Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology
Abstract
1. Introduction
2. Vertebrate Animal Models
2.1. Humans
2.1.1. Neisseria gonorrhoeae Studies
2.1.2. Neisseria lactamica Studies
2.1.3. Neisseria meningitidis Vaccine Studies
2.2. Non-Human Primates (NHPs)
2.2.1. Chimpanzee (Pan troglodytes)
2.2.2. Rhesus Macaque (Macaca mulatta)
2.2.3. Other Monkeys
2.3. Mouse
2.3.1. Mouse Models for Pathobiology Research
- Studies with N. gonorrhoeae.
| Compounds Tested in the Female Mouse Model of Neisseria gonorrhoeae Genital Tract Infection | Reference | |
|---|---|---|
| Antimicrobial | JSF-2659 [8-(6-fluoro-8-(methylamino)-2-((2-methylpyrimidin-5-yl)oxy)-9H-pyrimido[4,5-b]indol-4-yl)-2-oxa-8-azaspiro[4.5]decan-3-yl))methanol] | [130] |
| Moenomycin (phosphoglycolipid targeting peptidoglycan glycosyltransferase) | [131] | |
| PTC-847 and PTC-672 active on ribonucleotide reductase | [132] | |
| Auranofin (gold-containing) | [133] | |
| Acetazolamide (carbonic anhydrase inhibitor) | [134] | |
| TP0480066, a topoisomerase inhibitor | [135] | |
| SCH-79797 | [136] | |
| REDX05931 (tricyclic topoisomerase inhibitor) | [137] | |
| Resorufin pentyl ether (analogue of resazurin) | [138] | |
| Antibiotic | MBX-4132 (acylaminooxadiazoles) | [139] |
| Cephalosporins | [140] | |
| Cefixime and ceftriaxone | [141,142] | |
| Fluoroquinolone | [143] | |
| Vaginal microbicide | Semen-derived enhancer of viral infection (SEVI) | [144] |
| Lactobacillus crispatus producing hydrogen peroxide | [145] | |
| Porphyrin based compound with gallium | [146] | |
| CarraGuard, Ushercell, [poly]sodium 4-styrene sulfonate (T-PSS), PRO 2000, ACIDFORM, cellulose acetate phthalate (CAP), and BufferGel | [147] | |
| Immunotherapeutic | Complement factor H-based immunotherapeutic | [148,149,150] |
| C4BP-IgM protein | [151] | |
| Aminomethyl spectinomycins (semisynthetic analogues of spectinomycin) | [152] | |
| Anti-transforming growth factor β (TGF-β) antibody | [153] | |
| Vaccine candidate | Meningococcal 4CmenB | [154,155] |
| TMCP2 (peptide vaccine mimicking 2C7 oligosaccharide epitope in gonococcal LOS) | [156,157] | |
| Detoxified meningococcal outer membrane vesicle (dOMV) deficient in PorA, PorB, and RmpM | [158] | |
| MetQ lipoprotein formulated with CpG | [159] | |
| Hybrid transferrin binding protein B (from Haemophilus parasuis) with neisserial TbpA loop 10 | [160] | |
| Recombinant human IgG1 chimeric variant of MAb 2C7 containing an E430G Fc modification (2C7_E430G) | [161,162] | |
| MAb 2C7 | [163] | |
| 2C7 epitope in multiple antigen peptide formulation | [164] | |
| Intranasal gonococcal OMV plus microencapsulated interleukin-12 (IL-12) | [165] | |
| Gonococcal OMVs plus microencapsulated IL-12 | [166] | |
| Gonococcal OMV (intranasally administered) | [167] | |
| Gonococcal PI-B synthetic peptide | [168] | |
| rrPorB-Virus Replicon Particles prime and boost (rrPorB), both administered via footpad | [169] | |
| Opa-loop specific antibodies (passive protection trial) | [170] | |
- Studies with N. meningitidis.
