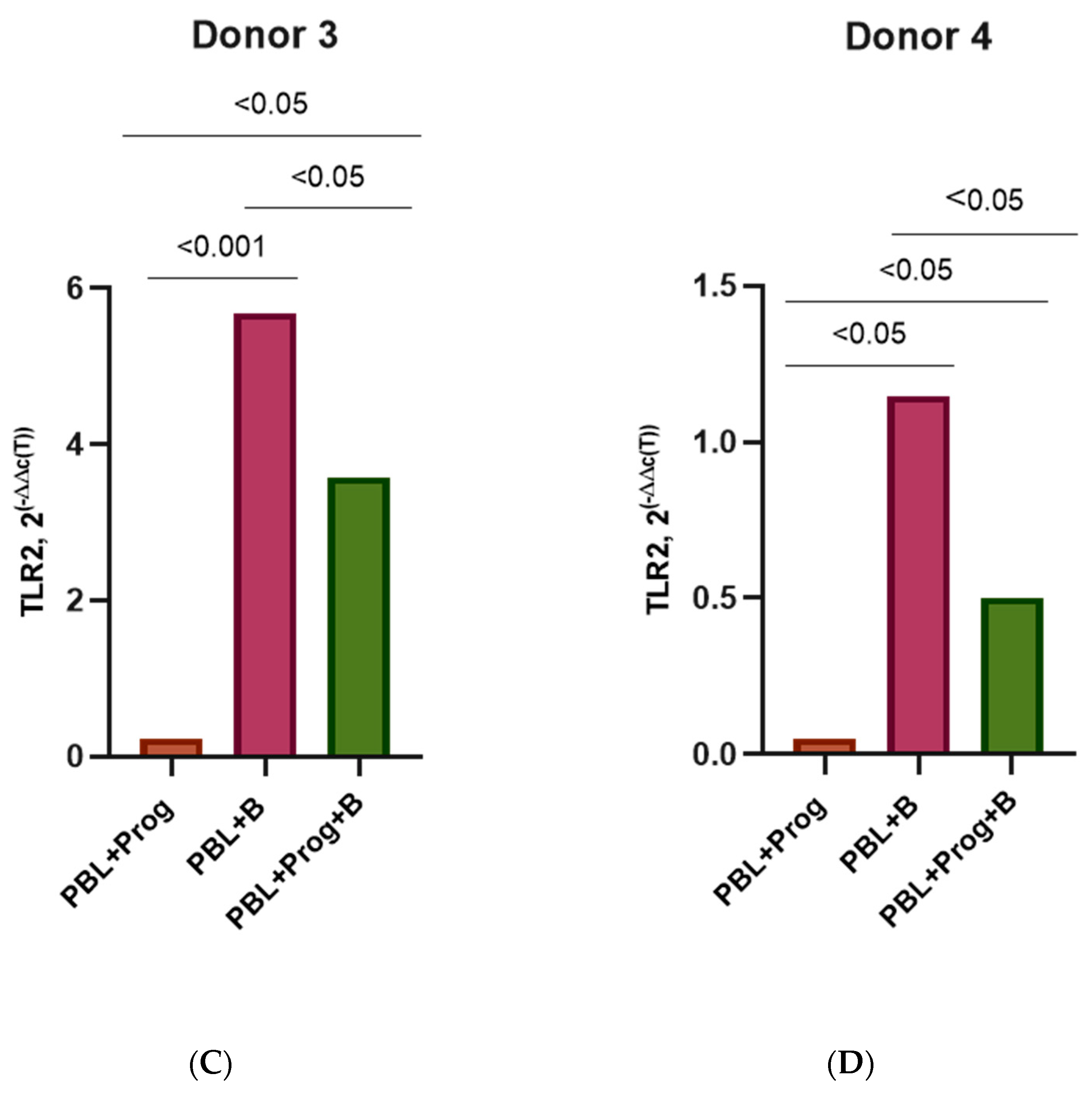
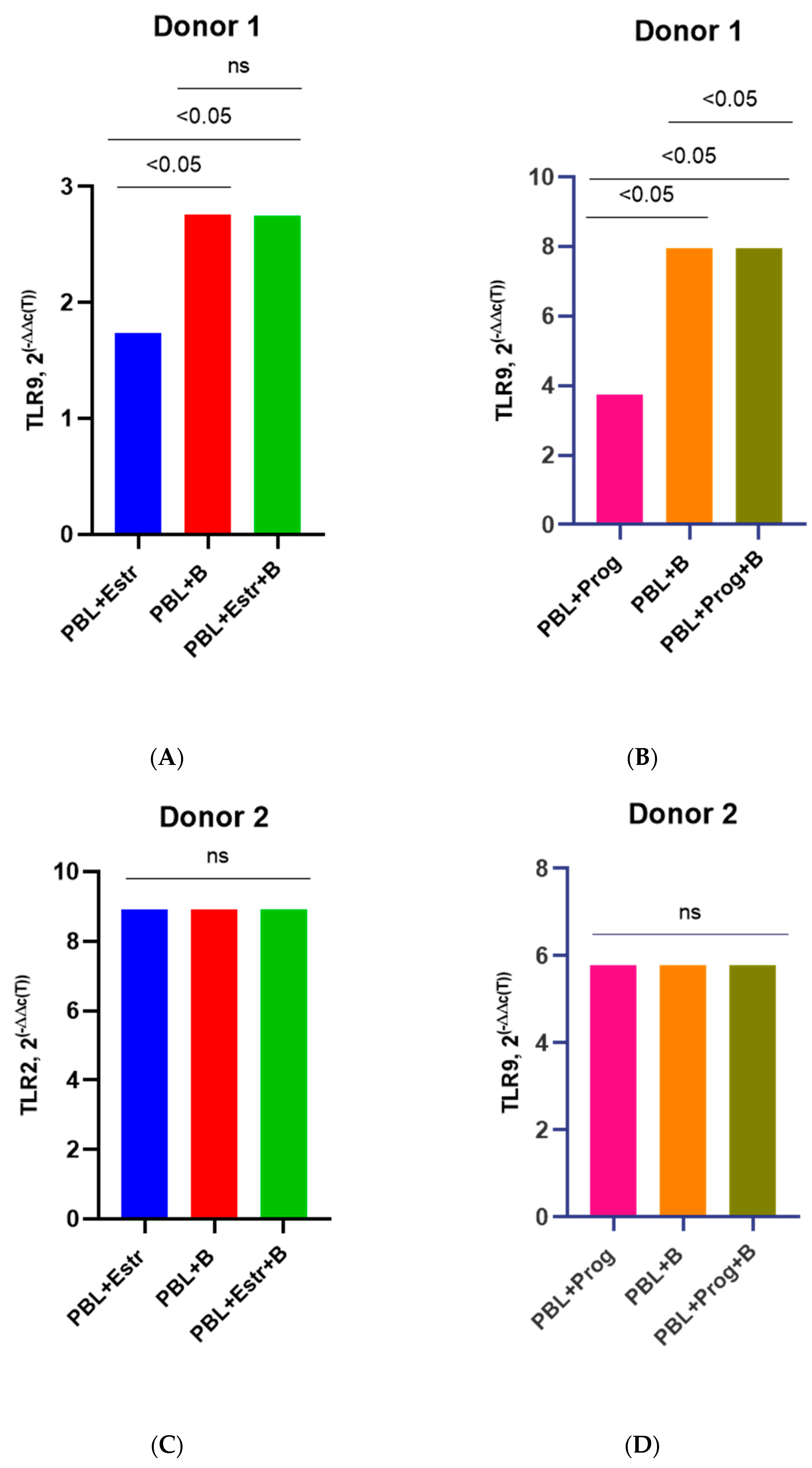
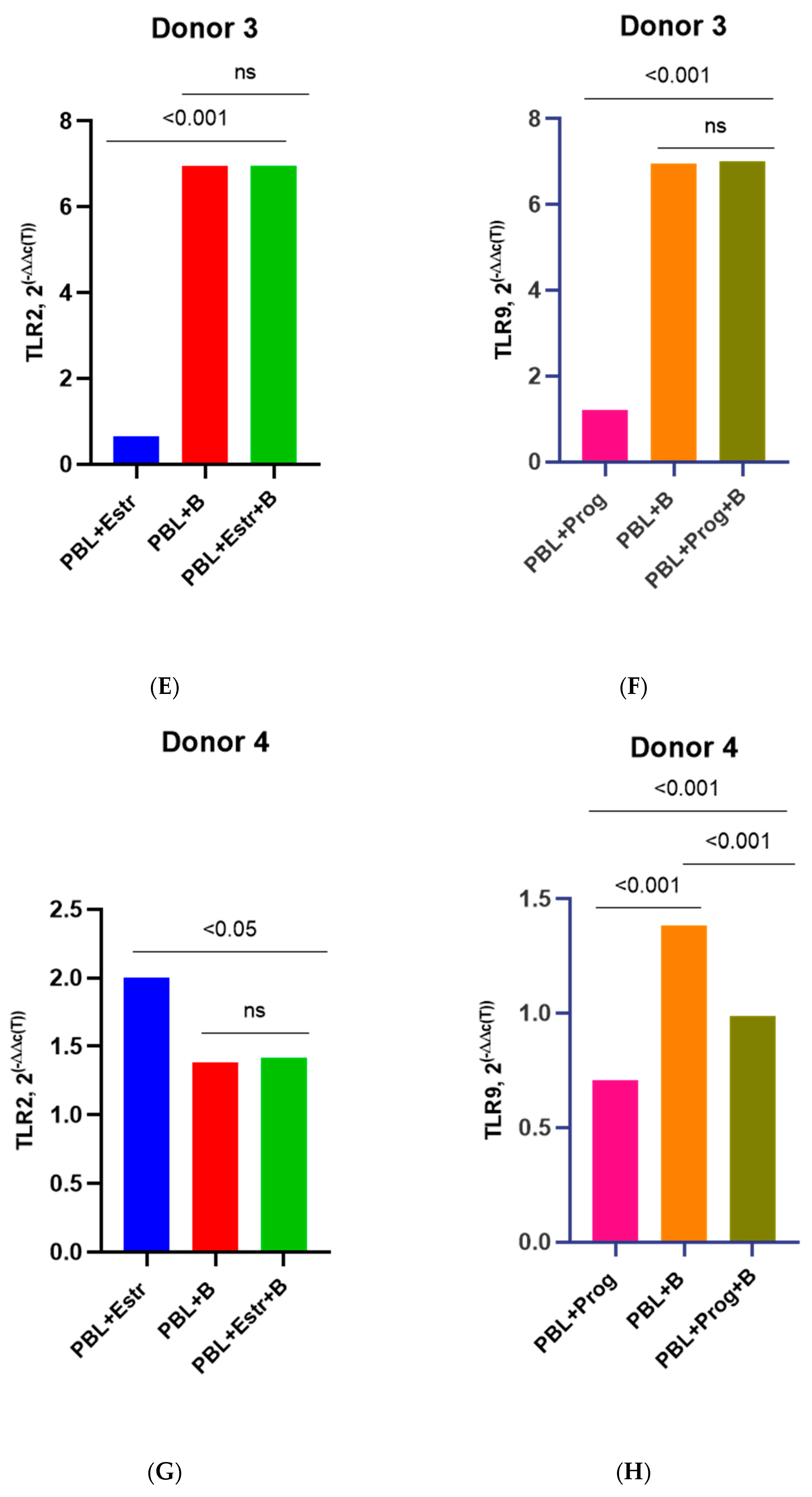
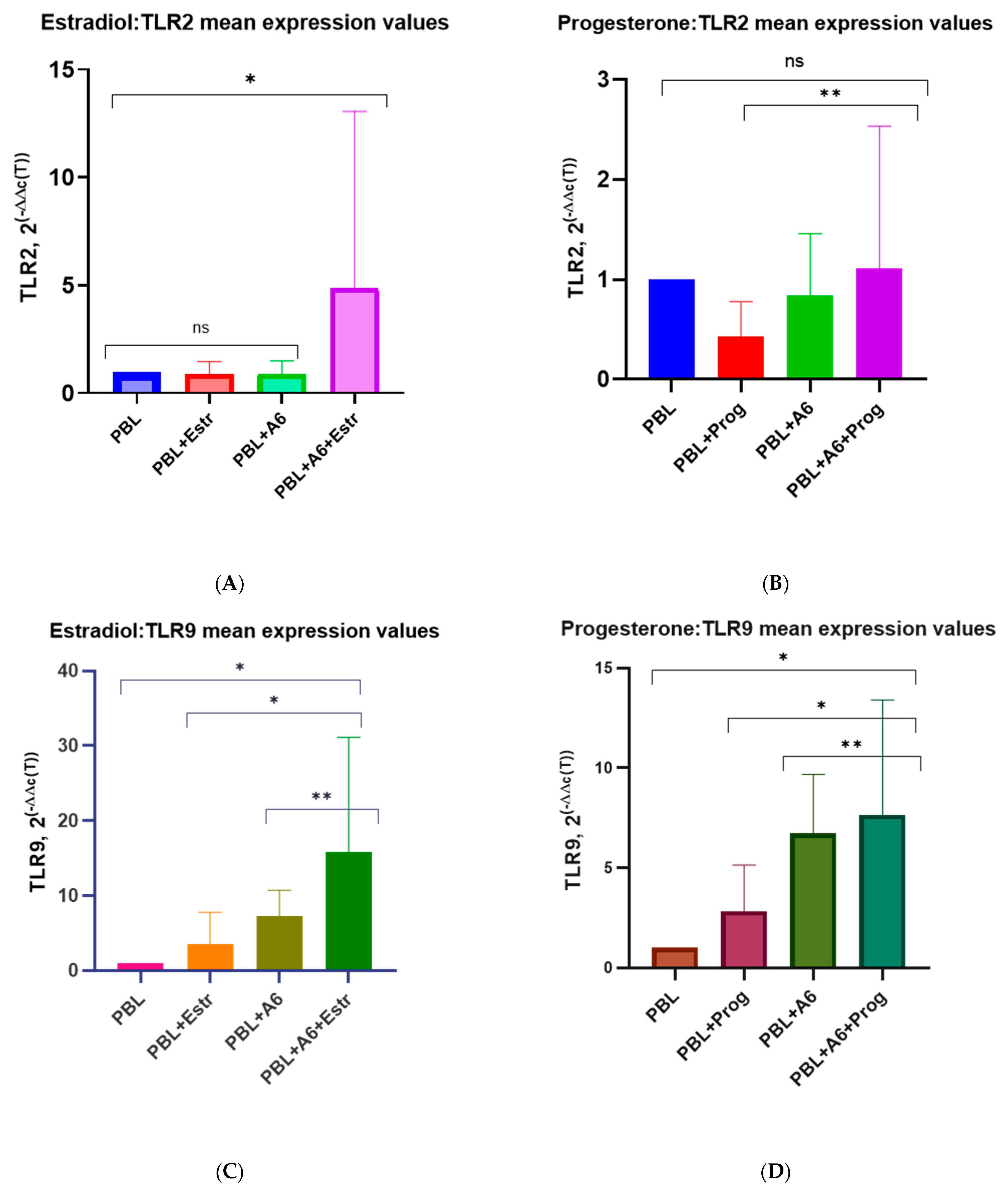
3.3. Effect of β-Estradiol and Progesterone on TLR2 and TLR9 Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 15 January 2023).
- AVERT. Global Information and Education on HIV and AIDS. Available online: https://www.avert.org/professionals/hiv-social-issues/key-affected-populations/women#:~:text=Today%2C%20women%20constitute%20more%20than%20half%20of%20all,for%20a%20disproportionate%20number%20of%20new%20HIV%20infections (accessed on 15 January 2023).
- UNAIDS. Women and HIV—A Spotlight on Adolescent Girls and Young Women. 2019. Available online: https://unaids-test.unaids.org/en/resources/documents/2019/women-and-hiv (accessed on 16 May 2023).
- United Nations Department of Economic and Social Affairs, Population Division (2022). World Family Planning 2022. Meeting the Changing Needs for Family Planning: Contraceptive Use by Age and Method. 2022. Available online: www.un.org/development/desa/pd/ (accessed on 15 January 2023).
- World Health Organization. Fact Sheet. Family Planning/Contraception Methods. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/family-planning-contraception (accessed on 15 January 2023).
- World Health Organization. Contraceptive Eligibility for Women at High Risk of HIV; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241550574 (accessed on 20 April 2023).
- United Nations, Department of Economic and Social Affairs, Population Division. Contraceptive Use by Method 2019: Data Booklet (ST/ESA/SER.A/435). 2019. Available online: https://www.un.org/development/desa/pd/content/contraceptive-use-method-2019 (accessed on 18 January 2023).
- United Nations. Population Division. Family Planning. Available online: https://www.un.org/development/desa/pd/content/family-planning-0 (accessed on 15 January 2023).
