Stage-Dependent Increase of Systemic Immune Activation and CCR5+CD4+ T Cells in Filarial Driven Lymphedema in Ghana and Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Parasitic Assessment
2.2. Ethics
2.3. Flow Cytometric Analysis
2.4. Statistical Analysis
3. Results
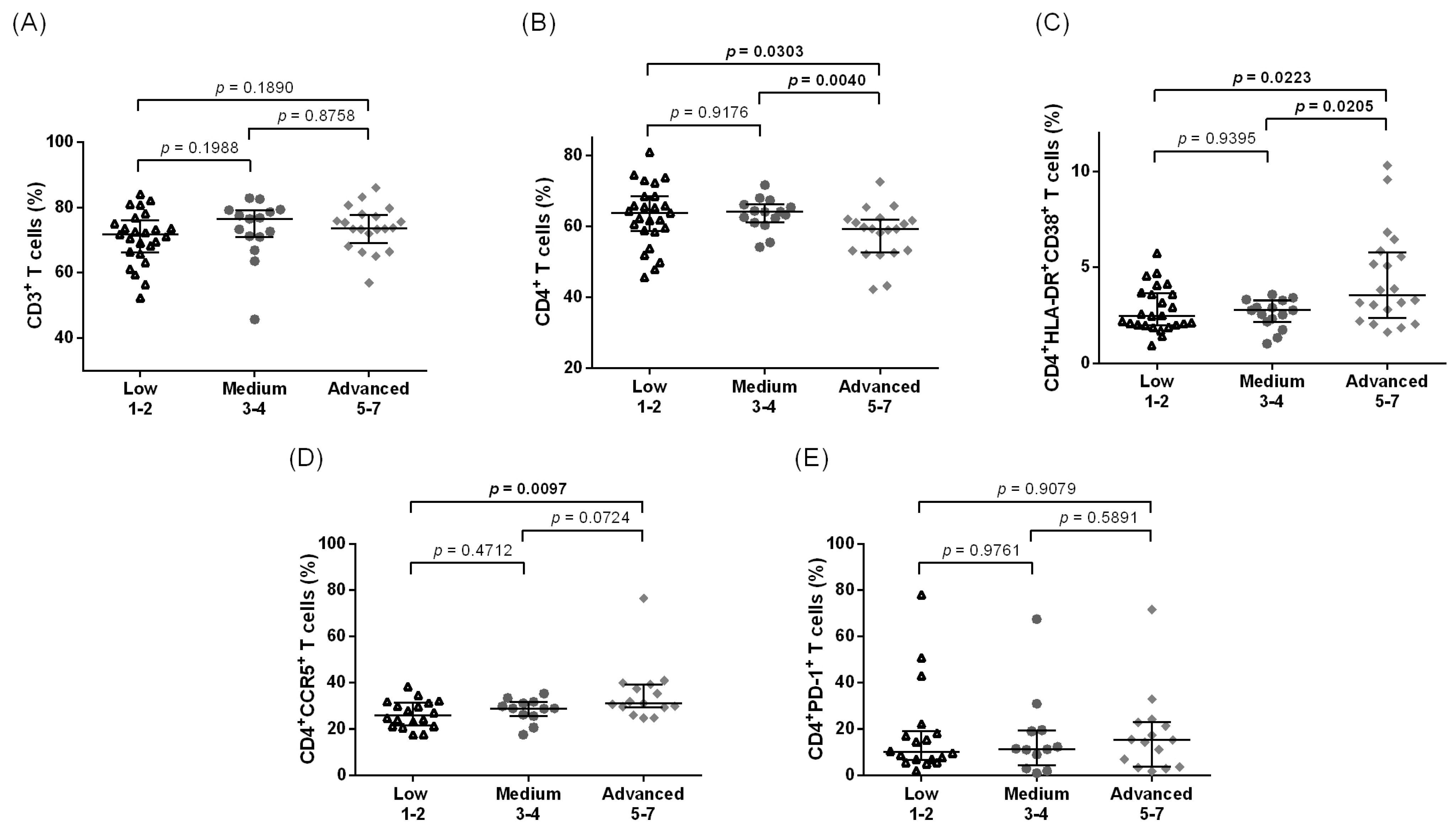
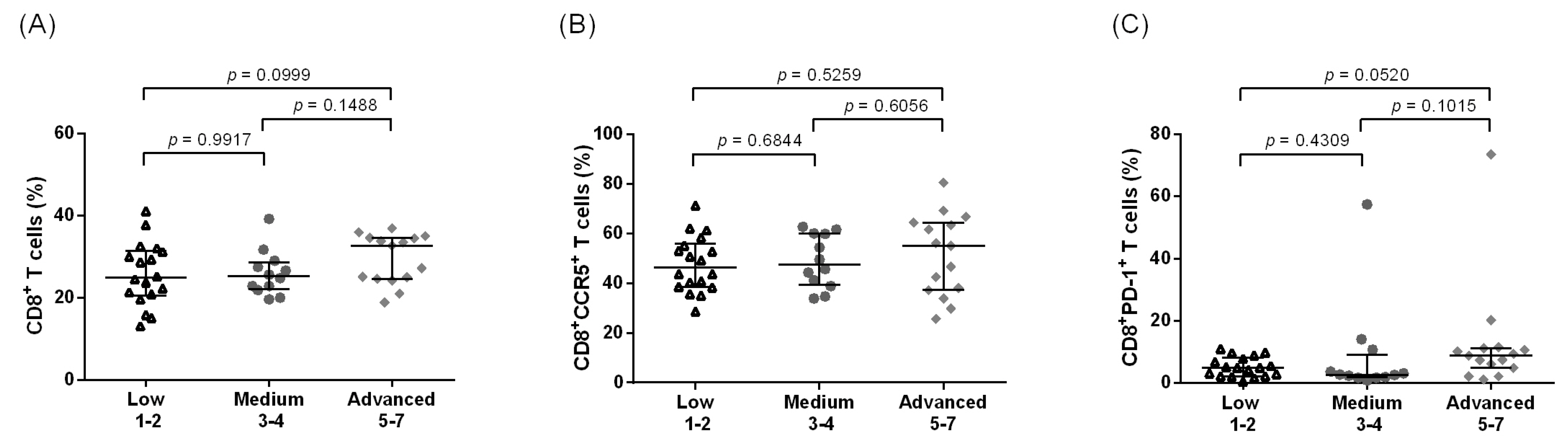
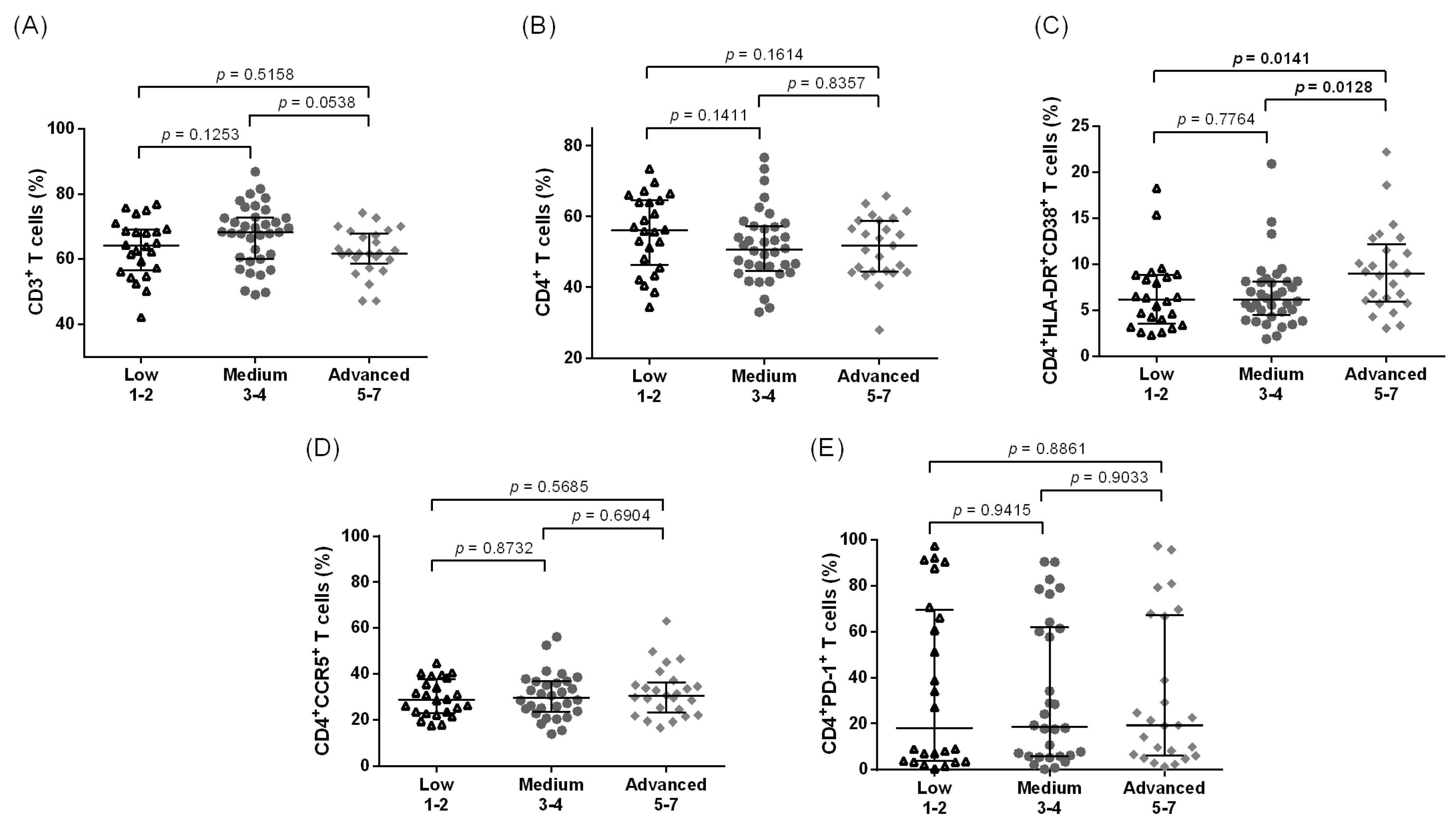
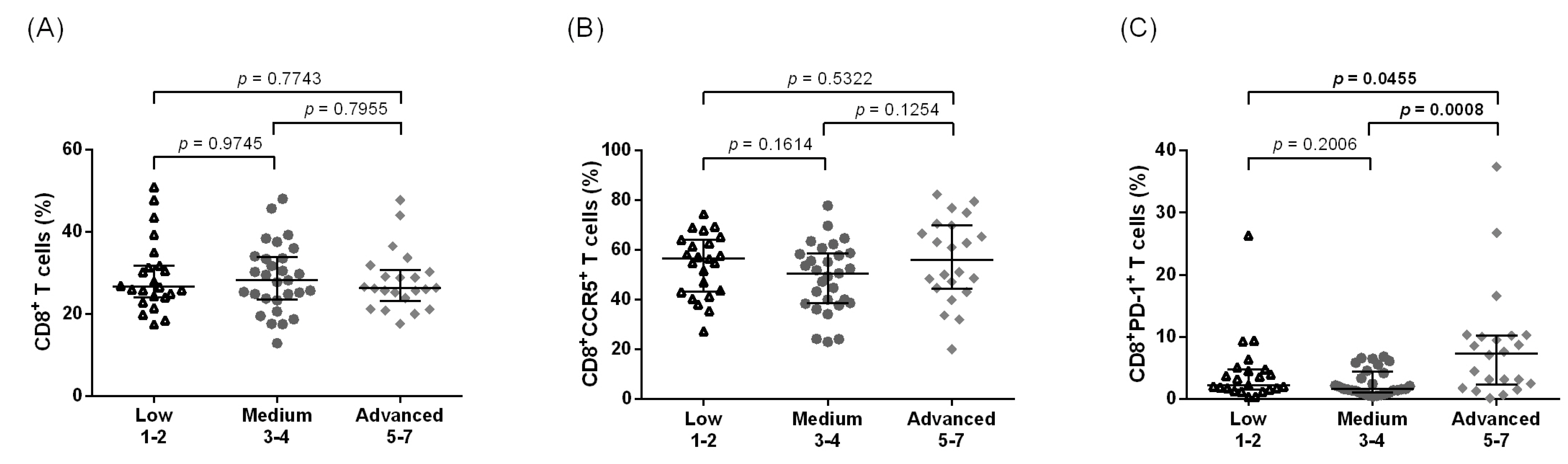
3.1. Increased Systemic CD4+ T Cell Activation among Individuals with Leg Lymphedema as Compared to Other Groups Residing in Filarial Endemic Areas
3.2. Increased Systemic Immune Activation and CCR5 Expression Are Associated with Advanced LE Stage in CD4+ T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The global distribution of lymphatic filariasis, 2000–2018: A geospatial analysis. Lancet Glob. Health 2020, 8, e1186–e1194. [CrossRef] [PubMed]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W.L.; Jamal, S.; Manokaran, G.; Lukomska, B.; Kubicka, U. Skin changes in filarial and non-filarial lymphoedema of the lower extremities. Trop. Med. Parasitol. Off. Organ Dtsch. Trop. Ges. Dtsch. Ges. Fur Tech. Zs. (GTZ) 1993, 44, 40–44. [Google Scholar]
- Pfarr, K.M.; Debrah, A.Y.; Specht, S.; Hoerauf, A. Filariasis and lymphoedema. Parasite Immunol. 2009, 31, 664–672. [Google Scholar] [CrossRef]
- Shenoy, R.K.; Kumaraswami, V.; Suma, T.K.; Rajan, K.; Radhakuttyamma, G. A double-blind, placebo-controlled study of the efficacy of oral penicillin, diethylcarbamazine or local treatment of the affected limb in preventing acute adenolymphangitis in lymphoedema caused by brugian filariasis. Ann. Trop. Med. Parasitol. 1999, 93, 367–377. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Programme to Eliminate Lymphatic Filariasis: Process Report; World Health Organization: Genava, Switzerland, 2019. [Google Scholar]
- Mnkai, J.; Marandu, T.F.; Mhidze, J.; Urio, A.; Maganga, L.; Haule, A.; Kavishe, G.; Ntapara, E.; Chiwerengo, N.; Clowes, P.; et al. Step towards elimination of Wuchereria bancrofti in Southwest Tanzania 10 years after mass drug administration with Albendazole and Ivermectin. PLoS Negl. Trop. Dis. 2022, 16, e0010044. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.D.; Mante, S. Caring for patients in the global programme to eliminate lymphatic filariasis. Int. Health 2020, 13, S48–S54. [Google Scholar] [CrossRef]