2.3.2. Mouse Models for Vaccine Research
2.4. Guinea Pig
2.5. Rabbit
2.6. Rat
2.6.1. Studies with N. gonorrhoeae
2.6.2. Studies with N. meningitidis
2.7. Horse
2.8. Chicken Embryo Model
2.9. Pig (Sus domesticus)
2.10. Zebrafish (Danio rerio)
3. Invertebrate Animal Models
3.1. Galleria Mellonella Larvae
3.2. Can Neisseria Infect Other Insects?
4. Can the 3Rs Be Applied to Neisseria Research?
In Vitro 3D Models and Organoids
5. Prospectus and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, G.; Tang, C.M.; Exley, R.M. Non-pathogenic Neisseria: Members of an abundant, multi-habitat, diverse genus. Microbiol. Read. Engl. 2015, 161, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.S. Location, Location, Location-Commensalism, Damage and Evolution of the Pathogenic Neisseria. J. Mol. Biol. 2019, 431, 3010–3014. [Google Scholar] [CrossRef]
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.S.; Criss, A.K. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: Neutrophilic host response, sustained infection, and clinical sequelae. Curr. Opin. Hematol. 2018, 25, 13–21. [Google Scholar] [CrossRef]
- Schoen, C.; Blom, J.; Claus, H.; Schramm-Gluck, A.; Brandt, P.; Muller, T.; Goesmann, A.; Joseph, B.; Konietzny, S.; Kurzai, O.; et al. Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 2008, 105, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; van Deuren, M. Classification and pathogenesis of meningococcal infections. In Neisseria Meningitidis: Advanced Methods and Protocols; Christodoulides, M., Ed.; Humana Press: New York, NY, USA, 2012; Volume 799, pp. 21–35. [Google Scholar]
- Humbert, M.V.; Christodoulides, M. Atypical, Yet Not Infrequent, Infections with Neisseria Species. Pathogens 2019, 9, 10. [Google Scholar] [CrossRef]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019, 97, 548–562P. [Google Scholar] [CrossRef]
- Neisser, A.L. Über eine der Gonorrhoe eigentümliche Micrococcusform. Cent. Med. Wissensch 1879, 17, 497–500. [Google Scholar]
- Benedek, T.G. Gonorrhea and the beginnings of clinical research ethics. Perspect. Biol. Med. 2005, 48, 54–73. [Google Scholar] [CrossRef]
- Harkness, A.H. The pathology of gonorrhoea. Br. J. Vener. Dis. 1948, 24, 137–147. [Google Scholar]
- Hunter, J. A Treatise on Venereal Disease; Longman: London, UK, 1835; Volume 2. [Google Scholar]
- Weiss, F. Le Microbe du Pus Blennorrhagique [The Microbe of Blenorrheal Pus]. Nancy (Nancy: Impr. Paul Sordoillet) 1880. [Google Scholar]
- Leistikow, L. Über bacterien bei den venerischen krankheiten [On bacteria in the venereal diseases]. Charite-Ann. 1882, 7, 750–772. [Google Scholar]
- Leistikow, L. Resultate seiner Untersuchungen uber die Tripperbacterien. Berl. Klin. Wochenschr. 1882, 19, 500. [Google Scholar]
- Bokai, A. Uber das contagium der acuten blennorhoe. Allge Med. Cent.-Zeit 1880, 49, 900–903. [Google Scholar]