- Lavreys, L.; Baeten, J.M.; Martin, H.L., Jr.; Overbaugh, J.; Mandaliya, K.; Ndinya-Achola, J.; Kreiss, J.K. Hormonal contraception and risk of HIV-1 acquisition: Results of a 10-year prospective study. Aids 2004, 18, 695–697. [Google Scholar] [CrossRef]
- Leclerc, P.M.; Dubois-Colas, N.; Garenne, M. Hormonal contraception and HIV prevalence in four African countries. Contraception 2008, 77, 371–376. [Google Scholar] [CrossRef]
- Morrison, C.S.; Chen, P.L.; Kwok, C.; Baeten, J.M.; Brown, J.; Crook, A.M.; Van Damme, L.; Delany-Moretlwe, S.; Francis, S.C.; Friedland, B.A.; et al. Hormonal contraception and the risk of HIV acquisition: An individual participant data meta-analysis. PLoS Med. 2015, 12, e1001778. [Google Scholar] [CrossRef] [PubMed]
- Ralph, L.J.; Gollub, E.L.; Jones, H.E. Hormonal contraceptive use and women’s risk of HIV acquisition: Priorities emerging from recent data. Curr. Opin. Obstet. Gynecol. 2015, 27, 487–495. [Google Scholar] [CrossRef]
- Heffron, R.; Donnell, D.; Rees, H.; Celum, C.; Mugo, N.; Were, E.; de Bruyn, G.; Nakku-Joloba, E.; Ngure, K.; Kiarie, J.; et al. Partners in Prevention HSV/HIV Transmission Study Team. Use of hormonal contraceptives and risk of HIV-1 transmission: A prospective cohort study. Lancet Infect. Dis. 2012, 12, 19–26. [Google Scholar] [CrossRef]
- Baeten, J.M.; Lavreys, L.; Overbaugh, J. The influence of hormonal contraceptive use on HIV-1 transmission and disease progression. Clin. Infect. Dis. 2007, 45, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.H.; Anahtar, M.N.; Cohen, K.E.; Moodley, A.; Padavattan, N.; Ismail, N.; Bowman, B.A.; Olson, G.S.; Mabhula, A.; Leslie, A.; et al. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in south African women: A prospective cohort study. Lancet Infect. Dis. 2016, 16, 441–448. [Google Scholar] [CrossRef]
- Hel, Z.; Stringer, E.; Mestecky, J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr. Rev. 2010, 31, 79–97. [Google Scholar] [CrossRef]
- Polis, C.B.; Curtis, K.M.; Hannaford, P.C.; Phillips, S.J.; Chipato, T.; Kiarie, J.N.; Westreich, D.J.; Steyn, P.S. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. Aids 2016, 30, 2665–2683. [Google Scholar] [CrossRef]
- Noguchi, L.M.; Richardson, B.A.; Baeten, J.M.; Hillier, S.L.; Balkus, J.E.; Chirenje, Z.M.; Bunge, K.; Ramjee, G.; Nair, G.; Palanee-Phillips, T.; et al. Risk of HIV-1 acquisition among women who use different types of progestin contraception in South Africa: A prospective cohort study. Lancet 2015, 15, S2352–S3018. [Google Scholar] [CrossRef]
- Morrison, C.S.; Richardson, B.A.; Mmiro, F.; Chipato, T.; Celentano, D.D.; Luoto, J.; Mugerwa, R.; Padian, N.; Rugpao, S.; Brown, J.M.; et al. Hormonal Contraception and the Risk of HIV Acquisition (HC-HIV) Study Group. Hormonal contraception and the risk of HIV acquisition. Aids 2007, 21, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.M.; Hannaford, P.C.; Rodriguez, M.I.; Chipato, T.; Steyn, P.S.; Kiarie, J.N. Hormonal contraception and HIV acquisition among women: An updated systematic review. BMJ Sex. Reprod. Health 2020, 46, 8–16. [Google Scholar] [CrossRef]
- Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. Lancet 2019, 394, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Maritz, M.F.; Ray, R.M.; Bick, A.J.; Tomasicchio, M.; Woodland, J.G.; Govender, Y.; Avenant, C.; Hapgood, J.P. Medroxyprogesterone acetate, unlike norethisterone, increases HIV-1 replication in human peripheral blood mononuclear cells and an indicator cell line, via mechanisms involving the glucocorticoid receptor, increased CD4/CD8 ratios and CCR5 levels. PLoS ONE 2018, 13, e0196043. [Google Scholar] [CrossRef] [PubMed]
- Sathyamala, C. Depot contraception and HIV: An exercise in obfuscation. BMJ 2019, 367, l5768. [Google Scholar] [CrossRef]
- Hapgood, J.P.; Kaushic, C.; Hel, Z. Hormonal Contraception and HIV-1 Acquisition: Biological Mechanisms. Endocr. Rev. 2018, 39, 36–78. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; Wang, W.; Huang, L.; Tu, J.; Baiamonte, L.; Stark, M.; Mills, M.; Hope, T.J.; Drobnis, E.Z.; et al. Effects of three long-acting reversible contraceptive methods on HIV target cells in the human uterine cervix and peripheral blood. Reprod. Biol. Endocrinol. 2019, 17, 26. [Google Scholar] [CrossRef]
- Matubu, A.; Hillier, S.L.; Meyn, L.A.; Stoner, K.A.; Mhlanga, F.; Mbizvo, M.; Maramba, A.; Chirenje, Z.M.; Achilles, S.L. Depot medroxyprogesterone acetate and norethisterone enanthate differentially impact T-cell responses and expression of immunosuppressive markers. Am. J. Reprod. Immunol. 2020, 83, e13210. [Google Scholar] [CrossRef]
