Latent Tuberculosis Patients Have an Increased Frequency of IFN-γ-Producing CD5+ B Cells, Which Respond Efficiently to Mycobacterial Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
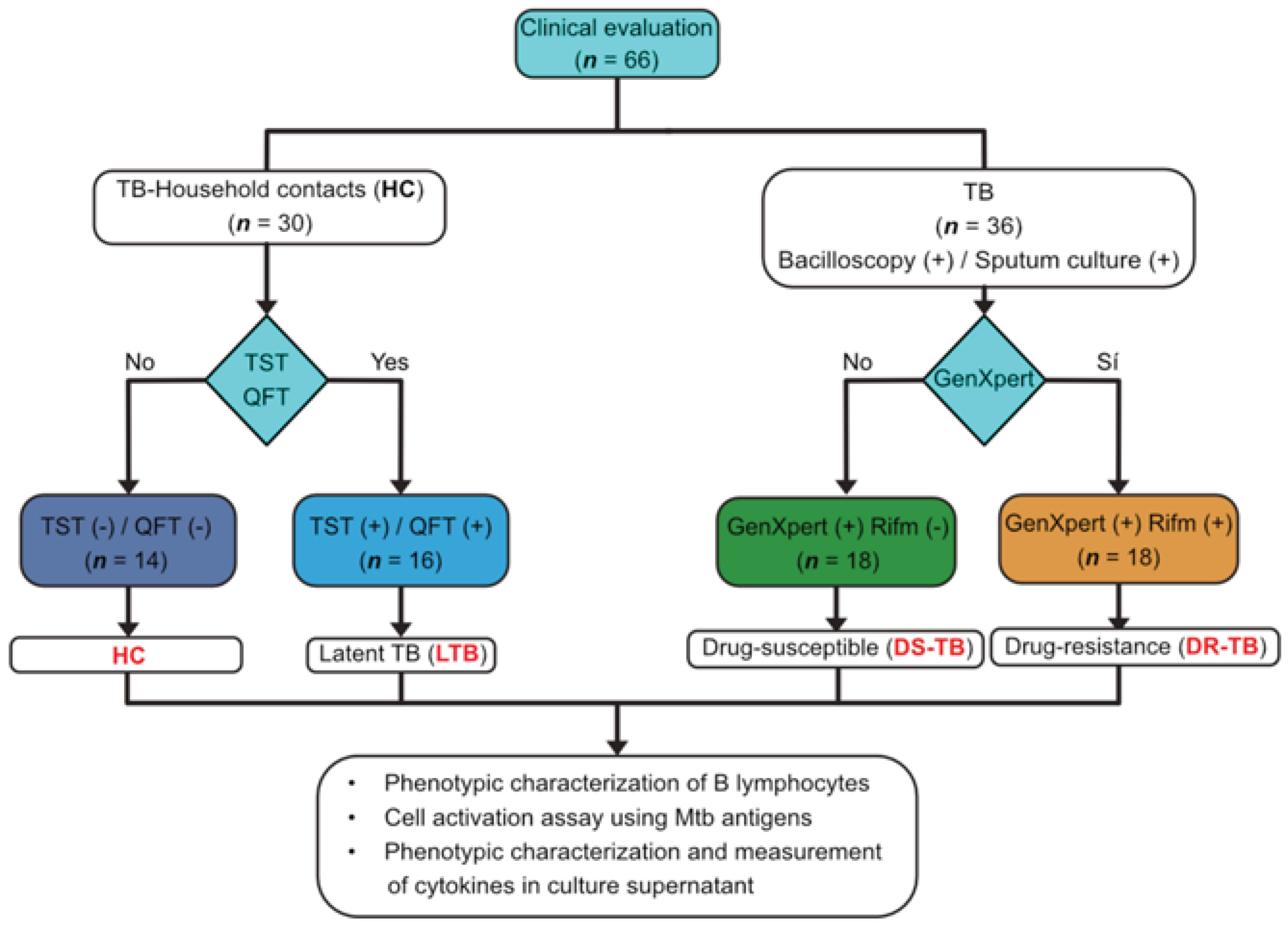
2.2. Study Populations
2.3. Cells
2.4. Multiparametric Flow Cytometry Analysis
2.5. PBMC’s Stimulation
2.6. Quantification of Intracellular IFN-γ and IL-10, upon In Vitro Stimulation with Mtb H37Rv Antigens
2.7. LEGENDplexTM Assay
2.8. Spearman Correlation Matrix
2.9. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. The Frequency of CD5+ B Cells Is Higher in LTB Than in ATB
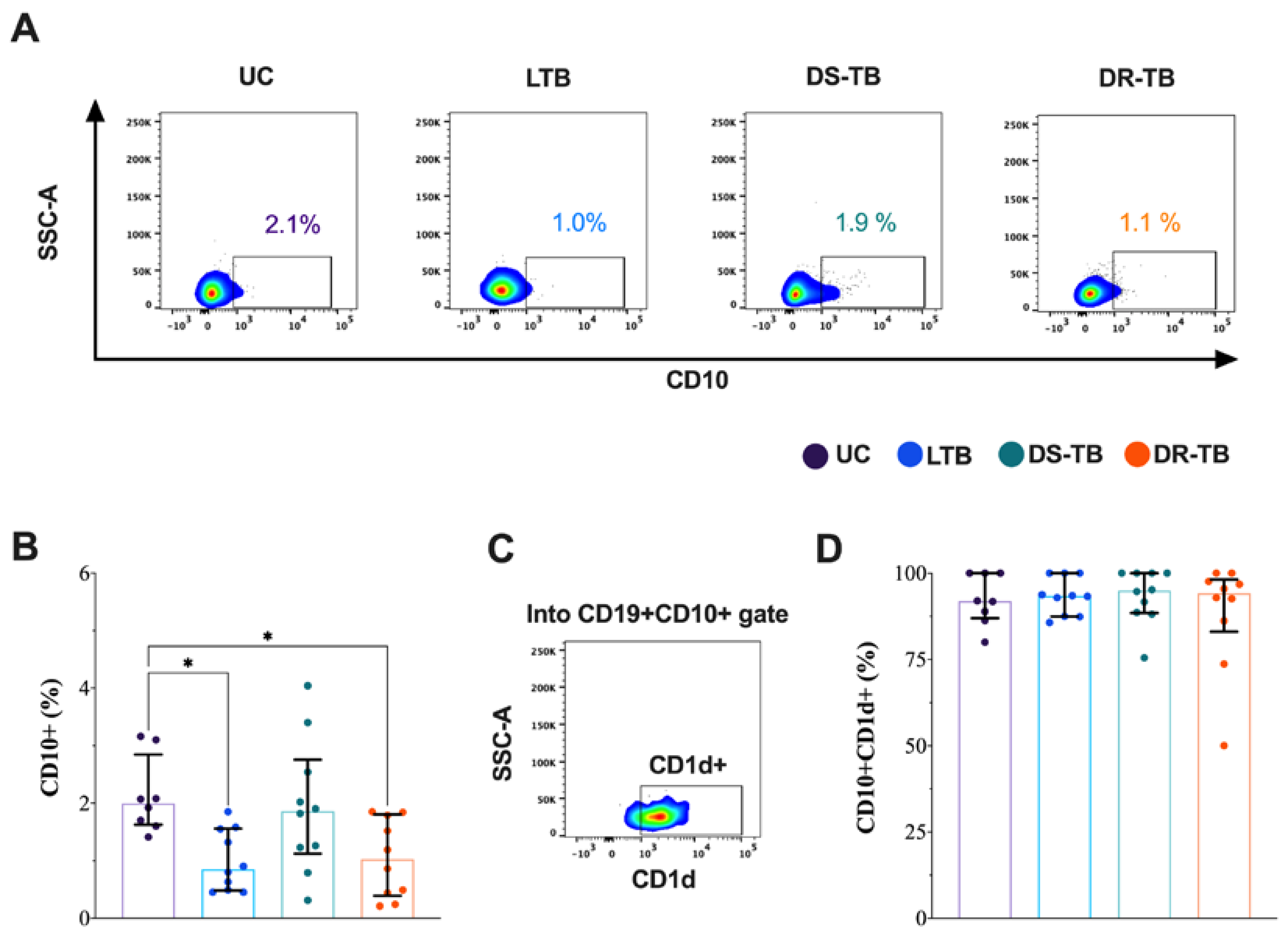
3.3. The CD10+ B Cells Frequency Is Decreased in LTB and DR-TB
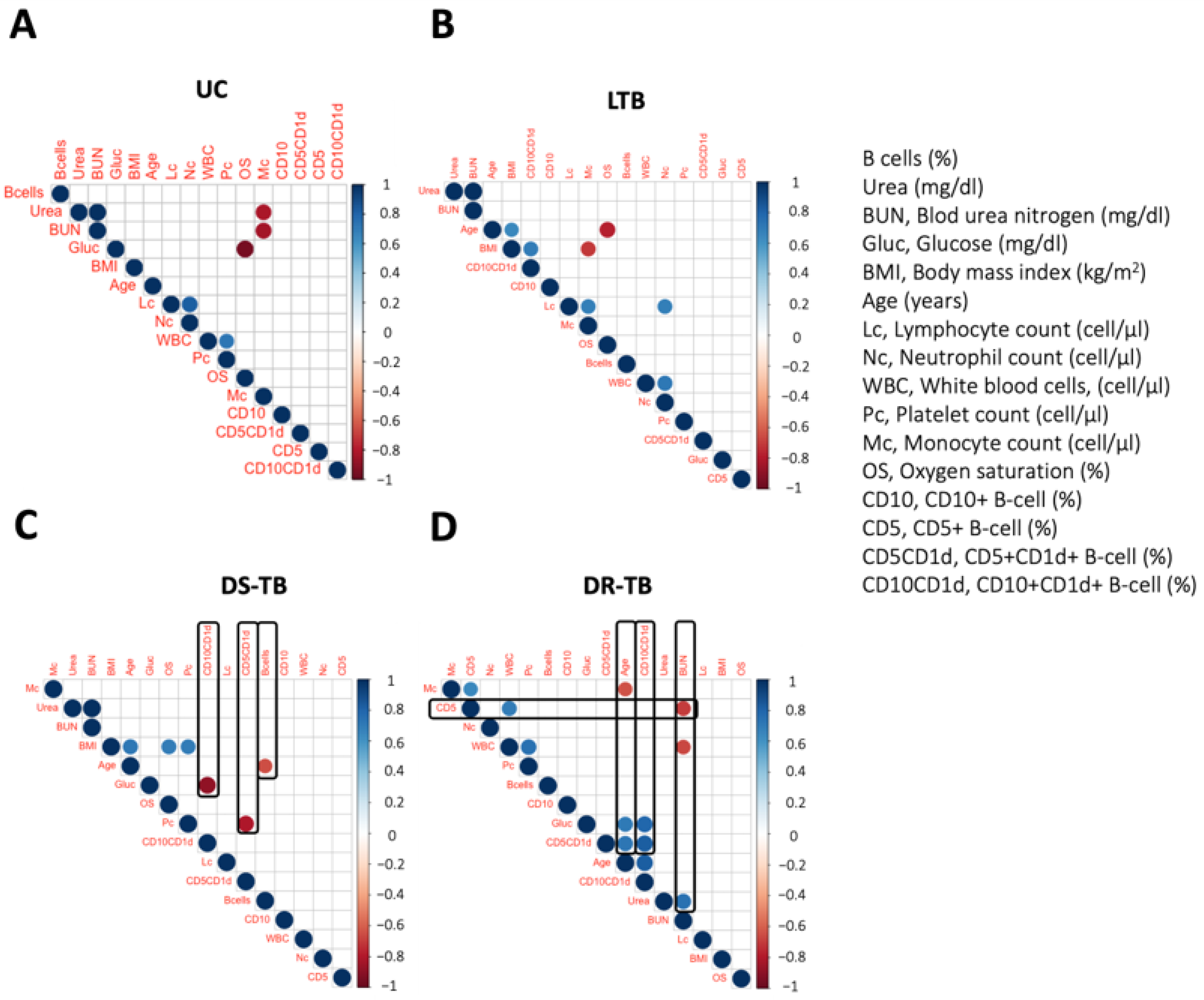
3.4. DR-TB Exhibits a Complex Network of Correlations between B Immunological Profile and Clinical Outcome
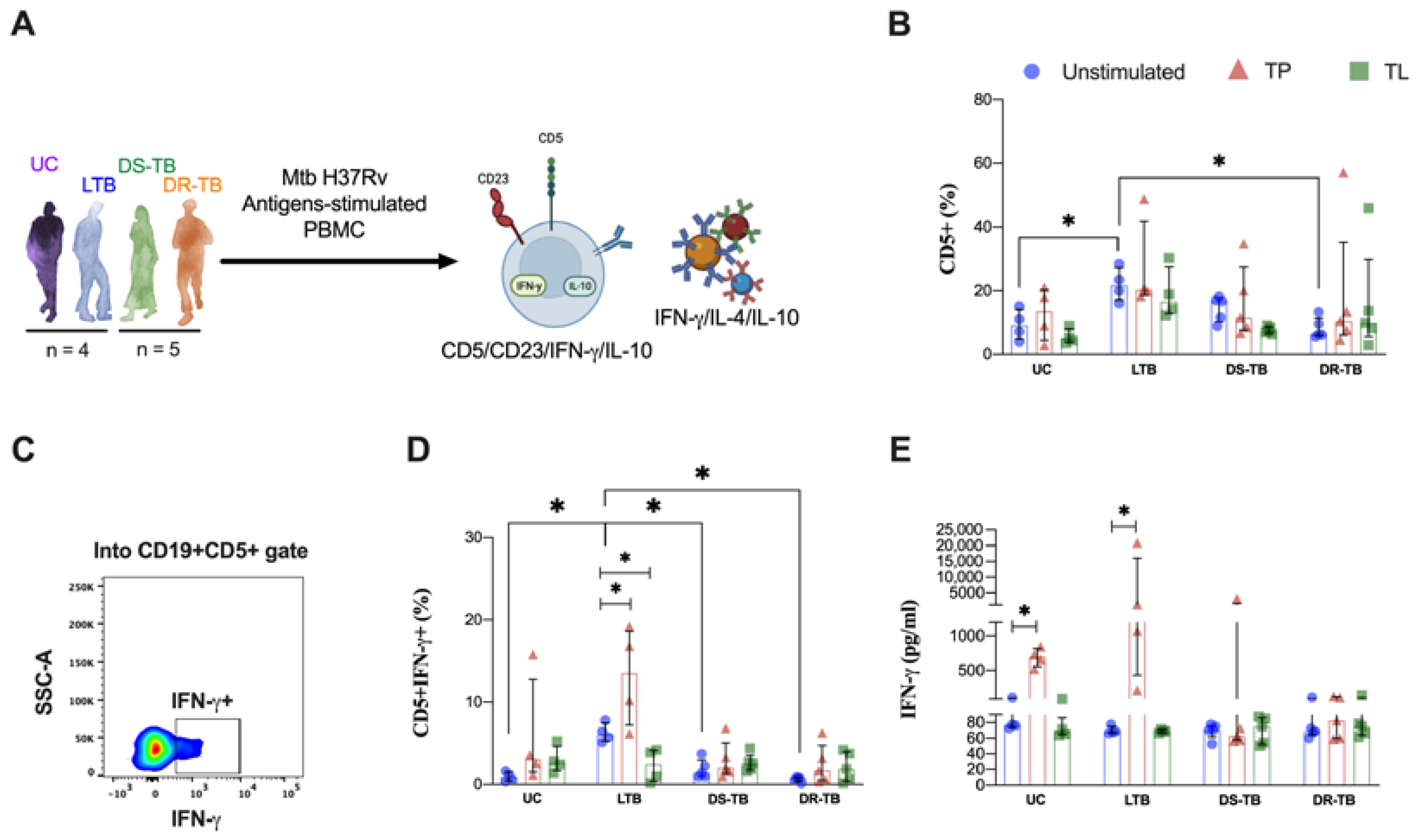
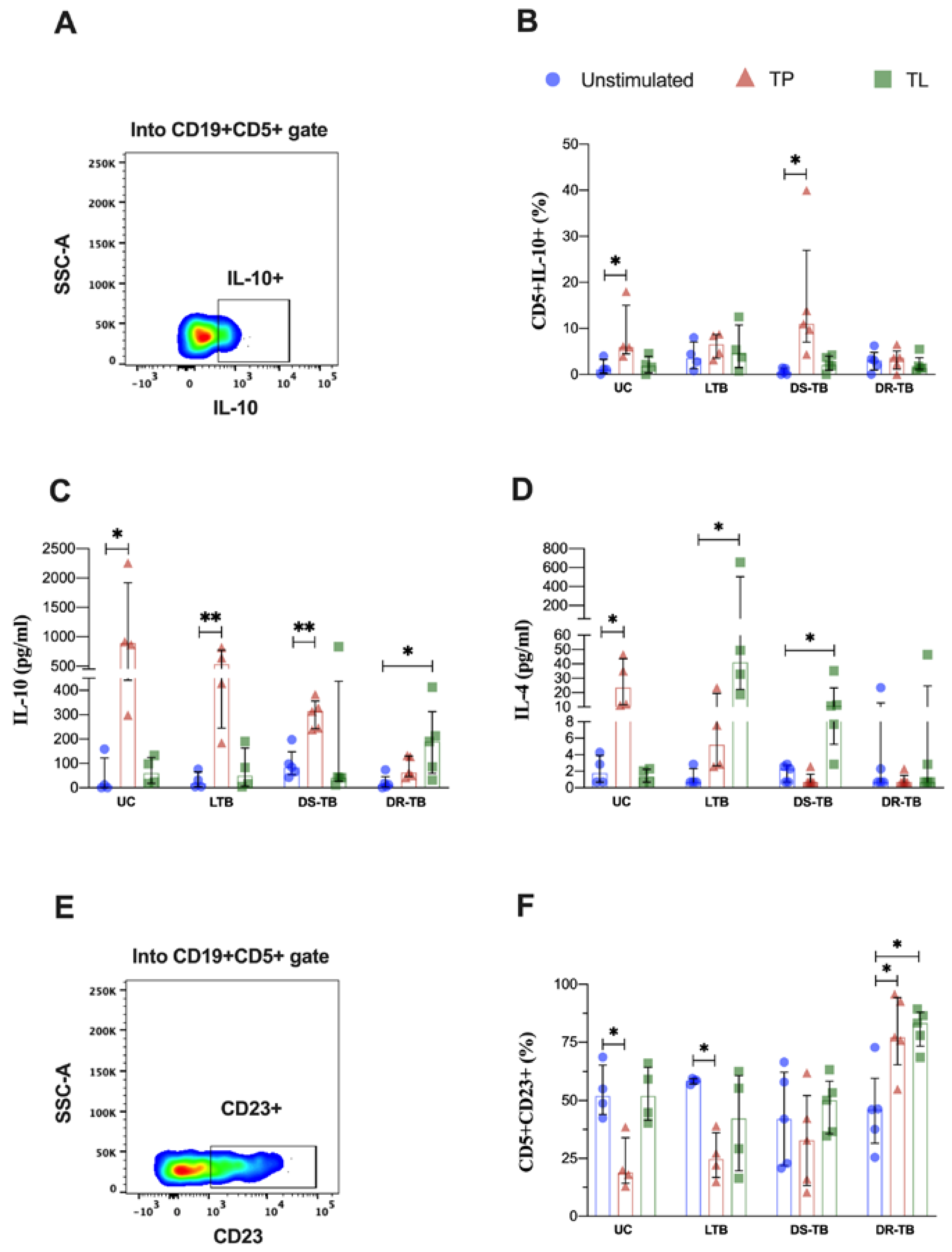
3.5. LTB Increases the Frequency of IFN-γ-Producing CD5+ B Cells in Response to Proteins from Mtb
3.6. LTB Produces High Levels of IL-10 and IL-4 in Response to Proteins and Lipids from Mtb, but It Is Independent of the B Cells Subpopulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forum of International Respiratory Societies; European Respiratory Society. The Global Impact of Respiratory Disease; Forum of International Respiratory Societies: Lausanne, Switzerland, 2017; ISBN 978-1-84984-087-3. [Google Scholar]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-Estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef]
- Lavalett, L.; Ortega, H.; Barrera, L.F. Infection of Monocytes from Tuberculosis Patients with Two Virulent Clinical Isolates of Mycobacterium tuberculosis Induces Alterations in Myeloid Effector Functions. Front. Cell. Infect. Microbiol. 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Rijnink, W.F.; Ottenhoff, T.H.M.; Joosten, S.A. B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front. Immunol. 2021, 12, 640168. [Google Scholar] [CrossRef]