- Bockhart, M. Beitrag zur aetiologie und pathologie des Harnrohrentrippers. Vrtlschr. Derm. Syph. 1883, 10, 3–18. [Google Scholar] [CrossRef]
- von Bumm, E. Der mikro-organismus der gonorrhöischen schleimhaut-erkrankungen, gonococcus Neisser [The microorganism of gonorrhea mucosal disease, “Gonococcus Neisser”]. Dtsch. Med. Wochenschr. 1885, 11, 508–509. [Google Scholar]
- von Bumm, E. Der Mikro-Organismus der Gonorrhoischen Schleimhaut-Erkrankungen ‘Gonococcus Neisser’. Nach Untersuchungen Beim Weibe und an der Conjunctiva der Neugeborenen Dargestellt [The Microorganism of Gonorrhea Mucosal Disease “Gonococcus Neisser”. After Investigations In the Female and the Conjunctiva of the Newborn Shown]; Bergmann: Weisbaden, Germany, 1885. [Google Scholar]
- Wertheim, E. Reinzuchtung des Gonococcous Neisser mittels des Plattenverfahrens. Dtsch. Med. Wochenschr. 1891, 17, 1351–1352. [Google Scholar] [CrossRef]
- Steinschneider. Uber die culture der gonokokken. Berl. Klin. Wochenschr. 1893, 30, 696–699, 728–731. [Google Scholar]
- Finger, E.; Ghon, A.; Schlagenhaufer, F. Beitrage zur biologie des gonococcus und zur pathologischen anatomie der gonorrhoischen processes. Arch. Dermatol. Syph. 1894, 28, 277–344. [Google Scholar] [CrossRef]
- Martin, E. Vulvo-vaginitis in children. J. Cutan G-U Med. 1892, 10, 415–427. [Google Scholar] [CrossRef]
- Heiman, H. Clinical and bacteriological study of the gonococcus (Neisser) as found in the male urethra and in the vuvlo-vaginal tract of children. Med. Rec. 1895, 47, 769–778. [Google Scholar]
- Jundell, I.; Ahman, C. Uber die reinzuchtung des gonococcus Neisser. Arch. Dermatol. Syph. 1897, 38, 59–68. [Google Scholar] [CrossRef]
- Waltmann, A.; Duncan, J.A.; Pier, G.B.; Cywes-Bentley, C.; Cohen, M.S.; Hobbs, M.M. Experimental Urethral Infection with Neisseria gonorrhoeae; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–17. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Duncan, J.A. Experimental Human Infection with Neisseria gonorrhoeae. Methods Mol. Biol. 2019, 1997, 431–452. [Google Scholar] [CrossRef] [PubMed]
- Brinton, C.; Wood, S.; Brwon, A.; Labik, A.; Bryan, J.; Lee, S.; Polen, S.; Tramont, E.; Sadoff, J.; Zollinger, W. The development of a Neisserial pilus vaccine for gonorrhea and meningococcal meningitis. Sem. Infect. Dis. Vol. IV Bact. Vaccines 1982, 4, 140–159. [Google Scholar]
- Tramont, E.C.; Boslego, J.W. Pilus vaccines. Vaccine 1985, 3, 3–10. [Google Scholar] [CrossRef]
- Boslego, J.W.; Tramont, E.C.; Chung, R.C.; Mcchesney, D.G.; Ciak, J.; Sadoff, J.C.; Piziak, M.V.; Brown, J.D.; Brinton, C.C.; Wood, S.W.; et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 1991, 9, 154–162. [Google Scholar] [CrossRef]
- Gulati, S.; Su, X.; Le, W.; Zheng, B.; Madico, G.; Ram, S.; Rice, P. Human studies in gonococcal infection: Do falied vaccine trials and clinical/transmission studies shed light? In Proceedings of the 20th International Pathogenic Neisseria Conference, Manchester, UK, 4–9 September 2016.