- Hapgood, J.P. Is the Injectable Contraceptive Depo-Medroxyprogesterone Acetate (DMPA-IM) Associated with an Increased Risk for HIV Acquisition? The Jury Is Still Out. AIDS Res. Hum. Retrov. 2020, 36, 357–366. [Google Scholar] [CrossRef]
- Jia, X.; Shao, Q.; Chaudhry, A.R.; Kinlock, B.L.; Izban, M.G.; Zhang, H.Y.; Villalta, F.; Hildreth, J.E.K.; Liu, B. Medroxyprogesterone Acetate (MPA) Enhances HIV-1 Accumulation and Release in Primary Cervical Epithelial Cells by Inhibiting Lysosomal Activity. Pathogens 2021, 10, 1192. [Google Scholar] [CrossRef]
- Singata-Madliki, M.; Lawrie, T.A.; Balakrishna, Y.; d’Hellencourt, F.C.; Hofmeyr, G.J. Behavioral effects of different contraceptive methods and HIV acquisition: An ancillary study of the ECHO randomized trial. Reprod. Health 2021, 18, 192. [Google Scholar] [CrossRef]
- Wessels, J.M.; Nguyen, P.V.; Vitali, D.; Mueller, K.; Vahedi, F.; Felker, A.M.; Dupont, H.A.; Bagri, P.; Verschoor, C.P.; Deshiere, A.; et al. Depot medroxyprogesterone acetate (DMPA) enhances susceptibility and increases the window of vulnerability to HIV-1 in humanized mice. Sci. Rep. 2021, 11, 3894. [Google Scholar] [CrossRef]
- Baeten, J.M.; Lavreys, L.; Sagar, M.; Kreiss, J.K.; Richardson, B.A.; Chohan, B.; Panteleeff, D.; Mandaliya, K.; Ndinya-Achola, J.O.; Overbaugh, J.; et al. Effect of contraceptive methods on natural history of HIV: Studies from the Mombasa cohort. J. Acquir. Immune Defic. Syndr. 2005, 38 (Suppl. 1), S18–S21. [Google Scholar] [CrossRef]
- Stringer, E.; Antonsen, E. Hormonal contraception and HIV disease progression. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Lavreys, L.; Baeten, J.M.; Chohan, V.; McClelland, R.S.; Hassan, W.M.; Richardson, B.A.; Mandaliya, K.; Ndinya-Achola, J.O.; Overbaugh, J. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin. Infect. Dis. 2006, 42, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Lavreys, L.; Baeten, J.M.; Kreiss, J.K.; Richardson, B.A.; Chohan, B.H.; Hassan, W.; Panteleeff, D.D.; Mandaliya, K.; Ndinya-Achola, J.O.; Overbaugh, J. Injectable contraceptive use and genital ulcer disease during the early phase of HIV-1 infection increase plasma virus load in women. J. Infect. Dis. 2004, 189, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Patel, M.V.; Wira, C.R. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J. Reprod. Immunol. 2013, 97, 74–84. [Google Scholar] [CrossRef]
- Devadas, K.; Biswas, S.; Ragupathy, V.; Lee, S.; Dayton, A.; Hewlett, I. Modulation of HIV replication in monocyte derived macrophages (MDM) by steroid hormones. PLoS ONE 2018, 13, e0191916. [Google Scholar] [CrossRef]
- Ragupathy, V.; Xue, W.; Tan, J.; Devadas, K.; Gao, Y.; Hewlett, I. Progesterone augments cell susceptibility to HIV-1 and HIV-1/HSV-2 co-infections. J. Mol. Endocrinol. 2016, 57, 185–199. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.; Biswas, N.; Patel, M.V.; Barr, F.D.; Crist, S.G.; Ochsenbauer, C.; Fahey, J.V.; Wira, C.R. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS ONE 2013, 8, e62069. [Google Scholar] [CrossRef]
- Tongo, M.; Harkins, G.W.; Dorfman, J.R.; Billings, E.; Tovanabutra, S.; de Oliveira, T.; Martin, D.P. Unravelling the complicated evolutionary and dissemination history of HIV-1M subtype A lineages. Virus Evol. 2018, 4, vey003. [Google Scholar] [CrossRef]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Fleminger, I.; Kirtley, S.; Williams, B.; Gouws-Williams, E.; Ghys, P.D. Global and regional molecular epidemiology of HIV-1, 1990–2015: A systematic review, global survey, and trend analysis. Lancet Infect. Dis. 2019, 9, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Mendes Da Silva, R.K.; Monteiro de Pina Araujo, I.I.; Venegas Maciera, K.; Gonçalves Morgado, M.; Lindenmeyer Guimarães, M. Genetic Characterization of a New HIV-1 Sub-Subtype A in Cabo Verde, Denominated A8. Viruses 2021, 13, 1093. [Google Scholar] [CrossRef]
- Serwin, K.; Urbańska, A.; Scheibe, K.; Witak-Jędra, M.; Jankowska, M.; Hlebowicz, M.; Bociąga-Jasik, M.; Kalinowska-Nowak, A.; Biała, M.; Ciepłucha, H.; et al. Molecular epidemiology and HIV-1 variant evolution in Poland between 2015 and 2019. Sci. Rep. 2021, 11, 16609. [Google Scholar] [CrossRef]
- Maksimenko, L.V.; Totmenin, A.V.; Gashnikova, M.P.; Astakhova, E.M.; Skudarnov, S.E.; Ostapova, T.S.; Yaschenko, S.V.; Meshkov, I.O.; Bocharov, E.F.; Maksyutov, R.A.; et al. Genetic Diversity of HIV-1 in Krasnoyarsk Krai: Area with High Levels of HIV-1 Recombination in Russia. BioMed Res. Int. 2020, 2020, 9057541. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.; Lebedeva, N.; Moskaleychik, F.; Pronin, A.; Kazennova, E.; Bobkova, M. Human Immunodeficiency Virus-1 Diversity in the Moscow Region, Russia: Phylodynamics of the Most Common Subtypes. Front. Microbiol. 2019, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Sivay, M.V.; Totmenin, A.V.; Zyryanova, D.P.; Osipova, I.P.; Nalimova, T.M.; Gashnikova, M.P.; Ivlev, V.V.; Meshkov, I.O.; Chokmorova, U.Z.; Narmatova, E. Characterization of HIV-1 Epidemic in Kyrgyzstan. Front. Microbiol. 2021, 12, 753675. [Google Scholar] [CrossRef]