- Horton, J.; Klarmann-Schulz, U.; Stephens, M.; Budge, P.J.; Coulibaly, Y.; Debrah, A.; Debrah, L.B.; Krishnasastry, S.; Mwingira, U.; Ngenya, A.; et al. The design and development of a multicentric protocol to investigate the impact of adjunctive doxycycline on the management of peripheral lymphoedema caused by lymphatic filariasis and podoconiosis. Parasit. Vectors 2020, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Nutman, T.B. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 2012, 34, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Nutman, T.B. Immunology of lymphatic filariasis. Parasite Immunol. 2014, 36, 338–346. [Google Scholar] [CrossRef]
- Ritter, M.; Osei-Mensah, J.; Debrah, L.B.; Kwarteng, A.; Mubarik, Y.; Debrah, A.Y.; Pfarr, K.; Hoerauf, A.; Layland, L.E. Wuchereria bancrofti-infected individuals harbor distinct IL-10-producing regulatory B and T cell subsets which are affected by anti-filarial treatment. PLoS Negl. Trop. Dis. 2019, 13, e0007436. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, R.; George, P.J.; Kumaran, P.; Nutman, T.B.; Babu, S. Interleukin-10- and transforming growth factor β-independent regulation of CD8⁺ T cells expressing type 1 and type 2 cytokines in human lymphatic filariasis. Clin. Vaccine Immunol. 2014, 21, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Kwarteng, A.; Asiedu, E.; Koranteng, K.K.; Asiedu, S.O. Highlighting the Relevance of CD8+ T Cells in Filarial Infections. Front. Immunol. 2021, 12, 714052. [Google Scholar] [CrossRef] [PubMed]
- Metenou, S.; Nutman, T.B. Regulatory T cell subsets in filarial infection and their function. Front. Immunol. 2013, 4, 305. [Google Scholar] [CrossRef]
- Kroidl, I.; Chachage, M.; Mnkai, J.; Nsojo, A.; Berninghoff, M.; Verweij, J.J.; Maganga, L.; Ntinginya, N.E.; Maboko, L.; Clowes, P.; et al. Wuchereria bancrofti infection is linked to systemic activation of CD4 and CD8 T cells. PLoS Negl. Trop. Dis. 2019, 13, e0007623. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Borrero-Wolff, D.; Ritter, M.; Arndts, K.; Wiszniewsky, A.; Debrah, L.B.; Debrah, A.Y.; Osei-Mensah, J.; Chachage, M.; Hoerauf, A.; et al. Distinct Immune Profiles of Exhausted Effector and Memory CD8+ T Cells in Individuals with Filarial Lymphedema. Front. Cell. Infect. Microbiol. 2021, 11, 680832. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Ritter, M.; Arndts, K.; Borrero-Wolff, D.; Wiszniewsky, A.; Debrah, L.B.; Debrah, A.Y.; Osei-Mensah, J.; Chachage, M.; Hoerauf, A.; et al. Filarial Lymphedema Patients Are Characterized by Exhausted CD4+ T Cells. Front. Cell Infect Microbiol 2021, 11, 767306. [Google Scholar] [CrossRef]