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu. Rev. Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Sparling, P.F.; Cohen, M.S.; Shafer, W.M.; Deal, C.D.; Jerse, A.E. Experimental Gonococcal Infection in Male Volunteers: Cumulative Experience with Neisseria gonorrhoeae Strains FA1090 and MS11mkC. Front. Microbiol. 2011, 2, 123. [Google Scholar] [CrossRef]
- Kellogg, D.S., Jr.; Peacock, W.L., Jr.; Deacon, W.E.; Brown, L.; Pirkle, D.I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 1963, 85, 1274–1279. [Google Scholar] [CrossRef]
- Isbey, S.F.; Alcorn, T.M.; Davis, R.H.; Haizlip, J.; Leone, P.A.; Cohen, M.S. Characterisation of Neisseria gonorrhoeae in semen during urethral infection in men. Genitourin. Med. 1997, 73, 378–382. [Google Scholar] [CrossRef]
- Ramsey, K.H.; Schneider, H.; Cross, A.S.; Boslego, J.W.; Hoover, D.L.; Staley, T.L.; Kuschner, R.A.; Deal, C.D. Inflammatory cytokines produced in response to experimental human gonorrhea. J. Infect. Dis. 1995, 172, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.A.; Schneider, H.; Lindstrom, J.A.; Boslego, J.W.; Warren, R.A.; Van de Verg, L.; Deal, C.D.; McClain, J.B.; Griffiss, J.M. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex. Transm. Dis. 2001, 28, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Griffiss, J.M.; Boslego, J.W.; Hitchcock, P.J.; Zahos, K.M.; Apicella, M.A. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J. Exp. Med. 1991, 174, 1601–1605. [Google Scholar] [CrossRef]
- Schneider, H.; Cross, A.S.; Kuschner, R.A.; Taylor, D.N.; Sadoff, J.C.; Boslego, J.W.; Deal, C.D. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J. Infect. Dis. 1995, 172, 180–185. [Google Scholar] [CrossRef]
- Schneider, H.; Schmidt, K.A.; Skillman, D.R.; Van De Verg, L.; Warren, R.L.; Wylie, H.J.; Sadoff, J.C.; Deal, C.D.; Cross, A.S. Sialylation lessens the infectivity of Neisseria gonorrhoeae MS11mkC. J. Infect. Dis. 1996, 173, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Jerse, A.E.; Cohen, M.S.; Drown, P.M.; Whicker, L.G.; Isbey, S.F.; Seifert, H.S.; Cannon, J.G. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 1994, 179, 911–920. [Google Scholar] [CrossRef]
- Schmidt, K.A.; Deal, C.D.; Kwan, M.; Thattassery, E.; Schneider, H. Neisseria gonorrhoeae MS11mkC opacity protein expression in vitro and during human volunteer infectivity studies. Sex. Transm. Dis. 2000, 27, 278–283. [Google Scholar] [CrossRef]
- Swanson, J.; Robbins, K.; Barrera, O.; Corwin, D.; Boslego, J.; Ciak, J.; Blake, M.S.; Koomey, J.M. Gonococcal pilin variants in experimental gonorrhoea. J. Exp. Med. 1987, 165, 1344–1357. [Google Scholar] [CrossRef]
- Seifert, H.S.; Wright, C.J.; Jerse, A.E.; Cohen, M.S.; Cannon, J.G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Investig. 1994, 93, 2744–2749. [Google Scholar] [CrossRef]
- Wright, C.J.; Jerse, A.E.; Cohen, M.S.; Cannon, J.G.; Seifert, H.S. Nonrepresentative PCR amplification of variable gene sequences in clinical specimens containing dilute, complex mixtures of microorganisms. J. Clin. Microbiol. 1994, 32, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, T.S.; Dempsey, J.A.F.; Cohen, M.S.; Cannon, J.G. Antigenic variation of gonococcal pilin expression in vivo: Analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiol. Read. 2001, 147, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, D.B.; Johnston, D.M.; Koymen, H.O.; Cohen, M.S.; Cannon, J.G. A Neisseria gonorrhoeae immunoglobulin A1 protease mutant is infectious in the human challenge model of urethral infection. Infect. Immun. 1999, 67, 3009–3013. [Google Scholar] [CrossRef]
