Challenges and Strategies for Developing Recombinant Vaccines against Leptospirosis: Role of Expression Platforms and Adjuvants in Achieving Protective Efficacy
Abstract
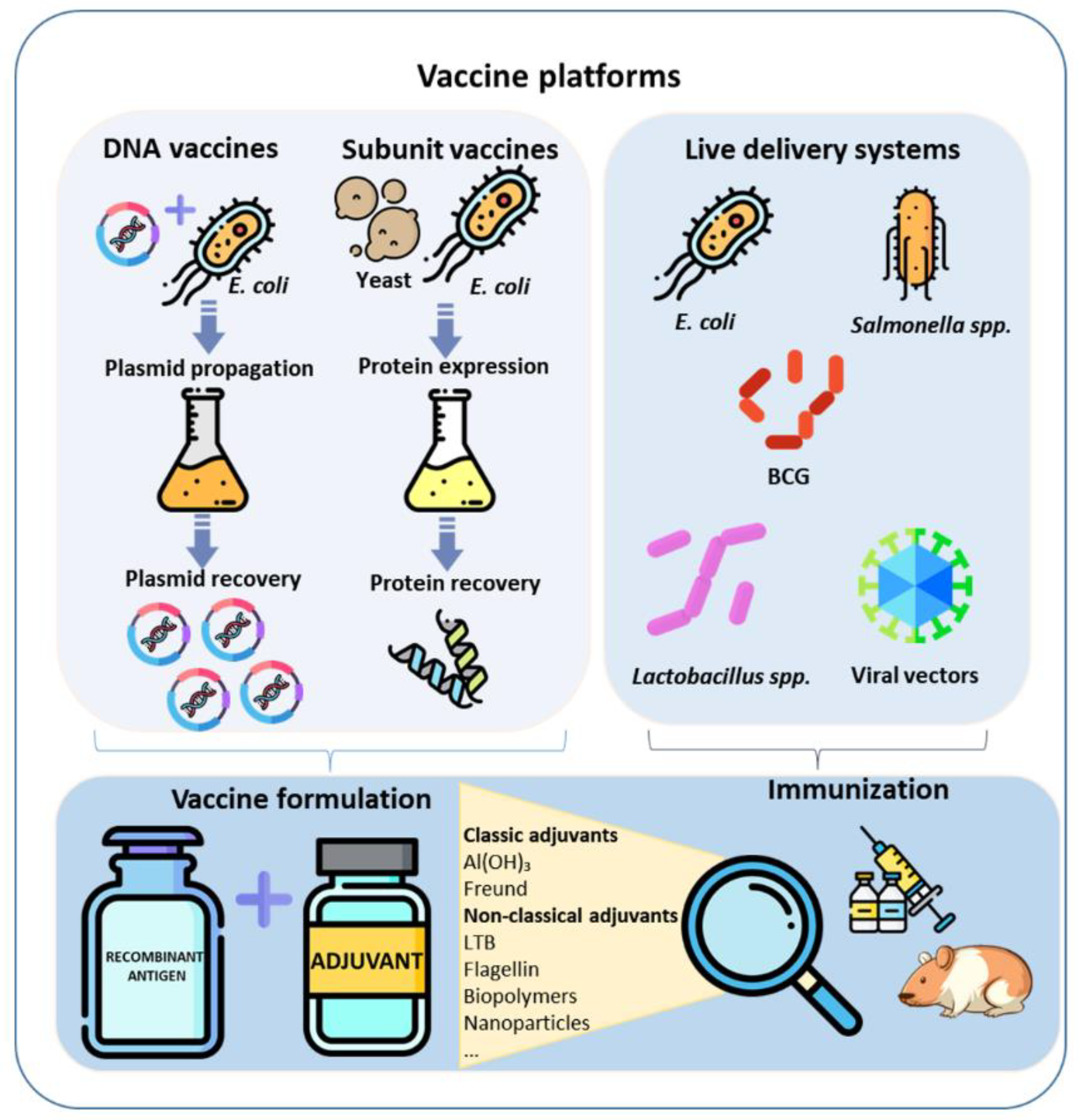
1. Introduction
2. Bacteria-Based Expression Platforms
3. Yeast-Based Expression Platforms
4. Live Recombinant Antigen Delivery Vehicles
4.1. Bacille Calmette-Guérin (BCG)
4.2. Lactobacillus
4.3. Escherichia coli
4.4. Salmonella
4.5. Viral Vectors
5. DNA Vaccines
6. Commercial Adjuvants
6.1. Aluminum Hydroxide
6.2. Freund’s Adjuvant
7. Pathogen Agonists and Other Adjuvants
7.1. Pathogen Agonists
7.2. The B Subunit of the Heat-Labile Toxin of E. coli (LTB)
7.3. Carbon and Halloysite Clay Nanotubes
8. Current Gaps and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adler, B.; de la Moctezuma, A.P. Leptospira and Leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gouveia, E.L.; Metcalfe, J.; de Carvalho, A.L.F.; Aires, T.S.F.; Villasboas-Bisneto, J.C.; Queirroz, A.; Santos, A.C.; Salgado, K.; Reis, M.G.; Ko, A.I. Leptospirosis-Associated Severe Pulmonary Hemorrhagic Syndrome, Salvador, Brazil. Emerg. Infect. Dis. 2008, 14, 505–508. [Google Scholar] [CrossRef]
- Ellis, W.A. Animal Leptospirosis. In Leptospira and Leptospirosis; Adler, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 99–138. [Google Scholar]
- Martins, G.; Oliveira, C.S.; Lilenbaum, W. Dynamics of Humoral Response in Naturally-Infected Cattle after Vaccination against Leptospirosis. Acta Trop. 2018, 187, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Khalid, M.K.N.M.; Amran, F.; Masuzawa, T.; et al. Revisiting the Taxonomy and Evolution of Pathogenicity of the Genus Leptospira through the Prism of Genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korba, A.A.; Lounici, H.; Kainiu, M.; Vincent, A.T.; Mariet, J.F.; Veyrier, F.J.; Goarant, C.; Picardeau, M. Leptospira Ainlahdjerensis Sp. Nov., Leptospira Ainazelensis Sp. Nov., Leptospira Abararensis Sp. Nov. and Leptospira Chreensis Sp. Nov., Four New Species Isolated from Water Sources in Algeria. Int. J. Syst. Evol. Microbiol. 2021, 71, 005148. [Google Scholar] [CrossRef]
- Verma, R.; Khanna, P.; Chawla, S. Whole-Cell Inactivated Leptospirosis Vaccine: Future Prospects. Hum. Vaccines Immunother. 2013, 9, 763–765. [Google Scholar] [CrossRef][Green Version]
- André-Fontaine, G.; Branger, C.; Gray, A.W.; Klaasen, H.L.B.M. Comparison of the Efficacy of Three Commercial Bacterins in Preventing Canine Leptospirosis. Vet. Rec. 2003, 153, 165–169. [Google Scholar] [CrossRef][Green Version]
- Suepaul, S.M.; Carrington, C.V.; Campbell, M.; Borde, G.; Adesiyun, A.A. Study on the Efficacy of Leptospira Vaccines Developed from Serovars Isolated from Trinidad and Comparison with Commercial Vaccines Using a Hamster Model. Vaccine 2010, 28, 5421–5426. [Google Scholar] [CrossRef]
- Sonada, R.B.; de Azevedo, S.S.; Soto, F.R.M.; da Costa, D.F.; de Morais, Z.M.; de Souza, G.O.; Gonçales, A.P.; Miraglia, F.; Vasconcellos, S.A. Efficacy of Leptospiral Commercial Vaccines on the Protection against an Autochtonous Strain Recovered in Brazil. Braz. J. Microbiol. 2018, 49, 347–350. [Google Scholar] [CrossRef]
- de Oliveira, N.R.; Jorge, S.; Maia, M.A.C.; Bunde, T.T.; Pedra, A.C.K.; Seixas Neto, A.C.P.; Oliveira, T.L.; Dellagostin, O.A. Protective Efficacy of Whole-Cell Inactivated Leptospira Vaccines Made Using Virulent or Avirulent Strains in a Hamster Model. Vaccine 2021, 39, 5626–5634. [Google Scholar] [CrossRef]
- Dellagostin, O.A.; Grassmann, A.A.; Rizzi, C.; Schuch, R.A.; Jorge, S.; Oliveira, T.L.; Mcbride, A.J.A.; Hartwig, D.D. Reverse Vaccinology: An Approach for Identifying Leptospiral Vaccine Candidates. Int. J. Mol. Sci. 2017, 18, 158. [Google Scholar] [CrossRef][Green Version]
- Felix, C.R.; Siedler, B.S.; Barbosa, L.N.; Timm, G.R.; McFadden, J.; McBride, A.J.A. An Overview of Human Leptospirosis Vaccine Design and Future Perspectives. Expert Opin. Drug Discov. 2020, 15, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.F.; Fernandes, L.G.V.; Cavenague, M.F.; Takahashi, M.B.; Santos, J.C.; Passalia, F.J.; Daroz, B.B.; Kochi, L.T.; Vieira, M.L.; Nascimento, A.L.T.O. Adjuvanted Leptospiral Vaccines: Challenges and Future Development of New Leptospirosis Vaccines. Vaccine 2019, 37, 3961–3973. [Google Scholar] [CrossRef]
- Grassmann, A.A.; Kremer, F.S.; dos Santos, J.C.; Souza, J.D.; da Pinto, L.S.; McBride, A.J.A. Discovery of Novel Leptospirosis Vaccine Candidates Using Reverse and Structural Vaccinology. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef][Green Version]
- Clark, T.; Cassidy-Hanley, D. Recombinant Subunit Vaccines: Potentials and Constraints. Dev. Biol. 2005, 121, 153–163. [Google Scholar]
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The Population Genetics of Commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Mahalik, S.; Sharma, A.K.; Mukherjee, K.J. Genome Engineering for Improved Recombinant Protein Expression in Escherichia coli. Microb. Cell Factories 2014, 13, 177. [Google Scholar] [CrossRef][Green Version]