- Dreyer, G.; Addiss, D.; Dreyer, P.; Noroes, J. Basic Lymphoedema Management: Treatment and Prevention of Problems Associated with Lymphatic Filariasis; Hollis Publishing Company: Hollis, NH, USA, 2004; ISBN 1-884186-17-3. [Google Scholar]
- Horn, S.; Ahmed, M.I.M.; Geldmacher, C.; Marandu, T.F.; Osei-Mensah, J.; Debrah, A.; Layland, L.E.; Hoerauf, A.; Kroidl, I. Flow cytometric analysis of cell lineage and immune activation markers using minimal amounts of human whole blood-Field method for remote settings. J. Immunol. Methods 2021, 491, 112989. [Google Scholar] [CrossRef]
- Babu, S.; Bhat, S.Q.; Pavan Kumar, N.; Lipira, A.B.; Kumar, S.; Karthik, C.; Kumaraswami, V.; Nutman, T.B. Filarial lymphedema is characterized by antigen-specific Th1 and th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl. Trop. Dis. 2009, 3, e420. [Google Scholar] [CrossRef]
- Babu, S.; Blauvelt, C.P.; Kumaraswami, V.; Nutman, T.B. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: Implications for parasite persistence. J. Immunol. 2006, 176, 3248–3256. [Google Scholar] [CrossRef]
- Metenou, S.; Dembele, B.; Konate, S.; Dolo, H.; Coulibaly, S.Y.; Coulibaly, Y.I.; Diallo, A.A.; Soumaoro, L.; Coulibaly, M.E.; Sanogo, D.; et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 2010, 184, 5375–5382. [Google Scholar] [CrossRef] [PubMed]
- Wammes, L.J.; Hamid, F.; Wiria, A.E.; Wibowo, H.; Sartono, E.; Maizels, R.M.; Smits, H.H.; Supali, T.; Yazdanbakhsh, M. Regulatory T cells in human lymphatic filariasis: Stronger functional activity in microfilaremics. PLoS Negl. Trop. Dis. 2012, 6, e1655. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Adu Mensah, D.; Debrah, L.B.; Gyamfi, P.A.; Rahamani, A.A.; Opoku, V.S.; Boateng, J.; Obeng, P.; Osei-Mensah, J.; Kroidl, I.; Klarmann-Schulz, U.; et al. Occurrence of Lymphatic Filariasis infection after 15 years of mass drug administration in two hotspot districts in the Upper East Region of Ghana. PLoS Negl. Trop. Dis. 2022, 16, e0010129. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Blauvelt, C.P.; Kumaraswami, V.; Nutman, T.B. Chemokine receptors of T cells and of B cells in lymphatic filarial infection: A role for CCR9 in pathogenesis. J. Infect. Dis. 2005, 191, 1018–1026. [Google Scholar] [CrossRef]