- Cornelissen, C.N.; Kelley, M.; Hobbs, M.M.; Anderson, J.E.; Cannon, J.G.; Cohen, M.S.; Sparling, P.F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 1998, 27, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.E.; Hobbs, M.M.; Biswas, G.D.; Sparling, P.F. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol. Microbiol. 2003, 48, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Gbesemete, D.; Laver, J.R.; de Graaf, H.; Ibrahim, M.; Vaughan, A.; Faust, S.; Gorringe, A.; Read, R.C. Protocol for a controlled human infection with genetically modified Neisseria lactamica expressing the meningococcal vaccine antigen NadA: A potent new technique for experimental medicine. BMJ Open 2019, 9, e026544. [Google Scholar] [CrossRef]
- Evans, C.M.; Pratt, C.B.; Matheson, M.; Vaughan, T.E.; Findlow, J.; Borrow, R.; Gorringe, A.R.; Read, R.C. Nasopharyngeal colonization by Neisseria lactamica and induction of protective immunity against Neisseria meningitidis. Clin. Infect. Dis. 2011, 52, 70–77. [Google Scholar] [CrossRef]
- Deasy, A.M.; Guccione, E.; Dale, A.P.; Andrews, N.; Evans, C.M.; Bennett, J.S.; Bratcher, H.B.; Maiden, M.C.; Gorringe, A.R.; Read, R.C. Nasal Inoculation of the Commensal Neisseria lactamica Inhibits Carriage of Neisseria meningitidis by Young Adults: A Controlled Human Infection Study. Clin. Infect. Dis. 2015, 60, 1512–1520. [Google Scholar] [CrossRef]
- Dale, A.P.; Theodosiou, A.A.; Gbesemete, D.F.; Guy, J.M.; Jones, E.F.; Hill, A.R.; Ibrahim, M.M.; de Graaf, H.; Ahmed, M.; Faust, S.N.; et al. Effect of colonisation with Neisseria lactamica on cross-reactive anti-meningococcal B-cell responses: A randomised, controlled, human infection trial. Lancet Microbe 2022, 3, e931–e943. [Google Scholar] [CrossRef]
- Pandey, A.; Cleary, D.W.; Laver, J.R.; Gorringe, A.; Deasy, A.M.; Dale, A.P.; Morris, P.D.; Didelot, X.; Maiden, M.C.J.; Read, R.C. Microevolution of Neisseria lactamica during nasopharyngeal colonisation induced by controlled human infection. Nat. Commun. 2018, 9, 4753. [Google Scholar] [CrossRef]
- Laver, J.R.; Gbesemete, D.; Dale, A.P.; Pounce, Z.C.; Webb, C.N.; Roche, E.F.; Guy, J.M.; Berreen, G.; Belogiannis, K.; Hill, A.R.; et al. A recombinant commensal bacteria elicits heterologous antigen-specific immune responses during pharyngeal carriage. Sci. Transl. Med. 2021, 13, eabe8573. [Google Scholar] [CrossRef]
- Weyand, N.J. Neisseria models of infection and persistence in the upper respiratory tract. Pathog. Dis. 2017, 75, ftx031. [Google Scholar] [CrossRef]
- Reenstierna, J. Impfversuche an Affen mit dem gonococcus Neisser. Arch. Derm. Syph. 1916, 121, 286. [Google Scholar] [CrossRef]
- Finklestein, F.; Timochina, M. Experimentelle Gonorrhoe. Sov. Vestn. Vener. Derm. 1935, 4, 97. [Google Scholar]
- Lucas, C.T.; Chandler, F., Jr.; Martin, J.E., Jr.; Schmale, J.D. Transfer of gonococcal urethritis from man to chimpanzee. An animal model for gonorrhea. JAMA 1971, 216, 1612–1614. [Google Scholar] [CrossRef]