- Legastelois, I.; Buffin, S.; Peubez, I.; Mignon, C.; Sodoyer, R.; Werle, B. Non-Conventional Expression Systems for the Production of Vaccine Proteins and Immunotherapeutic Molecules. Hum. Vaccin. Immunother. 2016, 13, 947–961. [Google Scholar] [CrossRef][Green Version]
- Rosales-Mendoza, S.; Angulo, C.; Meza, B. Food-Grade Organisms as Vaccine Biofactories and Oral Delivery Vehicles. Trends Biotechnol. 2016, 34, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.; Murphy, L.; Tyther, R. Post-Translational Modifications of Recombinant Proteins: Significance for Biopharmaceuticals. Mol. Biotechnol. 2008, 39, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.; Batista-Duharte, A.; González, E.; Zayas, C.; Balboa, J.; Cuello, M.; Cabrera, O.; Lastre, M.; Schijns, V.E.J.C. Human Prophylactic Vaccine Adjuvants and Their Determinant Role in New Vaccine Formulations. Braz. J. Med. Biol. Res. 2012, 45, 681–692. [Google Scholar] [CrossRef]
- Malmström, J.; Beck, M.; Schmidt, A.; Lange, V.; Deutsch, E.W.; Aebersold, R. Proteome-Wide Cellular Protein Concentrations of the Human Pathogen Leptospira Interrogans. Nature 2009, 460, 762–765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deveson Lucas, D.S.; Cullen, P.A.; Lo, M.; Srikram, A.; Sermswan, R.W.; Adler, B. Recombinant LipL32 and LigA from Leptospira Are Unable to Stimulate Protective Immunity against Leptospirosis in the Hamster Model. Vaccine 2011, 29, 3413–3418. [Google Scholar] [CrossRef]
- Humphryes, P.C.; Weeks, M.E.; AbuOun, M.; Thomson, G.; Núñez, A.; Coldham, N.G. Vaccination with Leptospiral Outer Membrane Lipoprotein LipL32 Reduces Kidney Invasion of Leptospira Interrogans Serovar Canicola in Hamsters. Clin. Vaccine Immunol. 2014, 21, 546–551. [Google Scholar] [CrossRef][Green Version]
- Grassmann, A.A.; Félix, S.R.; Dos Santos, C.X.; Amaral, M.G.; Neto, A.C.P.S.; Fagundes, M.Q.; Seixas, F.K.; Da Silva, É.F.; Conceiçao, F.R.; Dellagostin, O.A. Protection against Lethal Leptospirosis after Vaccination with LipL32 Coupled or Coadministered with the b Subunit of Escherichia coli Heat-Labile Enterotoxin. Clin. Vaccine Immunol. 2012, 19, 740–745. [Google Scholar] [CrossRef][Green Version]
- Branger, C.; Chatrenet, B.; Gauvrit, A.; Aviat, F.; Aubert, A.; Bach, J.M.; André-Fontaine, G. Protection against Leptospira interrogans Sensu Lato Challenge by DNA Immunization with the Gene Encoding Hemolysin-Associated Protein 1. Infect. Immun. 2005, 73, 4062–4069. [Google Scholar] [CrossRef][Green Version]
- Matsunaga, J.; Barocchi, M.A.; Croda, J.; Young, T.A.; Sanchez, Y.; Siqueira, I.; Bolin, C.A.; Reis, M.G.; Riley, L.W.; Haake, D.A.; et al. Pathogenic Leptospira Species Express Surface-Exposed Proteins Belonging to the Bacterial Immunoglobulin Superfamily. Mol. Microbiol. 2003, 49, 929–945. [Google Scholar] [CrossRef]
- Palaniappan, R.U.M.; McDonough, S.P.; Divers, T.J.; Chen, C.S.; Pan, M.J.; Matsumoto, M.; Chang, Y.F. Immunoprotection of Recombinant Leptospiral Immunoglobulin-like Protein A against Leptospira interrogans Serovar Pomona Infection. Infect. Immun. 2006, 74, 1745–1750. [Google Scholar] [CrossRef][Green Version]
- Forster, K.M.; Hartwig, D.D.; Oliveira, T.L.; Bacelo, K.L.; Schuch, R.; Amaral, M.G.; Dellagostin, O.A. DNA Prime-Protein Boost Based Vaccination with a Conserved Region of Leptospiral Immunoglobulin-like A and B Proteins Enhances Protection against Leptospirosis. Mem. Inst. Oswaldo Cruz 2015, 110, 989–995. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, C.E.P.; Bettin, E.B.; Bakry, A.F.A.A.; Neto, A.C.P.S.; Amaral, M.G.; Dellagostin, O.A. Evaluation of Different Strategies to Promote a Protective Immune Response against Leptospirosis Using a Recombinant LigA and LigB Chimera. Vaccine 2019, 37, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, R.; Salovey, A.; Guo, X.; Desir, G.; Vinetz, J.M. Vaccination With Leptospira interrogans PF07598 Gene Family-Encoded Virulence Modifying Proteins Protects Mice From Severe Leptospirosis and Reduces Bacterial Load in the Liver and Kidney. Front. Cell. Infect. Microbiol. 2022, 12, 822. [Google Scholar] [CrossRef]
- Witchell, T.D.; Eshghi, A.; Nally, J.E.; Hof, R.; Boulanger, M.J.; Wunder, E.A.; Ko, A.I.; Haake, D.A.; Cameron, C.E. Post-Translational Modification of LipL32 during Leptospira interrogans Infection. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bill, R.M. Playing Catch-up with Escherichia coli: Using Yeast to Increase Success Rates in Recombinant Protein Production Experiments. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef][Green Version]
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant Protein Production in Yeasts. Methods Mol. Biol. 2012, 824, 329–358. [Google Scholar] [CrossRef]
- Taguchi, S.; Ooi, T.; Mizuno, K.; Matsusaki, H. Advances and Needs for Endotoxin-Free Production Strains. Appl. Microbiol. Biotechnol. 2015, 99, 9349–9360. [Google Scholar] [CrossRef]
- Conde, R.; Cueva, R.; Pablo, G.; Polaina, J.; Larriba, G. A Search for Hyperglycosylation Signals in Yeast Glycoproteins. J. Biol. Chem. 2004, 279, 43789–43798. [Google Scholar] [CrossRef][Green Version]