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Di Marzio, P.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381, 661–666. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Kaminski, V.L.; Rodrigues, A.G.; Kulmann-Leal, B.; Chies, J.A.B. CCR5 and CCR5Δ32 in bacterial and parasitic infections: Thinking chemokine receptors outside the HIV box. Int. J. Immunogenet. 2020, 47, 261–285. [Google Scholar] [CrossRef]
- Klein, R.S. A moving target: The multiple roles of CCR5 in infectious diseases. J. Infect. Dis. 2008, 197, 183–186. [Google Scholar] [CrossRef]
- Kobayashi, K.; Umeda, K.; Ihara, F.; Tanaka, S.; Yamagishi, J.; Suzuki, Y.; Nishikawa, Y. Transcriptome analysis of the effect of C-C chemokine receptor 5 deficiency on cell response to Toxoplasma gondii in brain cells. BMC Genomics 2019, 20, 705. [Google Scholar] [CrossRef]
- Lederman, M.M.; Penn-Nicholson, A.; Cho, M.; Mosier, D. Biology of CCR5 and its role in HIV infection and treatment. JAMA 2006, 296, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, L. CCR5: From Natural Resistance to a New Anti-HIV Strategy. Viruses 2010, 2, 574–600. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.L.; Souza, P.R.; Pereira, C.A.; Fernandes, A.; Guabiraba, R.; Russo, R.C.; Vieira, L.Q.; Corrêa, A., Jr.; Teixeira, M.M.; Negrão-Corrêa, D. Experimental infection with Schistosoma mansoni in CCR5-deficient mice is associated with increased disease severity, as CCR5 plays a role in controlling granulomatous inflammation. Infect. Immun. 2011, 79, 1741–1749. [Google Scholar] [CrossRef]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Duramad, P.; Tager, I.B.; Holland, N.T. Cytokines and other immunological biomarkers in children’s environmental health studies. Toxicol. Lett. 2007, 172, 48–59. [Google Scholar] [CrossRef]
- Mandala, W.L.; Ananworanich, J.; Apornpong, T.; Kerr, S.J.; MacLennan, J.M.; Hanson, C.; Jaimulwong, T.; Gondwe, E.N.; Rosenblatt, H.M.; Bunupuradah, T.; et al. Control lymphocyte subsets: Can one country’s values serve for another’s? J. Allergy Clin. Immunol. 2014, 134, 759–761.e758. [Google Scholar] [CrossRef]