- Brown, W.J.; Lucas, C.T.; Kuhn, U.S. Gonorrhoea in the chimpanzee. Infection with laboratory-passed gonococci and by natural transmission. Br. J. Vener. Dis. 1972, 48, 177–178. [Google Scholar] [CrossRef]
- Brown, W.J.; Kraus, S.J.; Arko, R.J. Chimpanzee urethral meningococci. Br. J. Vener. Dis. 1973, 49, 88. [Google Scholar] [CrossRef] [PubMed]
- Kraus, S.J.; Brown, W.J.; Arko, R.J. Acquired and natural immunity to gonococcal infection in chimpanzees. J. Clin. Investig. 1975, 55, 1349–1356. [Google Scholar] [CrossRef]
- Arko, R.J.; Kraus, S.J.; Brown, W.J.; Buchanan, T.M.; Kuhn, U.S.G. Neisseria gonorrhoeae: Effects of systemic immunisation on resistance of chimpanzees to urethral infection. J. Infect. Dis. 1974, 130, 160–164. [Google Scholar] [CrossRef]
- Arko, R.J.; Duncan, W.P.; Brown, W.J.; Peacock, W.L.; Tomizawa, T. Immunity in infection with Neisseria gonorrhoeae: Duration and serological response in the chimpanzee. J. Infect. Dis. 1976, 133, 441–447. [Google Scholar] [CrossRef]
- Vedros, N.A.; Hoke, C.; Chun, P. Neisseria-Macacae Sp-Nov, a New Neisseria Species Isolated from the Oropharynges of Rhesus-Monkeys (Macaca-Mulatta). Int. J. Syst. Bacteriol. 1983, 33, 515–520. [Google Scholar] [CrossRef]
- Weyand, N.J.; Wertheimer, A.M.; Hobbs, T.R.; Sisko, J.L.; Taku, N.A.; Gregston, L.D.; Clary, S.; Higashi, D.L.; Biais, N.; Brown, L.M.; et al. Neisseria infection of rhesus macaques as a model to study colonization, transmission, persistence, and horizontal gene transfer. Proc. Natl. Acad. Sci. USA 2013, 110, 3059–3064. [Google Scholar] [CrossRef]
- Thapa, E.; Knauss, H.M.; Colvin, B.A.; Fischer, B.A.; Weyand, N.J. Persistence Dynamics of Antimicrobial-Resistant Neisseria in the Pharynx of Rhesus Macaques. Antimicrob. Agents Chemother. 2020, 64, e02232-19. [Google Scholar] [CrossRef] [PubMed]
- Granoff, D.M.; Costa, I.; Konar, M.; Giuntini, S.; Van Rompay, K.K.; Beernink, P.T. Binding of Complement Factor H (FH) Decreases Protective Anti-FH Binding Protein Antibody Responses of Infant Rhesus Macaques Immunized with a Meningococcal Serogroup B Vaccine. J. Infect. Dis. 2015, 212, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Konar, M.; Beernink, P.T.; Granoff, D.M. A Newly-Identified Polymorphism in Rhesus Macaque Complement Factor H Modulates Binding Affinity for Meningococcal FHbp. PLoS ONE 2015, 10, e0135996. [Google Scholar] [CrossRef] [PubMed]
- Beernink, P.T.; Shaughnessy, J.; Stefek, H.; Ram, S.; Granoff, D.M. Heterogeneity in rhesus macaque complement factor H binding to meningococcal factor H binding protein (FHbp) informs selection of primates to assess immunogenicity of FHbp-based vaccines. Clin. Vaccine Immunol. 2014, 21, 1505–1511. [Google Scholar] [CrossRef]
- Ngampasutadol, J.; Ram, S.; Blom, A.M.; Jarva, H.; Jerse, A.E.; Lien, E.; Goguen, J.; Gulati, S.; Rice, P.A. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc. Natl. Acad. Sci. USA 2005, 102, 17142–17147. [Google Scholar] [CrossRef]
- Jarva, H.; Ngampasutadol, J.; Ram, S.; Rice, P.A.; Villoutreix, B.O.; Blom, A.M. Molecular characterization of the interaction between porins of Neisseria gonorrhoeae and C4b-binding protein. J. Immunol. 2007, 179, 540–547. [Google Scholar] [CrossRef]