- Jacobs, A.J.; Mongkolsapaya, J.; Screaton, G.R.; McShane, H.; Wilkinson, R.J. Antibodies and Tuberculosis. Tuberculosis 2016, 101, 102–113. [Google Scholar] [CrossRef]
- Rao, M.; Valentini, D.; Poiret, T.; Dodoo, E.; Parida, S.; Zumla, A.; Brighenti, S.; Maeurer, M. B in TB: B Cells as Mediators of Clinically Relevant Immune Responses in Tuberculosis. Clin. Infect. Dis. 2015, 61, S225–S234. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, Y.A.; Park, J.H.; Cha, H.H.; Jeon, N.Y.; Lee, S.W.; Lee, S.O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; et al. An IFN-γ and TNF-α Dual Release Fluorospot Assay for Diagnosing Active Tuberculosis. Clin. Microbiol. Infect. 2020, 26, 928–934. [Google Scholar] [CrossRef]
- Silva, C.S.; Sundling, C.; Folkesson, E.; Fröberg, G.; Nobrega, C.; Canto-Gomes, J.; Chambers, B.J.; Lakshmikanth, T.; Brodin, P.; Bruchfeld, J.; et al. High Dimensional Immune Profiling Reveals Different Response Patterns in Active and Latent Tuberculosis Following Stimulation with Mycobacterial Glycolipids. Front. Immunol. 2021, 12, 727300. [Google Scholar] [CrossRef]
- Bénard, A.; Sakwa, I.; Schierloh, P.; Colom, A.; Mercier, I.; Tailleux, L.; Jouneau, L.; Boudinot, P.; Al-Saati, T.; Lang, R.; et al. B Cells Producing Type I IFN Modulate Macrophage Polarization in Tuberculosis. Am. J. Respir. Crit. Care Med. 2018, 197, 801–813. [Google Scholar] [CrossRef]
- Wong, J.B.; Hewitt, S.L.; Heltemes-Harris, L.M.; Mandal, M.; Johnson, K.; Rajewsky, K.; Koralov, S.B.; Clark, M.R.; Farrar, M.A.; Skok, J.A. B-1a Cells Acquire Their Unique Characteristics by Bypassing the Pre-BCR Selection Stage. Nat. Commun. 2019, 10, 4768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zheng, X.; Zhang, J.; Zhu, Y.; Zhu, X.; Liu, H.; Zeng, M.; Graner, M.W.; Zhou, B.; Chen, X. CD19+CD1d+CD5+ B Cell Frequencies Are Increased in Patients with Tuberculosis and Suppress Th17 Responses. Cell. Immunol. 