- Melrose, W.D. Lymphatic filariasis: New insights into an old disease. Int. J. Parasitol. 2002, 32, 947–960. [Google Scholar] [CrossRef]
- De Almeida, A.B.; Freedman, D.O. Epidemiology and immunopathology of bancroftian filariasis. Microbes Infect. 1999, 1, 1015–1022. [Google Scholar] [CrossRef]
- Pani, S.P.; Yuvaraj, J.; Vanamail, P.; Dhanda, V.; Michael, E.; Grenfell, B.T.; Bundy, D.A. Episodic adenolymphangitis and lymphoedema in patients with bancroftian filariasis. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, R.K. Clinical and pathological aspects of filarial lymphedema and its management. Korean J. Parasitol. 2008, 46, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, R.K.; Sandhya, K.; Suma, T.K.; Kumaraswami, V. A preliminary study of filariasis related acute adenolymphangitis with special reference to precipitating factors and treatment modalities. Southeast Asian J. Trop. Med. Public Health 1995, 26, 301–305. [Google Scholar] [PubMed]
- Asiedu, S.O.; Kwarteng, A.; Amewu, E.K.A.; Kini, P.; Aglomasa, B.C.; Forkuor, J.B. Financial burden impact quality of life among lymphatic Filariasis patients. BMC Public Health 2021, 21, 174. [Google Scholar] [CrossRef]
- Van ’t Noordende, A.T.; Aycheh, M.W.; Schippers, A. The impact of leprosy, podoconiosis and lymphatic filariasis on family quality of life: A qualitative study in Northwest Ethiopia. PLoS Negl. Trop. Dis. 2020, 14, e0008173. [Google Scholar] [CrossRef] [PubMed]
- Gyapong, J.O. Lymphatic filariasis in Ghana: From research to control. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Zeldenryk, L.M.; Gray, M.; Speare, R.; Gordon, S.; Melrose, W. The emerging story of disability associated with lymphatic filariasis: A critical review. PLoS Negl. Trop. Dis. 2011, 5, e1366. [Google Scholar] [CrossRef]

| Uninf. | Wb-inf. | LE | |
|---|---|---|---|
| n | 34 | 10 | 25 |
| Age, median (range) | 50 (30–81) | 45.50 (31–83) | 47.50 (32–64) |
| Females, n (%) | 21 (61.8) | 6 (60) | 22 (88) |
| Mean years living in the endemic area (range) | 41.76 (22–81) | 48 (28–83) | 49.31 (32–64) |
| Median MDA rounds (range) | 1 (1–6) | 3.50 (1–5) | 6 (1–10) |
| FTS results/TropBio result | −/− | +/+ | −/− |
| History of ADL attacks, n (%) | NA | NA | 25 (100) |
| Occupation, n (%) | |||
| Farmer | NA | NA | 18 (72) |
| Trader | NA | NA | 7 (28) |
| Weight, median (range) | NA | NA | 65.5 (53.5–100) |