- Ngampasutadol, J.; Ram, S.; Gulati, S.; Agarwal, S.; Li, C.; Visintin, A.; Monks, B.; Madico, G.; Rice, P.A. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J. Immunol. 2008, 180, 3426–3435. [Google Scholar] [CrossRef]
- Gray-Owen, S.D.; Schryvers, A.B. The interaction of primate transferrins with receptors on bacteria pathogenic to humans. Microb. Pathog. 1993, 14, 389–398. [Google Scholar] [CrossRef]
- Beernink, P.T.; Vianzon, V.; Lewis, L.A.; Moe, G.R.; Granoff, D.M. A Meningococcal Outer Membrane Vesicle Vaccine with Overexpressed Mutant FHbp Elicits Higher Protective Antibody Responses in Infant Rhesus Macaques than a Licensed Serogroup B Vaccine. mBio 2019, 10, e01231-19. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, Z.; Lin, J.; Xu, H.; Herbert, P.; Wang, X.M.; Mehl, J.T.; Ahl, P.L.; Dieter, L.; Russell, R.; et al. Improving the immunogenicity of a trivalent Neisseria meningitidis native outer membrane vesicle vaccine by genetic modification. Vaccine 2016, 34, 4250–4256. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, S.; Beernink, P.T.; Granoff, D.M. Effect of complement Factor H on anti-FHbp serum bactericidal antibody responses of infant rhesus macaques boosted with a licensed meningococcal serogroup B vaccine. Vaccine 2015, 33, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Koeberling, O.; Seubert, A.; Santos, G.; Colaprico, A.; Ugozzoli, M.; Donnelly, J.; Granoff, D.M. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 2011, 29, 4728–4734. [Google Scholar] [CrossRef]
- Devi, S.J.N.; Zollinger, W.D.; Snoy, P.J.; Tai, J.Y.; Costantini, P.; Norelli, F.; Rappuoli, R.; Frasch, C.E. Preclinical evaluation of group B Neisseria meningitidis and Escherichia coli K92 capsular polysaccharide-protein conjugate vaccines in juvenile rhesus monkeys. Infect. Immun. 1997, 65, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, W.D.; Moran, E.E.; Devi, S.J.; Frasch, C.E. Bactericidal antibody responses of juvenile rhesus monkeys immunized with group B Neisseria meningitidis capsular polysaccharide-protein conjugate vaccines. Infect. Immun. 1997, 65, 1053–1060. [Google Scholar] [CrossRef]
- Fusco, P.C.; Michon, F.; Tai, J.Y.; Blake, M.S. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and nonhuman primates. J. Infect. Dis. 1997, 175, 364–372. [Google Scholar] [CrossRef]
- Chabot, D.J.; Joyce, J.; Caulfield, M.; Cook, J.; Hepler, R.; Wang, S.; Vietri, N.J.; Ruthel, G.; Shoop, W.; Pitt, L.; et al. Efficacy of a capsule conjugate vaccine against inhalational anthrax in rabbits and monkeys. Vaccine 2012, 30, 846–852. [Google Scholar] [CrossRef]
- Chabot, D.J.; Ribot, W.J.; Joyce, J.; Cook, J.; Hepler, R.; Nahas, D.; Chua, J.; Friedlander, A.M. Protection of rhesus macaques against inhalational anthrax with a Bacillus anthracis capsule conjugate vaccine. Vaccine 2016, 34, 4012–4016. [Google Scholar] [CrossRef]
- Morera, Y.; Bequet-Romero, M.; Ayala, M.; Pérez, P.P.; Castro, J.; Sánchez, J.; Alba, J.S.; Ancízar, J.; Cosme, K.; Gavilondo, J.V. Antigen dose escalation study of a VEGF-based therapeutic cancer vaccine in non human primates. Vaccine 2012, 30, 368–377. [Google Scholar] [CrossRef]