2012, 274, 89–97. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.-D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.P.; Ettinger, R.; Shirota, Y.; Yarboro, C.H.; Illei, G.G.; Lipsky, P.E. Identification and Characterization of Circulating Human Transitional B Cells. Blood 2005, 105, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, A.; Moir, S.; Ho, J.; Wang, W.; Howell, M.L.; O’Shea, M.A.; Roby, G.A.; Rehm, C.A.; Mican, J.M.; Chun, T.-W.; et al. Appearance of Immature/Transitional B Cells in HIV-Infected Individuals with Advanced Disease: Correlation with Increased IL-7. Proc. Natl. Acad. Sci. USA 2006, 103, 2262–2267. [Google Scholar] [CrossRef]
- Organizat, W.H. WHO Consolidated Guidelines on Tuberculosis. Module 1: Prevention. Tuberculosis Preventive Treatment. Tuberc. Lung Dis. HIV Infect. 2021, 2, 86–92. [Google Scholar] [CrossRef]
- Monaco, G.; Chen, H.; Poidinger, M.; Chen, J.; de Magalhães, J.P.; Larbi, A. FlowAI: Automatic and Interactive Anomaly Discerning Tools for Flow Cytometry Data. Bioinformatics 2016, 32, 2473–2480. [Google Scholar] [CrossRef]
- Tiwari, M.; Gami, S.P. Pulmonary Hypertension in Pulmonary Tuberculosis—A Prognostic Indicator. Eur. Respir. Soc. J. 2017, 50, PA2432. [Google Scholar]
- Urbán-Solano, A.; Flores-Gonzalez, J.; Cruz-Lagunas, A.; Pérez-Rubio, G.; Buendia-Roldan, I.; Ramón-Luing, L.A.; Chavez-Galan, L. High Levels of PF4, VEGF-A, and Classical Monocytes Correlate with the Platelets Count and Inflammation during Active Tuberculosis. Front. Immunol. 2022, 13, 1016472. [Google Scholar] [CrossRef]
- Stögerer, T.; Stäger, S. Innate Immune Sensing by Cells of the Adaptive Immune System. Front. Immunol. 2020, 11, 1081. [Google Scholar] [CrossRef]