- Hartwig, D.D.; Oliveira, T.L.; Seixas, F.K.; Forster, K.M.; Rizzi, C.; Hartleben, C.P.; McBride, A.J.A.; Dellagostin, O.A. High Yield Expression of Leptospirosis Vaccine Candidates LigA and LipL32 in the Methylotrophic Yeast Pichia pastoris. Microb. Cell Fact. 2010, 9, 1–7. [Google Scholar] [CrossRef][Green Version]
- Govindan, P.; Manjusha, P.; Saravanan, K.M.; Natesan, V.; Salmen, S.H.; Alfarraj, S.; Wainwright, M.; Shakila, H. Expression and Preliminary Characterization of the Potential Vaccine Candidate LipL32 of Leptospirosis. Appl. Nanosci. 2021, 30, 1–15. [Google Scholar] [CrossRef]
- Hartwig, D.D.; Bacelo, K.L.; De Oliveira, P.D.; Oliveira, T.L.; Seixas, F.K.; Amaral, M.G.; Hartleben, C.P.; McBride, A.J.A.; Dellagostin, O.A. Mannosylated LigANI Produced in Pichia pastoris Protects Hamsters against Leptospirosis. Curr. Microbiol. 2014, 68, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Krebs, S.J.; Haigwood, N.L.; De Berardinis, P. Delivery Strategies for Novel Vaccine Formulations. World J. Virol. 2012, 1, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, P.; Madhavi, B. Vaccine Development and Delivery Strategies—A Glimpse. J. Vaccines Immunol. 2021, 7, 4–8. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and Oral Vaccination with Protein-Based Antigens: Advantages, Challenges and Formulation Strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef][Green Version]
- Ding, C.; Ma, J.; Dong, Q.; Liu, Q. Live Bacterial Vaccine Vector and Delivery Strategies of Heterologous Antigen: A Review. Immunol. Lett. 2018, 197, 70–77. [Google Scholar] [CrossRef]
- Broset, E.; Calvet Seral, J.; Arnal, C.; Uranga, S.; Kanno, A.I.; Leite, L.C.C.; Martín, C.; Gonzalo-Asensio, J. Engineering a New Vaccine Platform for Heterologous Antigen Delivery in Live-Attenuated Mycobacterium tuberculosis. Comput. Struct. Biotechnol. J. 2021, 19, 4273–4283. [Google Scholar] [CrossRef]
- Marques-Neto, L.M.; Piwowarska, Z.; Kanno, A.I.; Moraes, L.; Trentini, M.M.; Rodriguez, D.; Silva, J.L.S.C.; Leite, L.C.C. Thirty Years of Recombinant BCG: New Trends for a Centenary Vaccine. Expert Rev. Vaccines 2021, 20, 1001–1011. [Google Scholar] [CrossRef]
- Seixas, F.K.; Silva, E.F.; Hartwig, D.D.; Cerqueira, G.M.; Amaral, M.; Fagundes, M.Q.; Dossa, R.G.; Dellagostin, O.A. Recombinant Mycobacterium bovis BCG Expressing the LipL32 Antigen of Leptospira interrogans Protects Hamsters from Challenge. Vaccine 2007, 26, 88–95. [Google Scholar] [CrossRef]
- Oliveira, T.L.; Rizzi, C.; da Cunha, C.E.P.; Dorneles, J.; Seixas Neto, A.C.P.; Amaral, M.G.; Hartwig, D.D.; Dellagostin, O.A. Recombinant BCG Strains Expressing Chimeric Proteins Derived from Leptospira Protect Hamsters against Leptospirosis. Vaccine 2019, 37, 776–782. [Google Scholar] [CrossRef]
- Dorneles, J.; Madruga, A.B.; Seixas Neto, A.C.P.; Rizzi, C.; Bettin, É.B.; Hecktheuer, A.S.; de Castro, C.C.; Fernandes, C.G.; Oliveira, T.L.; Dellagostin, O.A. Protection against Leptospirosis Conferred by Mycobacterium bovis BCG Expressing Antigens from Leptospira interrogans. Vaccine 2020, 38, 8136–8144. [Google Scholar] [CrossRef]
- Bettin, E.B.; Dorneles, J.; Hecktheuer, A.S.; Madruga, A.B.; Seixas Neto, A.C.P.; Mcbride, A.J.A.; Oliveira, T.L.; Grassmann, A.A.; Dellagostin, O.A. TonB-Dependent Receptor Epitopes Expressed in M. Bovis BCG Induced Significant Protection in the Hamster Model of Leptospirosis. Appl. Microbiol. Biotechnol. 2022, 106, 173–184. [Google Scholar] [CrossRef]
- Barazzone, G.C.; Teixeira, A.F.; Azevedo, B.O.P.; Damiano, D.K.; Oliveira, M.P.; Nascimento, A.L.T.O.; Lopes, A.P.Y. Revisiting the Development of Vaccines Against Pathogenic Leptospira: Innovative Approaches, Present Challenges, and Future Perspectives. Front. Immunol. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Perdigón, G.; Alvarez, S.; Holgado, A.P.D.R. Immunoadjuvant Activity of Oral Lactobacillus casei: Influence of Dose on the Secretory Immune Response and Protective Capacity in Intestinal Infections. J. Dairy Res. 1991, 58, 485–496. [Google Scholar] [CrossRef]
- Del Rio, B.; Dattwyler, R.J.; Aroso, M.; Neves, V.; Meirelles, L.; Seegers, J.F.M.L.; Gomes-Solecki, M. Oral Immunization with Recombinant Lactobacillus plantarum Induces a Protective Immune Response in Mice with Lyme Disease. Clin. Vaccine Immunol. 2008, 15, 1429–1435. [Google Scholar] [CrossRef][Green Version]
- Welll, J.; Mercenier, A. Lactic Acid Bacteria as Mucosal Delivery Vehicles. In Genetics of Lactic Acid Bacteria; Wood, B., Warner, P., Eds.; Springer: Boston, MA, USA, 2003; pp. 261–290. ISBN 978-1-4613-4959-4. [Google Scholar]
- Taskila, S.; Ojamo, H. The Current Status and Future Expectations in Industrial Production of Lactic Acid by Lactic Acid Bacteria. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, M., Ed.; IntechOpen: London, UK, 2013; pp. 615–632. ISBN 978-953-51-0955-6. [Google Scholar]