| Ghana | Tanzania | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Low | Medium | Advanced | Total | Low | Medium | Advanced | |
| n | 60 | 25 | 15 | 20 | 84 | 24 | 36 | 24 |
| Age, median (range) | 47.50 (19–65) | 47 (26–65) | 47 (26–62) | 53 (19–64) | 48.5 (23–65) | 51.5 (23–65) | 51 (23–65) | 51 (23–65) |
| Females, n (%) | 53 (88.3) | 21 (84) | 15 (100) | 17 (85) | 49 (58.3) | 16 (66.7) | 22 (61.1) | 11 (45.8) |
| Mean years since lymphedema began (range) | 14 (2–40) | 9.82 (3–21) | 16.82 (4–33) | 17.59 (2–40) | 25.9 (1–49) | 25.90 (1–49) | 25.59 (1–45) | 25.97 (1–49) |
| Mean years living in the endemic area (range) | 47.70 (19–65) | 45.96 (26–65) | 47.53 (26–62) | 50 (19–64) | 41.7 (3–65) | 42.17 (3–65) | 42.27 (3–65) | 41.81 (3–65) |
| Median MDA rounds (range) | 5 (1–15) | 5 (1–13) | 5 (1–10) | 5.5 (2–15) | 4 (0–15) | 4 (0–15) | 4 (0–15) | 4 (0–15) |
| History of ADL attacks, n (%) | 59 (98.3) | 24 (96) | 15 (100) | 20 (100) | 82 (97.6) | 23 (95.8) | 35 (97.2) | 24 (100) |
| Weight, median (range) | 62.75 (42.5–90) | 58.5 (42.5–90) | 65.5 (53.5–82.5) | 64.25 (48–77) | 59.35 (40–125) | 62.2 (40–94.5) | 56.1 (42–100) | 61.4 (43–125) |
| Occupation, n (%) | ||||||||
| Farmer | 40 (66.7) | 19 (76) | 11 (73.3) | 10 (50) | 50 (59.5) | 14 (58.3) | 22 (61.1) | 14 (58.3) |
| Trader | 14 (23.3) | 4 (16) | 3 (20) | 7 (35) | NA | NA | NA | NA |
| Other | 6 (10) | 2 (8) | 1 (6.7) | 3 (15) | 34 (40.5) | 10 (41.7) | 14 (38.9) | 10 (41.7) |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | n | Coef. | 95% CI | p-Value | Coef. | 95% CI | p-Value |
| Site | |||||||
| Ghana * | 60 | 0.00 | - | - | 0.00 | - | - |
| Tanzania | 84 | −8.83 | −11.88 to −5.79 | <0.001 | −5.10 | −10.00 to 0.06 | 0.05 |
| Age (per year) | - | −0.01 | −0.16 to 0.13 | 0.87 | −0.01 | −0.39 to 0.37 | >0.90 |
| Sex | |||||||
| female * | 102 | 0.00 | - | - | 0.00 | - | - |
| male | 42 | −5.59 | −8.97 to −2.21 | 0.001 | −0.81 | −6.90 to 5.20 | 0.80 |
| Years living in the endemic area | - | −0.04 | −0.15 to 0.07 | 0.50 | −0.05 | −0.34 to 0.23 | 0.70 |
| Years with lymphedema | - | −0.14 | −0.29 to 0.02 | 0.07 | −0.14 | −0.35 to 0.07 | 0.20 |
| Rounds of MDA received | - | 0.17 | −0.33 to 0.67 | 0.51 | 0.26 | −0.32 to 0.84 | 0.40 |
| Stage grouping | |||||||
| Stages 1–2 * | 49 | 0.00 | - | - | 0.00 | - | - |
| Stages 3–4 | 51 | −2.43 | −6.04 to 1.19 | 0.19 | −2.10 | −7.30 to 3.10 | 0.40 |