- Ahmad, F.; Rani, A.; Alam, A.; Zarin, S.; Pandey, S.; Singh, H.; Hasnain, S.E.; Ehtesham, N.Z. Macrophage: A Cell with Many Faces and Functions in Tuberculosis. Front. Immunol. 2022, 13, 747799. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Downey, J.; Sanz, J.; Kaufmann, E.; Blankenhaus, B.; Pacis, A.; Pernet, E.; Ahmed, E.; Cardoso, S.; Nijnik, A.; et al. M. tuberculosis Reprograms Hematopoietic Stem Cells to Limit Myelopoiesis and Impair Trained Immunity. Cell 2020, 183, 752–770.e22. [Google Scholar] [CrossRef] [PubMed]
- La Manna, M.P.; Shekarkar-Azgomi, M.; Badami, G.D.; Tamburini, B.; Dieli, C.; Di Carlo, P.; Fasciana, T.; Marcianò, V.; Lo Sasso, B.; Giglio, R.V.; et al. Impact of Mycobacterium tuberculosis Infection on Human B Cell Compartment and Antibody Responses. Cells 2022, 11, 2906. [Google Scholar] [CrossRef]
- Joosten, S.A.; van Meijgaarden, K.E.; del Nonno, F.; Baiocchini, A.; Petrone, L.; Vanini, V.; Smits, H.H.; Palmieri, F.; Goletti, D.; Ottenhoff, T.H.M. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes Following Successful Treatment. PLoS Pathog. 2016, 12, e1005687. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Navarrete, N.A.; Ramon-Luing, L.A.; Muñoz-Torrico, M.; Preciado-García, M.; Medina-Quero, K.; Hernandez-Pando, R.; Chavez-Galan, L. Anti-Tuberculosis Chemotherapy Alters TNFR2 Expression on CD4+ Lymphocytes in Both Drug-Sensitive and -Resistant Tuberculosis: However, Only Drug-Resistant Tuberculosis Maintains a pro-Inflammatory Profile after a Long Time. Mol. Med. 2021, 27, 76. [Google Scholar] [CrossRef]
- Ocaña-Guzmán, R.; Téllez-Navarrete, N.A.; Ramón-Luing, L.A.; Herrera, I.; De Ita, M.; Carrillo-Alduenda, J.-L.; Choreño-Parra, J.A.; Medina-Quero, K.; Zúñiga, J.; Chávez-Galán, L. Leukocytes from Patients with Drug-Sensitive and Multidrug-Resistant Tuberculosis Exhibit Distinctive Profiles of Chemokine Receptor Expression and Migration Capacity. J. Immunol. Res. 2021, 2021, 6654220. [Google Scholar] [CrossRef]
- Cagatay, T.; Kiran, B.; Yurt, S.; Gulbaran, Z.; Kosar, F.; Cagatay, P. Levels of Tumour Necrosis Factor-Alpha and IL-1alpha in Newly Diagnosed and Multidrug Resistant Tuberculosis. Respirology 2005, 10, 290–294. [Google Scholar] [CrossRef]