- Wang, M.; Gao, Z.; Zhang, Y.; Pan, L. Lactic Acid Bacteria as Mucosal Delivery Vehicles: A Realistic Therapeutic Option. Appl. Microbiol. Biotechnol. 2016 10013 2016, 100, 5691–5701. [Google Scholar] [CrossRef] [PubMed]
- Potula, H.H.; Richer, L.; Werts, C.; Gomes-Solecki, M. Pre-Treatment with Lactobacillus plantarum Prevents Severe Pathogenesis in Mice Infected with Leptospira interrogans and May Be Associated with Recruitment of Myeloid Cells. PLoS Negl. Trop. Dis. 2017, 11, e0005870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lycke, N. Recent Progress in Mucosal Vaccine Development: Potential and Limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Lourdault, K.; Wang, L.; Vieira, A.; Matsunaga, J.; Melo, R.; Lewis, M.S.; Haake, D.A.; Gomes-solecki, M. Oral Immunization with Escherichia coli Expressing a Lipidated Form of LigA Protects Hamsters against Challenge with Leptospira interrogans Serovar Copenhageni. Infect. Immun. 2014, 82, 893–902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- del Rio, B.; Seegers, J.F.M.L.; Gomes-Solecki, M. Immune Response to Lactobacillus plantarum Expressing Borrelia burgdorferi OspA Is Modulated by the Lipid Modification of the Antigen. PLoS ONE 2010, 5, e11199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrol, M.E.W.; Jackett, P.S.; Aber, V.R.; Lowrie, D.B. Phagolysosome Formation, Cyclic Adenosine 3′:5′-Monophosphate and the Fate of Salmonella typhimurium within Mouse Peritoneal Macrophages. J. Gen. Microbiol. 1979, 110, 421–429. [Google Scholar] [CrossRef][Green Version]
- Roland, K.L.; Brenneman, K.E. Salmonella as a Vaccine Delivery Vehicle. Expert Rev. Vaccines 2013, 12, 1033–1045. [Google Scholar] [CrossRef][Green Version]
- Samakchan, N.; Thinwang, P.; Boonyom, R. Oral Immunization of Rat with Chromosomal Expression LipL32 in Attenuated Salmonella Vaccine Induces Immune Respond against Pathogenic Leptospira. Clin. Exp. Vaccine Res. 2021, 10, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sean, T.C.; Bhavya, K.S.; Sahithya, C.S.; Chan-drasekaran, S.; Palanisamy, R.; Robinson, E.R.; Subbiah, S.K.; Mok, P.L. Leptospiral Infection, Pathogenesis and Its Diagnosis—A Review. Pathogens 2021, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.P.; Leite, L.C.C. Recombinant Vaccines and the Development of New Vaccine Strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef][Green Version]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. Vaccines 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Branger, C.; Sonrier, C.; Chatrenet, B.; Klonjkowski, B.; Ruvoen-Clouet, N.; Aubert, A.; André -Fontaine, G.; Eloit, M. Identification of the Hemolysis-Associated Protein 1 as a Cross-Protective Immunogen of Leptospira interrogans by Adenovirus-Mediated Vaccination. Infect. Immun. 2001, 69, 6831–6838. [Google Scholar] [CrossRef][Green Version]
- Shafaati, M.; Saidijam, M.; Soleimani, M.; Hazrati, F.; Mirzaei, R.; Amirheidari, B.; Tanzadehpanah, H.; Karampoor, S.; Kazemi, S.; Yavari, B.; et al. A Brief Review on DNA Vaccines in the Era of COVID-19. Future Virol. 2022, 17, 49–66. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Ulmer, J.B.; Liu, M.A. DNA Vaccines. Dev. Biol. Stand. 1997, 15, 617–648. [Google Scholar] [CrossRef]
- Silveira, M.M.; Conceição, F.R.; Mendonça, M.; Garcia Moreira, G.M.S.; Da Cunha, C.E.P.; Conrad, N.L.; De Oliveira, P.D.; Hartwig, D.D.; De Leon, P.M.M.; Moreira, Â.N. Saccharomyces Boulardii Improves Humoral Immune Response to DNA Vaccines against Leptospirosis. J. Med. Microbiol. 2017, 66, 184–190. [Google Scholar] [CrossRef]
- Forster, K.M.; Hartwig, D.D.; Seixas, F.K.; Bacelo, K.L.; Amaral, M.; Hartleben, C.P.; Dellagostin, O.A. A Conserved Region of Leptospiral Immunoglobulin-like A and B Proteins as a DNA Vaccine Elicits a Prophylactic Immune Response against Leptospirosis. Clin. Vaccine Immunol. 2013, 20, 725–731. [Google Scholar] [CrossRef][Green Version]
- Hartwig, D.D.; Forster, K.M.; Oliveira, T.L.; Amaral, M.; McBride, A.J.A.; Dellagostin, O.A. A Prime-Boost Strategy Using the Novel Vaccine Candidate, LemA, Protects Hamsters against Leptospirosis. Clin. Vaccine Immunol. 2013, 20, 747–752. [Google Scholar] [CrossRef]
- Raja, V.; Sobana, S.; Mercy, C.S.A.; Cotto, B.; Bora, D.P.; Natarajaseenivasan, K. Heterologous DNA Prime-Protein Boost Immunization with RecA and FliD Offers Cross-Clade Protection against Leptospiral Infection. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef][Green Version]
- Buaklin, A.; Palaga, T.; Hannaman, D.; Kerdkaew, R.; Patarakul, K.; Jacquet, A. Optimization of the Immunogenicity of a DNA Vaccine Encoding a Bacterial Outer Membrane Lipoprotein. Mol. Biotechnol. 2014, 56, 903–910. [Google Scholar] [CrossRef]