| Stages 5–7 | 44 | −4.70 | −8.39 to −1.00 | 0.01 | −5.00 | −10.00 to −0.35 | 0.067 |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | n | Coef. | 95% CI | p-Value | Coef. | 95% CI | p-Value |
| Site | |||||||
| Ghana * | 60 | 0.00 | - | - | 0.00 | - | - |
| Tanzania | 84 | 4.28 | 3.14 to 5.41 | <0.001 | 3.8 | 2.10 to 5.60 | <0.001 |
| Age (per year) | - | 0.08 | 0.03 to 0.14 | 0.002 | 0.02 | −0.11 to 0.15 | 0.80 |
| Sex | |||||||
| Female * | 102 | 0.00 | - | - | 0.00 | - | - |
| Male | 42 | 0.13 | −1.17 to 1.44 | 0.84 | 0.18 | −1.90 to 2.30 | 0.90 |
| Years living in the endemic area | - | 0.04 | −0.001 to 0.8 | 0.06 | 0.06 | −0.04 to 0.16 | 0.20 |
| Years with lymphedema | - | 0.02 | −0.04 to 0.08 | 0.49 | 0.00 | −0.07 to 0.07 | >0.90 |
| Rounds of MDA received | - | −0.06 | −0.26 to 0.13 | 0.51 | −0.16 | −0.36 to 0.04 | 0.12 |
| Stage grouping | |||||||
| Stages 1–2 * | 49 | 0.00 | - | - | 0.00 | - | - |
| Stages 3–4 | 51 | −0.10 | −1.41 to 1.21 | 0.89 | −0.01 | −1.80 to 1.80 | >0.9 |
| Stages 5–7 | 44 | 2.23 | 0.89 to 3.57 | 0.001 | 3.30 | 1.40 to 5.10 | <0.001 |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | n | Coef. | 95% CI | p-Value | Coef. | 95% CI | p-Value |
| Site | |||||||
| Ghana * | 45 | 0.00 | - | - | 0.00 | - | - |
| Tanzania | 70 | 0.01 | −0.02 to 0.05 | 0.49 | −0.03 | −0.10 to 0.04 | 0.40 |
| Age (per year) | - | 0.00 | 0.00 to 0.003 | 0.26 | 0.00 | −0.01 to 0.004 | 0.71 |
| Sex | |||||||
| Female * | 81 | 0.00 | - | - | 0.00 | - | - |
| Male | 34 | −0.03 | −0.07 to 0.11 | 0.15 | −0.02 | −0.10 to 0.06 | 0.57 |
| Years living in the endemic area | - | 0.00 | −0.001 to 0.002 | 0.67 | 0.00 | −0.004 to 0.003 | 0.67 |
| Years with lymphedema | - | 0.002 | −0.484 to 0.004 | 0.06 | 0.003 | 0.00 to 0.01 | 0.03 |
| Rounds of MDA received | - | 0.00 | −0.01 to 0.01 | 0.92 | −0.001 | −0.01 to 0.01 | 0.69 |
| Stage grouping | |||||||
| Stages 1–2 * | 39 | 0.00 | - | - | 0.00 | - | - |
| Stages 3–4 | 39 | 0.01 | −0.03 to 0.06 | 0.54 | −0.02 | −0.09 to 0.04 | 0.49 |
| Stages 5–7 | 37 | 0.39 | −0.01 to 0.08 | 0.08 | 0.06 | −0.01 to 0.12 | 0.09 |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | n | Coef. | 95% CI | p-Value | Coef. | 95% CI | p-Value |
| Site | |||||||
| Ghana * | 45 | 0.00 | - | - | 0.00 | - | - |
| Tanzania | 65 | 0.08 | −0.07 to 0.003 | <0.001 | −0.04 | −0.09 to 0.02 | 0.15 |