- Abreu, M.T.; Carvalheiro, H.; Rodrigues-Sousa, T.; Domingos, A.; Segorbe-Luis, A.; Rodrigues-Santos, P.; Souto-Carneiro, M.M. Alterations in the Peripheral Blood B Cell Subpopulations of Multidrug-Resistant Tuberculosis Patients. Clin. Exp. Med. 2014, 14, 423–429. [Google Scholar] [CrossRef]
- Lee, J.; Kornfeld, H. Interferon-γ Regulates the Death of M. tuberculosis-Infected Macrophages. J. Cell Death 2010, 3, JCD.S2822. [Google Scholar] [CrossRef]
- Ahmed, M.; Mackenzie, J.; Tezera, L.; Krause, R.; Truebody, B.; Garay-Baquero, D.; Vallejo, A.; Govender, K.; Adamson, J.; Fisher, H.; et al. Mycobacterium tuberculosis Senses Host Interferon-γ via the Membrane Protein MmpL10. Commun. Biol. 2022, 5, 1317. [Google Scholar] [CrossRef]
- Kotb, A.; Ismail, S.; Kimito, I.; Mohamed, W.; Salman, A.; Mohammed, A.A. Increased CD5+ B-Cells Are Associated with Autoimmune Phenomena in Lepromatous Leprosy Patients. J. Infect. Public Health 2019, 12, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Stabel, J.R.; Bannantine, J.P.; Eda, S.; Robbe-Austerman, S. Induction of B Cell Responses upon Experimental Infection of Neonatal Calves with Mycobacterium Avium Subsp. Paratuberculosis. Clin. Vaccine Immunol. 2011, 18, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Stabel, J.R.; Bannantine, J.P.; Humphrey, S. B Cell Phenotypes and Maturation States in Cows Naturally Infected with Mycobacterium Avium Subsp. Paratuberculosis. PLoS ONE 2022, 17, e0278313. [Google Scholar] [CrossRef] [PubMed]
- Grönwall, C.; Vas, J.; Silverman, G.J. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 282–286. [Google Scholar] [CrossRef]
- Ordoñez, C.; Savage, H.P.; Tarajia, M.; Rivera, R.; Weeks-Galindo, C.; Sambrano, D.; Riley, L.; Fernandez, P.L.; Baumgarth, N.; Goodridge, A. Both B-1a and B-1b Cells Exposed to Mycobacterium tuberculosis Lipids Differentiate into IgM Antibody-Secreting Cells. Immunology 2018, 154, 613–623. [Google Scholar] [CrossRef]