- Aibekova, L.; Foley, B.; Hortelano, G.; Raees, M.; Abdraimov, S.; Toichuev, R.; Ali, S. Molecular epidemiology of HIV-1 subtype A in former Soviet Union countries. PLoS ONE 2018, 13, e0191891. [Google Scholar] [CrossRef]
- Bobkova, M. Current status of HIV-1 diversity and drug resistance monitoring in the former USSR. AIDS Rev. 2013, 15, 204–212. [Google Scholar] [PubMed]
- Kostaki, E.-G.; Flampouris, A.; Karamitros, T.; Chueca, N.; Alvarez, M.; Casas, P.; Alejos, B.; Hatzakis, A.; Garcia, F.; Paraskevis, D. Spatiotemporal Characteristics of the Largest HIV-1 CRF02_AG Outbreak in Spain: Evidence for Onward Transmissions. Front. Microbiol. 2019, 10, 370. [Google Scholar] [CrossRef]
- Maggiorella, M.T.; Sanarico, N.; Brindicci, G.; Monno, L.; Santoro, C.R.; Coppola, N.; Cuomo, N.; Azzurri, A.; Francesco, C.; Filippo, L.; et al. High HIV-1 diversity in immigrants resident in Italy (2008–2017). Sci. Rep. 2020, 10, 3226. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.G.; Ibanescu, R.I.; Hardy, I.; Roger, M. Genotypic and Phylogenetic Insights on Prevention of the Spread of HIV-1 and Drug Resistance in “Real-World” Settings. Viruses 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Manet, C.; Montagutelli, X. Host genetic susceptibility to viral infections: The role of type I interferon induction. Genes Immun. 2020, 21, 365–379. [Google Scholar] [CrossRef]
- Elhabyan, A.; Elyaacoub, S.; Sanad, E.; Abukhadra, A.; Elhabyan, A.; Dinu, V. The role of host genetics in susceptibility to severe viral infections in humans and insights into host genetics of severe COVID-19: A systematic review. Virus Res. 2020, 289, 198163. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Rathore, A.; Vidyant, S.; Kakkar, K.; Dhole, T.N. Chemokines and chemokine receptors in susceptibility to HIV-1 infection and progression to AIDS Dis. Markers 2012, 32, 143–151. [Google Scholar] [CrossRef]
- Chatterjee, K. Host genetic factors in susceptibility to HIV-1 infection and progression to AIDS. J. Genet. 2010, 89, 109–116. [Google Scholar] [CrossRef]
- Lama, J.; Planelles, V. Host factors influencing susceptibility to HIV infection and AIDS progression. Retrovirology 2007, 4, 52. [Google Scholar] [CrossRef]
- Fernandez, M.V.; Hoffman, H.K.; Pezeshkian, N.; Tedbury, P.R.; van Engelenburg, S.B.; Freed, E.O. Elucidating the Basis for Permissivity of the MT-4 T-Cell Line to Replication of an HIV-1 Mutant Lacking the gp41 Cytoplasmic Tail. J. Virol. 2020, 94, e01334-20. [Google Scholar] [CrossRef]
- Miyoshi, I.; Kubonishi, I.; Yoshimoto, S.; Akagi, T.; Ohtsuki, Y.; Shiraishi, Y.; Nagata, K.; Hinuma, Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 1981, 294, 770–771. [Google Scholar] [CrossRef]
- Katagiri, D.; Hayashi, H.; Victoriano, A.F.B.; Okamoto, T.; Onozaki, K. Estrogen stimulates transcription of human immunodeficiency virus type 1 (HIV-1). Int. Immunopharmacol. 2006, 6, 170–181. [Google Scholar] [CrossRef]
- Sampah, M.E.S.; Laird, G.M.; Blankson, J.N.; Siliciano, R.F.; Coleman, J.S. Medroxyprogesterone acetate increases HIV-1 infection of unstimulated peripheral blood mononuclear cells in vitro. Aids 2015, 29, 1137–1146. [Google Scholar] [CrossRef]
- Ragupathy, V.; Devadas, K.; Tang, S.; Wood, O.; Lee, S.; Dastyer, A.; Wang, X.; Dayton, A.; Hewlett, I. Effect of sex steroid hormones on replication and transmission of major HIV subtypes. J. Steroid Biochem. Mol. Biol. 2013, 138, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Perno, C.F. HIV-1 Genetic Variability and Clinical Implications. ISRN Microbiol. 2013, 2013, 481314. [Google Scholar] [CrossRef]
- Taylor, B.S.; Sobieszczyk, M.E.; McCutchan, F.E.; Hammer, S.M. The challenge of HIV-1 subtype diversity. N. Engl. J. Med. 2008, 358, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Bbosa, N.; Kaleebu, P.; Ssemwanga, D. HIV subtype diversity worldwide. Curr. Opin. HIV Aids 2019, 14, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Ciccozzi, M.; Parolin, C.; Borsetti, A. Molecular Epidemiology of HIV-1 in African Countries: A Comprehensive Overview. Pathogens 2020, 9, 1072. [Google Scholar] [CrossRef]
- Foley, B.T.; Leitner, T.; Paraskevis, D.; Peeters, M. Primate immunodeficiency virus classification and nomenclature: Review. Infect. Genet. Evol. 2016, 46, 150–158. [Google Scholar] [CrossRef]