- Umthong, S.; Buaklin, A.; Jacquet, A.; Sangjun, N.; Kerdkaew, R.; Patarakul, K.; Palaga, T. Immunogenicity of a DNA and Recombinant Protein Vaccine Combining LipL32 and Loa22 for Leptospirosis Using Chitosan as a Delivery System. J. Microbiol. Biotechnol. 2015, 25, 526–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Govindan, P.; Pitchaikani, S.; Kandasamy, S.; Rajan, M.; Shakila, H.; Eed, E.M.; Elfasakhany, A.; Pugazhendhi, A. Biomacromolecules of Chitosan—Bacopa Saponin Based LipL32 Gene Delivery System for Leptospirosis Therapy. Environ. Res. 2021, 202, 111699. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. Toll-Free Vaccines? Nat. Biotechnol. 2007, 25, 303–305. [Google Scholar] [CrossRef]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New Horizons in Adjuvants for Vaccine Development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. Methods Mol. Biol. 2022, 2412, 145–178. [Google Scholar] [CrossRef]
- Vernel-Pauillac, F.; Werts, C. Recent Findings Related to Immune Responses against Leptospirosis and Novel Strategies to Prevent Infection. Microbes Infect. 2018, 20, 578–588. [Google Scholar] [CrossRef][Green Version]
- Lindblad, E.B.; Duroux, L. Mineral Adjuvants. In Immunopotentiators in Modern Vaccines; Schijns, V., O’Hagan, D., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 347–375. ISBN 9780128040195. [Google Scholar]
- Cain, D.W.; Sanders, S.E.; Cunningham, M.M.; Kelsoe, G. Disparate Adjuvant Properties among Three Formulations of “Alum”. Vaccine 2013, 31, 653–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum Adjuvant: Some of the Tricks of the Oldest Adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Oleszycka, E.; Lavelle, E.C. Immunomodulatory Properties of the Vaccine Adjuvant Alum. Curr. Opin. Immunol. 2014, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kwissa, M.; Lindblad, E.B.; Schirmbeck, R.; Reimann, J. Codelivery of a DNA Vaccine and a Protein Vaccine with Aluminum Phosphate Stimulates a Potent and Multivalent Immune Response. J. Mol. Med. 2003, 81, 502–510. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Dewitt, C.M.; Chastain, M.; Friedman, A.; Donnelly, J.J.; McClements, W.L.; Caulfield, M.J.; Bohannon, K.E.; Volkin, D.B.; Evans, R.K. Enhancement of DNA Vaccine Potency Using Conventional Aluminum Adjuvants. Vaccine 1999, 18, 18–28. [Google Scholar] [CrossRef]
- Yan, W.; Faisal, S.M.; McDonough, S.P.; Divers, T.J.; Barr, S.C.; Chang, C.F.; Pan, M.J.; Chang, Y.F. Immunogenicity and Protective Efficacy of Recombinant Leptospira Immunoglobulin-like Protein B (RLigB) in a Hamster Challenge Model. Microbes Infect. 2009, 11, 230–237. [Google Scholar] [CrossRef]
- Conrad, N.L.; McBride, F.W.D.C.; Souza, J.D.; Silveira, M.M.; Felix, S.; Mendonc, K.S.; Santos, C.S.; Athanazio, D.A.; Medeiros, M.A.; Reis, M.G.; et al. LigB Subunit Vaccine Confers Sterile Immunity against Challenge in the Hamster Model of Leptospirosis. PLoS Negl. Trop. Dis. 2017, 11, e0005441. [Google Scholar] [CrossRef][Green Version]
- Fernandes, L.G.V.; Teixeira, A.F.; Filho, A.F.S.; Souza, G.O.; Vasconcellos, S.A.; Heinemann, M.B.; Romero, E.C.; Nascimento, A.L.T.O. Immune Response and Protective Profile Elicited by a Multi-Epitope Chimeric Protein Derived from Leptospira interrogans. Int. J. Infect. Dis. 2017, 57, 61–69. [Google Scholar] [CrossRef][Green Version]
- Stills, H.F. Adjuvants and Antibody Production: Dispelling the Myths Associated with Freund’s Complete and Other Adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef][Green Version]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef]
- Miller, L.H.; Saul, A.; Mahanty, S. Revisiting Freund’s Incomplete Adjuvant for Vaccines in the Developing World. Trends Parasitol. 2005, 21, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Billiau, A.; Matthys, P. Modes of Action of Freund’s Adjuvants in Experimental Models of Autoimmune Diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.M.; Safhi, M.M.; Kannadasan, M.; Sukumaran, N. Vaccine Adjuvants—Current Status and Prospects on Controlled Release Adjuvancity. Saudi Pharm. J. 2011, 19, 197–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, É.F.; Medeiros, M.A.; McBride, A.J.A.; Matsunaga, J.; Esteves, G.S.; Ramos, J.G.R.; Santos, C.S.; Croda, J.; Homma, A.; Dellagostin, O.A.; et al. The Terminal Portion of Leptospiral Immunoglobulin-like Protein LigA Confers Protective Immunity against Lethal Infection in the Hamster Model of Leptospirosis. Vaccine 2007, 25, 6277–6286. [Google Scholar] [CrossRef]
- Coutinho, M.L.; Choy, H.A.; Kelley, M.M.; Matsunaga, J.; Babbitt, J.T.; Lewis, M.S.; Aleixo, J.A.G.; Haake, D.A. A LigA Three-Domain Region Protects Hamsters from Lethal Infection by Leptospira interrogans. PLoS Negl. Trop. Dis. 2011, 5, 1–10. [Google Scholar] [CrossRef]
- Evangelista, K.V.; Lourdault, K.; Matsunaga, J.; Haake, D.A. Immunoprotective Properties of Recombinant LigA and LigB in a Hamster Model of Acute Leptospirosis. PLoS ONE 2017, 12, e0180004. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Lyu, J.; Xu, J.; Zhang, P.; Zhang, S.; Chen, Y.; Wang, Y.; Chen, G. The Related Mechanism of Complete Freund’s Adjuvant-Induced Chronic Inflammation Pain Based on Metabolomics Analysis. Biomed. Chromatogr. 2021, 35, e5020. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef][Green Version]