- Nziza, N.; Cizmeci, D.; Davies, L.; Irvine, E.B.; Jung, W.; Fenderson, B.A.; de Kock, M.; Hanekom, W.A.; Franken, K.L.M.C.; Day, C.L.; et al. Defining Discriminatory Antibody Fingerprints in Active and Latent Tuberculosis. Front. Immunol. 2022, 13, 856906. [Google Scholar] [CrossRef] [PubMed]
- Maekura, R.; Kitada, S.; Osada-Oka, M.; Tateishi, Y.; Ozeki, Y.; Fujicawa, T.; Miki, M.; Jyunnko, O.; Mori, M.; Matsumoto, S. Serum Antibody Profiles in Individuals with Latent Mycobacterium tuberculosis Infection. Microbiol. Immunol. 2019, 63, 130–138. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, H.; Zhang, W.; Yazaki, P.J.; Zhang, Z.; Le, K.; Li, W.; Lee, H.; Kwak, L.; Forman, S.; et al. CD5 Binds to Interleukin-6 and Induces a Feed-Forward Loop with the Transcription Factor STAT3 in B Cells to Promote Cancer. Immunity 2016, 44, 913–923. [Google Scholar] [CrossRef]
- Chen, Q.; Mosovsky, K.L.; Ross, A.C. Retinoic Acid and α-Galactosylceramide Regulate the Expression of Costimulatory Receptors and Transcription Factors Responsible for B Cell Activation and Differentiation. Immunobiology 2013, 218, 1477–1487. [Google Scholar] [CrossRef]
- Palanichamy, A.; Barnard, J.; Zheng, B.; Owen, T.; Quach, T.; Wei, C.; Looney, R.J.; Sanz, I.; Anolik, J.H. Novel Human Transitional B Cell Populations Revealed by B Cell Depletion Therapy. J. Immunol. 2009, 182, 5982–5993. [Google Scholar] [CrossRef]
- Mats, B. Translating Transitions—How to Decipher Peripheral Human B Celldevelopment. J. Biomed. Res. 2015, 29, 264. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, Y.V.N.; Brelaz, M.C.A.; de Andrade Lemoine Neves, J.K.; Ferraz, J.C.; Pereira, V.R.A. Role of TNF-Alpha, IFN-Gamma, and IL-10 in the Development of Pulmonary Tuberculosis. Pulm. Med. 2012, 2012, 745483. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Liu, X.; Han, C.; Xu, S.; Xie, B.; Zhang, Q.; Gu, Y.; Hou, J.; Qian, L.; Qian, C.; et al. Identification of IFN-γ-Producing Innate B Cells. Cell Res. 2014, 24, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Bianco, A.; Perna, F.; D’Auria, D.; Grella, E.; Moscariello, E.; Sanduzzi, A. T Lymphocyte Phenotypic Profile in Lung Segments Affected by Cavitary and Non-Cavitary Tuberculosis. Clin. Exp. Immunol. 2003, 132, 283–288. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, I.C.; Wagman, C.; Stanley, K.; Beltran, C.; Ronacher, K.; Walzl, G.; Loxton, A.G. Successful TB Treatment Induces B-Cells Expressing FASL and IL5RA MRNA. Oncotarget 2017, 8, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Heffler, E.; Crimi, C.; Maglio, A.; Vatrella, A.; Pelaia, G.; Canonica, G.W. Interleukins 4 and 13 in Asthma: Key Pathophysiologic Cytokines and Druggable Molecular Targets. Front. Pharmacol. 2022, 13, 851940. [Google Scholar] [CrossRef]

| Variable | UC [A] (n = 14) | LTB [B] (n = 16) | DS-TB [C] (n = 18) | DR-TB [D] (n = 18) | p-Value |
|---|---|---|---|---|---|
| Demographic characteristics 1 | |||||
| Age, years | 28 (22–41) | 50 (45–57) | 36 (25–42) | 45 (34–59) | * [A vs. B] * [B vs. C] |
| Male, n (%) 2 | 10 (67) | 6 (37) | 8 (44) | 12 (67) | ns |
| Female, n (%) 2 | 5 (33) | 10 (63) | 10 (56) | 6 (33) | ns |
| Body mass index, kg/m2 | 27 (23–28) | 30 (25–33) | 21 (19–24) | 22 (18–25) | ** [A vs. C] * [A vs. D] *** [B vs. C] *** [B vs. D] |
| Oxygen saturation, % | 95 (91–95) | 95 (92–98) | 96 (94–96) | 95 (90–97) | ns |
| BCG vaccine, n2 | 13 | 14 | 15 | 15 | ns |
| Clinical characteristics, n (%) 2 | |||||
| Coexisting conditions | |||||
| Type 2 diabetes | 0 (0) | 1 (7) | 3 (17) | 10 (56) | *** [A vs. D] ** [B vs. D] ** [C vs. D] |
| Hypertension Dis. | 0 (0) | 2 (13) | 0 (0) | 2 (11) | ns |
| Blood cell count (×103 cell/μL) 1 | |||||
| White blood cells | 6 (5–7) | 7 (6–8) | 9 (7–12) | 8 (6–10) | ** [A vs. C] * [A vs. D] * [B vs. C] |
| Lymphocyte count | 2 (2–3) | 2 (2–3) | 2 (1–2) | 2 (1–2) | ns |
| Monocyte count | 0.4 (0.1–0.5) | 0.4 (0.4–0.5) | 0.6 (0.5–0.9) | 0.6 (0.5–0.9) | ** [A vs. C] ** [A vs. D] *** [B vs. C] ** [B vs. D] |