- Leal, E.; Villanova, F.E. Diversity of HIV-1 subtype B: Implications to the origin of BF recombinants. PLoS ONE 2010, 5, e11833. [Google Scholar] [CrossRef]
- Xie, H.; Nie, J.; Chen, Q.; Huang, W.; Wang, Y. Comparison of the genotypic and phenotypic properties of HIV-1 standard subtype B and subtype B/B’ env molecular clones derived from infections in China. Emerg. Microbes Infect. 2018, 7, 90. [Google Scholar] [CrossRef]
- Cabrera-Muñoz, E.; Fuentes-Romero, L.L.; Zamora-Chávez, J.; Camacho-Arroyo, I.; Soto-Ramírez, L.E. Effects of progesterone on the content of CCR5 and CXCR4 coreceptors in PBMCs of seropositive and exposed but uninfected Mexican women to HIV-1. J. Steroid Biochem. Mol. Bio. 2012, 132, 66–72. [Google Scholar] [CrossRef]
- Sheffield, J.S.; Wendel, G.D., Jr.; McIntire, D.D.; Norgard, M.V. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod. Sci. 2009, 16, 20–31. [Google Scholar] [CrossRef]
- Dominguez, F.; Galan, A.; Martin, J.J.; Remohi, J.; Pellicer, A.; Simon, C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol. Hum. Reprod. 2003, 9, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sciaranghella, G.; Wang, C.; Hu, H.; Anastos, K.; Merhi, Z.; Nowicki, M.; Stanczyk, F.Z.; Greenblatt, R.M.; Cohen, M.; Golub, E.T.; et al. CCR5 expression levels in HIV-uninfected women receiving hormonal contraception. J. Infect. Dis. 2015, 212, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Kapembwa, M.S.; Gotch, F.; Patterson, S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4+ T cells in the cervical epithelium of healthy women. J. Reprod. Immunol. 2002, 54, 117–131. [Google Scholar] [CrossRef]
- Feng, Y.; Broder, C.C.; Kennedy, P.E.; Berger, E.A. HIV-1 Entry Cofactor: Functional cDNA Cloning of a Seven-Transmembrane, G Protein-Coupled Receptor. Science 1996, 272, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Di Marzio, P.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381, 661–666. [Google Scholar] [CrossRef]
- Dragic, T.; Litwin, V.; Allaway, G.P.; Martin, S.R.; Huang, Y.; Nagashima, K.A.; Cayanan, C.; Maddon, P.J.; Koup, R.A.; Moore, J.P.; et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996, 381, 667–673. [Google Scholar] [CrossRef]
- Calado, M.; Matoso, P.; Santos-Costa, Q.; Espirito-Santo, M.; Machado, J.; Rosado, L.; Antunes, F.; Mansinho, K.; Lopes, M.N.; Maltez, F. Coreceptor usage by HIV-1 and HIV-2 primary isolates: The relevance of CCR8 chemokine receptor as an alternative coreceptor. Virology 2010, 408, 174–182. [Google Scholar] [CrossRef]
- Wang, Z.; Shang, H.; Jiang, Y. Chemokines and Chemokine Receptors: Accomplices for Human Immunodeficiency Virus Infection and Latency. Front. Immunol. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Hatse, S.; Huskens, D.; Princen, K.; Vermeire, K.; Bridger, G.J.; De Clercq, E.; Rosenkilde, M.M.; Schwartz, T.W.; Schols, D. Modest human immunodeficiency virus coreceptor function of CXCR3 is strongly enhanced by mimicking the CXCR4 ligand binding pocket in the CXCR3 receptor. J. Virol. 2007, 81, 3632–3639. [Google Scholar] [CrossRef]
- Limou, S.; Coulonges, C.; Herbeck, J.T.; van Manen, D.; An, P.; Le Clerc, S.; Delaneau, O.; Diop, G.; Taing, L.; Montes, M.; et al. Multiple-cohort genetic association study reveals CXCR6 as a new chemokine receptor involved in long-term nonprogression to AIDS. J. Infect. Dis. 2010, 202, 908–915. [Google Scholar] [CrossRef]
- Sharron, M.; Pöhlmann, S.; Price, K.; Lolis, E.; Tsang, M.; Kirchhoff, F.; Doms, R.W.; Lee, B. Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood 2000, 96, 41–49. [Google Scholar] [CrossRef]
- D’huys, T.; Claes, S.; Van Loy, T.; Schols, D. CXCR7/ACKR3-targeting ligands interfere with X7 HIV-1 and HIV-2 entry and replication in human host cells. Heliyon 2018, 4, e00557. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Soda, Y.; Kanbe, K.; Liu, H.Y.; Mukai, R.; Kitamura, T.; Hoshino, H. A putative G protein-coupled receptor, RDC1, is a novel coreceptor for human and simian immunodeficiency viruses. J. Virol. 2000, 74, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Doijen, J.; Van Loy, T.; De Haes, W.; Landuyt, B.; Luyten, W.; Schoofs, L.; Schols, D. Signaling properties of the human chemokine receptors CXCR4 and CXCR7 by cellular electric impedance measurements. PLoS ONE 2017, 12, e0185354. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Arya, R.K.; Trivedi, A.K.; Sanyal, S.; Baral, R.; Dormond, O.; Briscoe, D.M.; Datta, D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013, 24, 41–49. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Browne, E.P. The Role of Toll-Like Receptors in Retroviral Infection. Microorganisms 2020, 8, 1787. [Google Scholar] [CrossRef]