- Ong, G.H.; Lian, B.S.X.; Kawasaki, T.; Kawai, T. Exploration of Pattern Recognition Receptor Agonists as Candidate Adjuvants. Front. Cell. Infect. Microbiol. 2021, 0, 968. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine Adjuvants: Understanding the Structure and Mechanism of Adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR Signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sbrogio-Almeida, M.E.; Mosca, T.; Massis, L.M.; Abrahamsohn, I.A.; Ferreira, L.C.S. Host and Bacterial Factors Affecting Induction of Immune Responses to Flagellin Expressed by Attenuated Salmonella Vaccine Strains. Infect. Immun. 2004, 72, 2546–2555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akira, S.; Takeda, K. Toll-like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Monaris, D.; Sbrogio-Almeida, M.E.; Dib, C.C.; Canhamero, T.A.; Souza, G.O.; Vasconcellos, S.A.; Ferreira, L.C.S.; Abreu, P.A.E. Protective Immunity and Reduced Renal Colonization Induced by Vaccines Containing Recombinant Leptospira interrogans Outer Membrane Proteins and Flagellin Adjuvant. Clin. Vaccine Immunol. 2015, 22, 965–973. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in Xanthan Gum Production, Modifications and Its Applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Takeuchi, A.; Kamiryou, Y.; Yamada, H.; Eto, M.; Shibata, K.; Haruna, K.; Naito, S.; Yoshikai, Y. Oral Administration of Xanthan Gum Enhances Antitumor Activity through Toll-like Receptor 4. Int. Immunopharmacol. 2009, 9, 1562–1567. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. β-Glucan Recognition by the Innate Immune System. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Silveira, M.M.; Conceição, F.R.; Mendonça, M.; Moreira, G.M.S.G.; Pouey Da Cunha, C.E.; Rizzi, C.; Hartwig, D.D.; da Silveira Moreira, A.; Tondo Vendrusculo, C.; Moreira, A.N. Biopolymer Xanthan: A New Adjuvant for DNA Vaccines. Braz. Arch. Biol. Technol. 2020, 63, e20190090. [Google Scholar] [CrossRef]
- Bacelo, K.L.; Hartwig, D.D.; Seixas, F.K.; Schuch, R.; Moreira, A.D.S.; Amaral, M.; Collares, T.; Vendrusculo, C.T.; McBride, A.J.A.; Dellagostin, O.A. Xanthan Gum as an Adjuvant in a Subunit Vaccine Preparation against Leptospirosis. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Oliveira, T.L.; Bacelo, K.L.; Schuch, R.A.; Seixas, F.K.; Collares, T.; Rodrigues, O.E.D.; Vargas, J.; do Nascimento, R.O.; Dellagostin, O.A.; Hartwig, D.D. Immune Response in Hamsters Immunised with a Recombinant Fragment of LigA from Leptospira interrogans, Associated with Carrier Molecules. Mem. Inst. Oswaldo Cruz 2016, 111, 712–716. [Google Scholar] [CrossRef][Green Version]
- da Hora, V.P.; Conceição, F.R.; Dellagostin, O.A.; Doolan, D.L. Non-Toxic Derivatives of LT as Potent Adjuvants. Vaccine 2011, 29, 1538–1544. [Google Scholar] [CrossRef]
- Bignon, A.; Watt, A.P.; Linterman, M.A. Escherichia coli Heat-Labile Enterotoxin B Limits T Cells Activation by Promoting Immature Dendritic Cells and Enhancing Regulatory T Cell Function. Front. Immunol. 2017, 15, 560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghazali-Bina, M.; Pourmand, M.R.; Mirshafiey, A.; Bakhtiari, R.; Khaledi, A.; Kazemian, H.; Afshar, D.; Getso, M.I.; Eshraghi, S. Vaccine Potential of Lena and Lcpa Proteins of Leptospira interrogans in Combination with Escherichia coli Heat-Labile Enterotoxin, b Subunit (LTB). Iran. J. Microbiol. 2019, 11, 39–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheinberg, D.A.; McDevitt, M.R.; Dao, T.; Mulvey, J.J.; Feinberg, E.; Alidori, S. Carbon Nanotubes as Vaccine Scaffolds. Adv. Drug Deliv. Rev. 2013, 65, 2016–2022. [Google Scholar] [CrossRef][Green Version]
- Niyogi, S.; Hamon, M.A.; Hu, H.; Zhao, B.; Bhowmik, P.; Sen, R.; Itkis, M.E.; Haddon, R.C. Chemistry of Single-Walled Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1105–1113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Hartwig, D.D.; Bacelo, K.L.; Oliveira, T.L.; Schuch, R.; Seixas, F.K.; Collares, T.; Rodrigues, O.; Hartleben, C.P.; Dellagostin, O.A. The Use of Halloysite Clay and Carboxyl-Functionalised Multi-Walled Carbon Nanotubes for Recombinant LipL32 Antigen Delivery Enhanced the IgG Response. Mem. Inst. Oswaldo Cruz 2015, 110, 134–137. [Google Scholar] [CrossRef][Green Version]
- Lin, X.; Xiao, G.; Luo, D.; Kong, L.; Chen, X.; Sun, D.; Yan, J. Chimeric Epitope Vaccine against Leptospira interrogans Infection and Induced Specific Immunity in Guinea Pigs. BMC Microbiol. 2016, 16, 241. [Google Scholar] [CrossRef][Green Version]
- Techawiwattanaboon, T.; Barnier-Quer, C.; Palaga, T.; Jacquet, A.; Collin, N.; Sangjun, N.; Komanee, P.; Piboonpocanun, S.; Patarakul, K. Reduced Renal Colonization and Enhanced Protection by Leptospiral Factor H Binding Proteins as a Multisubunit Vaccine Against Leptospirosis in Hamsters. Vaccines 2019, 7, 95. [Google Scholar] [CrossRef][Green Version]
- Varma, V.P.; Kadivella, M.; Kumar, A.; Kavela, S.; Faisal, S.M. LigA Formulated in AS04 or Montanide ISA720VG Induced Superior Immune Response Compared to Alum, Which Correlated to Protective Efficacy in a Hamster Model of Leptospirosis. Front. Immunol. 2022, 13, 985802. [Google Scholar] [CrossRef]