| Neutrophil count | 3 (2–4) | 4 (3–5) | 6 (5–8) | 5 (3.0–9) | *** [A vs. C] * [A vs. D] ** [B vs. C] |
| Platelet count | 238 (225–307) | 241 (222–275) | 478 (365–514) | 280 (195–384) | **** [A vs. C] *** [B vs. C] ** [C vs. D] |
| Biochemical blood parameters, mg/dL 1 | |||||
| Glucose | 94 (86–102) | 98 (94–104) | 98 (92–149) | 133 (90–150) | * [A vs. D] |
| Urea | 22 (17–27) | 21 (18–28) | 20 (16–23) | 19 (17–25) | ns |
| Blood urea nitrogen | 10 (8–13) | 10 (8–13) | 9 (7–11) | 9 (7–11) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Gonzalez, J.; Ramón-Luing, L.A.; Romero-Tendilla, J.; Urbán-Solano, A.; Cruz-Lagunas, A.; Chavez-Galan, L. Latent Tuberculosis Patients Have an Increased Frequency of IFN-γ-Producing CD5+ B Cells, Which Respond Efficiently to Mycobacterial Proteins. Pathogens 2023, 12, 818. https://doi.org/10.3390/pathogens12060818
Flores-Gonzalez J, Ramón-Luing LA, Romero-Tendilla J, Urbán-Solano A, Cruz-Lagunas A, Chavez-Galan L. Latent Tuberculosis Patients Have an Increased Frequency of IFN-γ-Producing CD5+ B Cells, Which Respond Efficiently to Mycobacterial Proteins. Pathogens. 2023; 12(6):818. https://doi.org/10.3390/pathogens12060818
Chicago/Turabian StyleFlores-Gonzalez, Julio, Lucero A. Ramón-Luing, Jesus Romero-Tendilla, Alexia Urbán-Solano, Alfredo Cruz-Lagunas, and Leslie Chavez-Galan. 2023. "Latent Tuberculosis Patients Have an Increased Frequency of IFN-γ-Producing CD5+ B Cells, Which Respond Efficiently to Mycobacterial Proteins" Pathogens 12, no. 6: 818. https://doi.org/10.3390/pathogens12060818
APA StyleFlores-Gonzalez, J., Ramón-Luing, L. A., Romero-Tendilla, J., Urbán-Solano, A., Cruz-Lagunas, A., & Chavez-Galan, L. (2023). Latent Tuberculosis Patients Have an Increased Frequency of IFN-γ-Producing CD5+ B Cells, Which Respond Efficiently to Mycobacterial Proteins. Pathogens, 12(6), 818. https://doi.org/10.3390/pathogens12060818