- Nguyen, H.; Gazy, N.; Venketaraman, V. A Role of Intracellular Toll-Like Receptors (3, 7, and 9) in Response to Mycobacterium tuberculosis and Co-Infection with HIV. Int. J. Mol. Sci. 2020, 21, 6148. [Google Scholar] [CrossRef]
- Hernández, J.C.; Stevenson, M.; Latz, E.; Urcuqui-Inchima, S. HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro. Aids Res. Hum. Retrovir. 2012, 28, 1313–1328. [Google Scholar] [CrossRef]
- Rozman, M.; Zidovec-Lepej, S.; Jambrosic, K.; Babić, M.; Drmić Hofman, I. Role of TLRs in HIV-1 Infection and Potential of TLR Agonists in HIV-1 Vaccine Development and Treatment Strategies. Pathogens 2023, 12, 92. [Google Scholar] [CrossRef]
- Bolduc, J.-F.; Ouellet, M.; Hany, L.; Tremblay, M.J. Toll-like receptor 2 ligation enhances HIV-1 replication in activated CCR6+CD4+ T cells by increasing virus entry and establishing a more permissive environment to infection. J. Virol. 2017, 91, e01402-16. [Google Scholar] [CrossRef]
- Vallejo, A.; Molina-Pinelo, S.; De Felipe, B.; Abad-Fernández, M.; González-Escribano, M.F.; Leal, M.; Soriano-Sarabia, N. Toll-like receptor 9 1635A/G polymorphism is associated with HIV-1 rebound after four weeks of interruption of antiretroviral therapy. J. Acquir. Immune. Defic. Syndr. 2020, 85, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Laplana, M.; Bravo, M.J.; Fernández-Fuertes, M.; Ruiz-Garcia, C.; Alarcón-Martin, E.; Colmenero, J.D.; Caruz, A.; Fibla, J.; Real, L.M.; Royo, J.L. Toll like receptor 2 promoter -196 to -174 deletion affects CD4 levels along HIV infection progression. J. Infect. Dis. 2020, 222, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Punke, E.B.; Mehmetoglu-Gurbuz, T.; Peralta, D.P.; Garg, H. TLR9 polymorphism correlates with immune activation, CD4 decline and plasma IP10 levels in HIV patients. BMC Infect. Dis. 2019, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Beima-Sofie, K.; Bigham, A.; Lingappa, J.; Wamalwa, D.; Mackelprang, R.; Bamshad, M.; Maleche-Obimbo, E.; Richardson, B.; John-Stewart, G. Toll-like receptor variants are associated with infant HIV-1 acquisition and peak plasma HIV-1 RNA level. Aids 2013, 27, 2431–2439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nordone, S.K.; Ignacio, G.A.; Su, L.; Sempowski, G.D.; Golenbock, D.T.; Li, L.; Dean, G.A. Failure of TLR4-driven NF-kappa B activation to stimulate virus replication in models of HIV type 1 activation. Aids Res. Hum. Retrovir. 2007, 23, 1387–1395. [Google Scholar] [CrossRef]
- Rollenhagen, C.; Asin, S.N. Enhanced HIV-1 replication in ex vivo ectocervical tissues from post-menopausal women correlates with increased inflammatory responses. Mucosal. Immunol. 2011, 4, 671–681. [Google Scholar] [CrossRef]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef]
- Saba, E.; Origoni, M.; Taccagni, G.; Ferrari, D.; Doglioni, C.; Nava, A.; Lisco, A.; Grivel, J.C.; Margolis, L.; Poli, G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal. Immunol. 2013, 6, 1081–1090. [Google Scholar] [CrossRef]
- Asin, S.N.; Eszterhas, S.K.; Rollenhagen, C.; Heimberg, A.M.; Howell, A.L. HIV Type 1 Infection in Women: Increased Transcription of HIV Type 1 in Ectocervical Tissue Explants. J. Infect. Dis. 2009, 200, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Curlin, M.E.; Leelawiwat, W.; Dunne, E.F.; Chonwattana, W.; Mock, P.A.; Mueanpai, F.; Thep-Amnuay, S.; Whitehead, S.J.; McNicholl, J.M. Cyclic changes in HIV shedding from the female genital tract during the menstrual cycle. J. Infect. Dis. 2013, 207, 1616–1620. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosik, M.; Berezhnaya, E.; Bystritskaya, E.; Kiseleva, I.; Lobach, O.; Kireev, D.; Svitich, O. Female Sex Hormones Upregulate the Replication Activity of HIV-1 Sub-Subtype A6 and CRF02_AG but Not HIV-1 Subtype B. Pathogens 2023, 12, 880. https://doi.org/10.3390/pathogens12070880
Nosik M, Berezhnaya E, Bystritskaya E, Kiseleva I, Lobach O, Kireev D, Svitich O. Female Sex Hormones Upregulate the Replication Activity of HIV-1 Sub-Subtype A6 and CRF02_AG but Not HIV-1 Subtype B. Pathogens. 2023; 12(7):880. https://doi.org/10.3390/pathogens12070880
Chicago/Turabian StyleNosik, Marina, Elena Berezhnaya, Elizaveta Bystritskaya, Irina Kiseleva, Olga Lobach, Dmitry Kireev, and Oxana Svitich. 2023. "Female Sex Hormones Upregulate the Replication Activity of HIV-1 Sub-Subtype A6 and CRF02_AG but Not HIV-1 Subtype B" Pathogens 12, no. 7: 880. https://doi.org/10.3390/pathogens12070880
APA StyleNosik, M., Berezhnaya, E., Bystritskaya, E., Kiseleva, I., Lobach, O., Kireev, D., & Svitich, O. (2023). Female Sex Hormones Upregulate the Replication Activity of HIV-1 Sub-Subtype A6 and CRF02_AG but Not HIV-1 Subtype B. Pathogens, 12(7), 880. https://doi.org/10.3390/pathogens12070880