- Ptak, C.P.; Akif, M.; Hsieh, C.L.; Devarajan, A.; He, P.; Xu, Y.; Oswald, R.E.; Chang, Y.F. Comparative Screening of Recombinant Antigen Thermostability for Improved Leptospirosis Vaccine Design. Biotechnol. Bioeng. 2019, 116, 260–271. [Google Scholar] [CrossRef]
- Maia, M.A.C.; Bettin, E.B.; Barbosa, L.N.; de Oliveira, N.R.; Bunde, T.T.; Pedra, A.C.K.; Rosa, G.A.; Rosa, E.E.B.; Neto, A.C.P.S.; Dellagostin, O.A.; et al. Challenges for the Development of a Universal Vaccine against Leptospirosis Revealed by the Evaluation of 22 Vaccine Candidates. Front. Cell. Infect. Microbiol. 2022, 12, 940966. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Cavenague, M.F.; Kochi, L.T.; Fernandes, L.G.; Souza, G.O.; Francisco, A.; Filho, D.S.; Vasconcellos, S.A.; Heinemann, M.B.; Nascimento, A.L.T.O. Immunoprotective Activity Induced by Leptospiral Outer Membrane Proteins in Hamster Model of Acute Leptospirosis. Front. Immunol. 2020, 11, 568694. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K. New Disease Old Vaccine: Is Recombinant BCG Vaccine an Answer for COVID-19? Cell. Immunol. 2020, 356. [Google Scholar] [CrossRef] [PubMed]
- Mouhoub, E.; Domenech, P.; Ndao, M.; Reed, M.B. The Diverse Applications of Recombinant BCG-Based Vaccines to Target Infectious Diseases Other Than Tuberculosis: An Overview. Front. Microbiol. 2021, 12, 3199. [Google Scholar] [CrossRef] [PubMed]
- Naiman, B.M.; Alt, D.; Bolin, C.A.; Zuerner, R.; Baldwin, C.L. Protective Killed Leptospira borgpetersenii Vaccine Induces Potent Th1 Immunity Comprising Responses by CD4 and γδ T Lymphocytes. Infect. Immun. 2001, 69, 7550–7558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, R.A.; Blumerman, S.; Gay, C.; Bolin, C.; Duby, R.; Baldwin, C.L. Comparison of Three Different Leptospiral Vaccines for Induction of a Type 1 Immune Response to Leptospira borgpetersenii Serovar Hardjo. Vaccine 2003, 21, 4448–4458. [Google Scholar] [CrossRef]
- Santecchia, I.; Vernel-Pauillac, F.; Rasid, O.; Quuintin, J.; Gomes-Solecki, M.; Bonecaid, I.G.; Wertsid, C. Innate Immune Memory through TLR2 and NOD2 Contributes to the Control of Leptospira interrogans Infection. PLoS Pathog. 2019, 15, e1007811. [Google Scholar] [CrossRef]
- Prapong, S.; Tansiri, Y.; Sritrakul, T.; Sripattanakul, S.; Sopitthummakhun, A.; Katzenmeier, G.; Hsieh, C.L.; McDonough, S.P.; Prapong, T.; Chang, Y.F. Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates. Trop Med. Infect. Dis. 2022, 8, 6. [Google Scholar] [CrossRef]
- Llanos Salinas, S.P.; Castillo Sánchez, L.O.; Castañeda Miranda, G.; Rodríguez Reyes, E.A.; Ordoñez López, L.; Mena Bañuelos, R.; Alcaraz Sosa, L.E.; Núñez Carrera, M.G.; José Manuel, R.O.; Carmona Gasca, C.A.; et al. GspD, The Type II Secretion System Secretin of Leptospira, Protects Hamsters against Lethal Infection with a Virulent L. interrogans Isolate. Vaccines 2020, 8, 759. [Google Scholar] [CrossRef]
| Vaccine Approaches | Challenge Parameters | Protection Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antigen | Expression/ Delivery Platform | Dose a | Adjuvant b | Via c | Model | LD50 d | Type e | Vaccine Protection f | Negative Control Lethality g | Sterile Immunity | Experiment Repetitions h | Ref. | |
| Culture | qPCR | ||||||||||||
| LipL32 | |||||||||||||
| LipL32full | Adenovirus (Vectorized) | 3×/109 PFU/21 d | NA | IM | Gerbils | NA | HE | 86.6% (26/30) | 51% (17/33) | NA | NA | 2 | [70] |
| E. coli (Subunit) | 3×/50 µg/14 d | FA | SC | Gerbils | NA | HE | 0% (0/15) | 86.6% (13/15) | NA | NA | 2 | [29] | |
| Alum + QS21 | 50% (7/14) | 40% (6/15) | |||||||||||
| pcDNA (aut) (DNA) | 2×/100 µg/21 d | NA | IM | 60% (9/15) | 65% (13/20) | ||||||||
| pcDNA (grip) (DNA) | NA | ||||||||||||
| LipL32full | E. coli (Subunit) | 2×/43.5 µg/14 d | LTB | IM | Hamster | 5× | HO | 80–87% (12/15–13/15) | 60–73% (9/15–11/15) | NA | NA | 3 | [28] |
| LipL32full | rBCG (Vectorized) | 2×/106 CFU/21 d | NA | IP | Hamster | NA | HO | 12.5–55.9% (1/8–19/34) | 88% (30/34) | Yes | NA | 3 | [49] |
| LipL32full | E. coli (Subunit) | 1×/868 pmol | Alum | SC | Hamster | NA | HO | 20% (1/5) | 100% (5/5) | Yes | NA | 1 | [27] |
| LipL32full | E. coli (Subunit) | 2×/30 µg/14 d | Alum | NA | Hamster | NA | HO | 0% (0/10) | 80–100% (2/10–10/10) | NA | NA | 1 | [26] |
| LipL32155–200 | |||||||||||||
| LipL32full | E. coli (Subunit) | 2×/50 µg/14 d | Alum | NA | Hamster | NA | HO | 0% (0/6) | NA | NA | NA | 1 | [121] |
| COOH-MWCNTs | |||||||||||||
| HNTs | |||||||||||||
| LipL32full | rBCG (Vectorized) | 2×/106 CFU/21 d | NA | SC | Hamster | 5× | HO | 90–100% (9/10–10/10) | 100% (10/10) | Yes | Yes | 2 | [51] |
| Chimeras | |||||||||||||
| LemA28–157-LigAni943–1224 LipL32224–272-LemA28–157 | rBCG (Vectorized) | 2×/106 CFU/21 d | NA | SC | Hamster | 5× | HO | 100% (10/10) | 100% (10/10) | Yes | Yes | 2 | [51] |
| 100% (10/10) | 2 | ||||||||||||
| LipL32224–272-LemA28–157-LigAni943–1224 | rBCG (Vectorized) | 2×/106 CFU/21 d | NA | SC | Hamster | 5× | HO | 80–100% (8/10–10/10) | 100% (10/10) | Yes | Yes | 1 | [50] |