| Age (per year) | - | 0.00 | −0.003 to 0.001 | 0.39 | −0.002 | −0.01 to 0.002 | 0.28 |
| Sex | |||||||
| Female * | 80 | 0.00 | - | - | 0.00 | - | - |
| Male | 30 | 0.02 | −0.03 to 0.06 | 0.40 | −0.004 | −0.07 to 0.06 | 0.88 |
| Years living in the endemic area | - | 0.00 | −0.001 to 0.001 | 0.96 | 0.001 | −0.02 to 0.004 | 0.42 |
| Years with lymphedema | - | 0.00 | 0.00 to 0.003 | 0.13 | 0.00 | −0.001 to 0.003 | 0.43 |
| Rounds of MDA received | - | 0.00 | −0.01 to 0.002 | 0.20 | −0.003 | −0.008 to 0.003 | 0.36 |
| Stage grouping | |||||||
| Stages 1–2 * | 38 | 0.00 | - | - | 0.00 | - | - |
| Stages 3–4 | 38 | 0.01 | −0.04 to 0.05 | 0.81 | 0.02 | −0.03 to 0.07 | 0.48 |
| Stages 5–7 | 34 | 0.06 | 0.02 to 0.11 | 0.01 | 0.05 | −0.008 to 0.10 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahamani, A.A.; Horn, S.; Ritter, M.; Feichtner, A.; Osei-Mensah, J.; Serwaa Opoku, V.; Batsa Debrah, L.; Marandu, T.F.; Haule, A.; Mhidze, J.; et al. Stage-Dependent Increase of Systemic Immune Activation and CCR5+CD4+ T Cells in Filarial Driven Lymphedema in Ghana and Tanzania. Pathogens 2023, 12, 809. https://doi.org/10.3390/pathogens12060809
Rahamani AA, Horn S, Ritter M, Feichtner A, Osei-Mensah J, Serwaa Opoku V, Batsa Debrah L, Marandu TF, Haule A, Mhidze J, et al. Stage-Dependent Increase of Systemic Immune Activation and CCR5+CD4+ T Cells in Filarial Driven Lymphedema in Ghana and Tanzania. Pathogens. 2023; 12(6):809. https://doi.org/10.3390/pathogens12060809
Chicago/Turabian StyleRahamani, Abu Abudu, Sacha Horn, Manuel Ritter, Anja Feichtner, Jubin Osei-Mensah, Vera Serwaa Opoku, Linda Batsa Debrah, Thomas F. Marandu, Antelmo Haule, Jacklina Mhidze, and et al. 2023. "Stage-Dependent Increase of Systemic Immune Activation and CCR5+CD4+ T Cells in Filarial Driven Lymphedema in Ghana and Tanzania" Pathogens 12, no. 6: 809. https://doi.org/10.3390/pathogens12060809
APA StyleRahamani, A. A., Horn, S., Ritter, M., Feichtner, A., Osei-Mensah, J., Serwaa Opoku, V., Batsa Debrah, L., Marandu, T. F., Haule, A., Mhidze, J., Ngenya, A., Demetrius, M., Klarmann-Schulz, U., Hoelscher, M., Geldmacher, C., Hoerauf, A., Kalinga, A., Debrah, A. Y., & Kroidl, I. (2023). Stage-Dependent Increase of Systemic Immune Activation and CCR5+CD4+ T Cells in Filarial Driven Lymphedema in Ghana and Tanzania. Pathogens, 12(6), 809. https://doi.org/10.3390/pathogens12060809