| LigAni943–1224-LigBrep131–645 | E. coli (Subunit) E. coli (Subunit) pTARGET-chimera (DNA) Prime-boost * | 2×/50 µg/14 d | Alum | IM | Hamster | 5× | HO | 100% (8/8) | 100% (8/8) | No | No | 1 | [33] |
| 2×/50 µg/14 d | ISA 50 V2 | 100% (8/8) | |||||||||||
| 2×/100 µg/14 d | NA | 25% (2/8) | |||||||||||
| 2×/100 µg DNA + 50 µg protein/14 d | Alum (boost) | 100% (8/8) | |||||||||||
| LigAni631–1224-LigBrep19–672 | E. coli (Subunit) | 3×/100 µg/14 d | FA | SC | Hamster | 500× | HO | 100% (8/8) | 100% (5/5–6/6) | No | NA | 3 | [100] |
| LigAni852–1107- Mce131–207- Lsa45190–250- OmpL1153–221- LipL41213–276 | E. coli (Subunit) | 2×/50 µg/14 d | Alum | SC | Hamster | NA | HO | 33–50% (3/9 –3/6) | 80–100% (8/10–6/6) | No | NA | 2 | [92] |
| MPLA | 50–60% (3/6 –6/10) | ||||||||||||
| DDA | 0% (0/10) | ||||||||||||
| OmpL187–98; 173–191-LipL32133–160; 201–218- LipL2197–112; 176–184 | E. coli (Subunit) | 3×/200 µg/14 d | Alum | SC | guinea pigs | 2× | HO | 80% (4/5) | 100% (5/5) | Yes | NA | 1 | [122] |
| Ligs | |||||||||||||
| LigAni625–1224 | E. coli (Subunit) | 2×/50 µg/14 d | NA | SC | Hamster | 5× | HO | 0% (0/12) | 100% (12/12) | NA | NA | 2 | [114] |
| Alum | 67% (8/12) | ||||||||||||
| COOH-MWCNTs | 0% (0/12) | ||||||||||||
| CpG ODNs | 17% (2/12) | ||||||||||||
| COOH-MWCNTs + CpG ODNs | 17% (2/12) | ||||||||||||
| LigAni625–1229 | E. coli (Subunit) | 2×/50 µg/14 d | NA Alum CpG Xanthan Xanthan + CpG | SC | Hamster | 36× | HO | 0% (0/6) 66.7% (4/6) 16.7% (1/6) 100% (6/6) 100% (6/6) | 83.3–100% (5/6–6/6) | No | NA | 2 | [113] |
| LigAni629–1229 | E. coli (Subunit) | 2×/50 µg/15 d | Alum | SC | Hamster | 100× | HO | 100% (10/10) | 70–100% (7/10–10/10) | No | NA | 2 | [108] |
| LigAni629–1224 | E. coli (Subunit) | 3×/20 µg/14 d | LMQ | SC/ IM | Hamster | 20× | HO | 60% (3/5) | 100% (5/5) | No | NA | 1 | [123] |
| LigAni631–1224 | E. coli (Subunit) | 3×/100 µg/14 d | FA | SC | Hamster | 500× | HO | 100% (8/8) | 100% (5/5–6/6) | No | No | 3 | [100] |
| LigAni631–1224 | E. coli (Subunit) | 3×/100 µg/14 d | FA | SC | Hamster | NA | HO | 100% (8/8) | 100% (0/8) | No | NA | 2 | [99] |
| LigAni631–1033 | 50% (4/8) | ||||||||||||
| LigAni631–851 | 0% (0/8) | ||||||||||||
| LigAni852–1224 | 100% (8/8) | ||||||||||||
| LigAni852–1124 | 100% (8/8) | ||||||||||||
| LigAni943–1224 | 100% (8/8) | ||||||||||||
| LigAni943–1124 | 25% (2/8) | ||||||||||||
| LigAni1034–1224 | 50% (4/8) | ||||||||||||
| LigAni624–1224 | rBCG (Vectorized) | 2×/106 CFU/21 d | NA | SC | Hamster | 5× | HO | 100% (10/10) | 100% (10/10) | Yes | Yes | 2 | [51] |
| LigA-LAV (domains 8-13) | E. coli (Subunit) | 2×/50–25 µg/21 d | Alum AS04 Montanide | SC | Hamster | 100× | HO | 50% (3/6) 67% (4/6) 83% (5/6) | 100% (6/6) | NA | No | 3 | [124] |
| LigAni629–1229 LigBni629–1112 LigBrep1–628 | pTARGET-chimera (DNA) | 2×/100 µg/21 d | Alum | IM | Hamster | 5× | HE | 0% (0/8) | 100% (6/6) | No No Yes | NA | 1 | [74] |
| 0% (0/8) | |||||||||||||
| 62.5% (5/8) | |||||||||||||
| LigBrep19–672 | E. coli (Subunit) | 3×/100 µg/14 d | FA | SC | Hamster | 500× | HO | 37.5% (3/8) | 100% (6/6–8/8) | No | No | 3 | [100] |
| LigBrep131–645 | E. coli (Subunit) | 2×/20–100 µg/14 d | Alum | IM | Hamster | 10× | HO | 85.7–100% (8/10–10/10) | 70–100% (7/10–10/10) | Yes | Yes | 7 | [91] |
| LigBrep1–628 | E. coli (Subunit) pTARGET (DNA) pTARGET (DNA) Prime-boost * | 2×/100 µg/21 d | Alum | IM | Hamster | 5× | HE | 0% (0/6) | 100% (5/5) | Yes | NA | 1 | [32] |
| Alum | 40% (2/5) | ||||||||||||
| NA | 0% (0/6) | ||||||||||||
| Alum | 83.3% (5/6) | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, N.R.; Santos, F.D.S.; dos Santos, V.A.C.; Maia, M.A.C.; Oliveira, T.L.; Dellagostin, O.A. Challenges and Strategies for Developing Recombinant Vaccines against Leptospirosis: Role of Expression Platforms and Adjuvants in Achieving Protective Efficacy. Pathogens 2023, 12, 787. https://doi.org/10.3390/pathogens12060787
de Oliveira NR, Santos FDS, dos Santos VAC, Maia MAC, Oliveira TL, Dellagostin OA. Challenges and Strategies for Developing Recombinant Vaccines against Leptospirosis: Role of Expression Platforms and Adjuvants in Achieving Protective Efficacy. Pathogens. 2023; 12(6):787. https://doi.org/10.3390/pathogens12060787
Chicago/Turabian Stylede Oliveira, Natasha Rodrigues, Francisco Denis Souza Santos, Vitória Adrielly Catschor dos Santos, Mara Andrade Colares Maia, Thaís Larré Oliveira, and Odir Antônio Dellagostin. 2023. "Challenges and Strategies for Developing Recombinant Vaccines against Leptospirosis: Role of Expression Platforms and Adjuvants in Achieving Protective Efficacy" Pathogens 12, no. 6: 787. https://doi.org/10.3390/pathogens12060787
APA Stylede Oliveira, N. R., Santos, F. D. S., dos Santos, V. A. C., Maia, M. A. C., Oliveira, T. L., & Dellagostin, O. A. (2023). Challenges and Strategies for Developing Recombinant Vaccines against Leptospirosis: Role of Expression Platforms and Adjuvants in Achieving Protective Efficacy. Pathogens, 12(6), 787. https://doi.org/10.3390/pathogens12060787