- Przysiecki, C.; Lucas, B.; Mitchell, R.; Carapau, D.; Wen, Z.; Xu, H.; Wang, X.M.; Nahas, D.; Wu, C.; Hepler, R.; et al. Sporozoite neutralizing antibodies elicited in mice and rhesus macaques immunized with a Plasmodium falciparum repeat peptide conjugated to meningococcal outer membrane protein complex. Front. Cell Infect. Microbiol. 2012, 2, 146. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Izquierdo, A.; Alvarez, M.; Rosario, D.; Prado, I.; López, C.; Martínez, R.; Castro, J.; Santana, E.; Hermida, L.; et al. Immunogenicity and protective efficacy of a recombinant fusion protein containing the domain III of the dengue 1 envelope protein in non-human primates. Antivir. Res 2008, 80, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Przysiecki, C.; Flanagan, E.; Bello-Irizarry, S.N.; Ionescu, R.; Muratova, O.; Dobrescu, G.; Lambert, L.; Keister, D.; Rippeon, Y.; et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. USA 2006, 103, 18243–18248. [Google Scholar] [CrossRef]
- Fan, J.; Liang, X.; Horton, M.S.; Perry, H.C.; Citron, M.P.; Heidecker, G.J.; Fu, T.M.; Joyce, J.; Przysiecki, C.T.; Keller, P.M.; et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine 2004, 22, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Vella, P.P.; Marburg, S.; Staub, J.M.; Kniskern, P.J.; Miller, W.; Hagopian, A.; Ip, C.; Tolman, R.L.; Rusk, C.M.; Chupak, L.S.; et al. Immunogenicity of conjugate vaccines consisting of pneumococcal capsular polysaccharide types 6B, 14, 19F, and 23F and a meningococcal outer membrane protein complex. Infect. Immun. 1992, 60, 4977–4983. [Google Scholar] [CrossRef]
- McNeely, T.B.; Liu, X.; Bringman, T.; Donnelly, J.J. Effect of individual conjugate dose on immunogenicity of type 6B pneumococcal polysaccharide-N. meningitidis outer membrane protein complex conjugate vaccines in infant rhesus monkeys. Vaccine 2000, 18, 2808–2816. [Google Scholar] [CrossRef]
- Liu, M.A.; Friedman, A.; Oliff, A.I.; Tai, J.; Martinez, D.; Deck, R.R.; Shieh, J.T.C.; Jenkins, T.D.; Donnelly, J.J.; Hawe, L.A. A vaccine carrier derived from neisseria-meningitidis with mitogenic activity for lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 4633–4637. [Google Scholar] [CrossRef]
- Vella, P.P.; Ellis, R.W. Immunogenicity of Haemophilus influenzae type b conjugate vaccines in infant rhesus monkeys. Pediatr. Res. 1991, 29, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Bowie, W.R.; Digiacomo, R.F.; Holmes, K.K.; Gale, J.L. Genital inoculation of male Macaca fascicularis with Neisseria gonorrhoeae and Ureaplasma urealyticum. Br. J. Vener. Dis. 1978, 54, 235–238. [Google Scholar] [CrossRef]
- Harris, S.L.; Zhu, D.; Murphy, E.; McNeil, L.K.; Wang, X.; Mayer, L.W.; Harrison, L.H.; Jansen, K.U.; Anderson, A.S. Preclinical evidence for the potential of a bivalent fHBP vaccine to prevent Neisseria meningitidis Serogroup C Disease. Hum. Vaccine 2011, 7 (Suppl. 1), 68–74. [Google Scholar] [CrossRef]
- Rouppe van der Voort, E.M.; Schuller, M.; Holst, J.; de Vries, P.; van der Ley, P.; van den Dobbelsteen, G.; Poolman, J. Immunogenicity studies with a genetically engineered hexavalent PorA and a wild-type meningococcal group B outer membrane vesicle vaccine in infant cynomolgus monkeys. Vaccine 2000, 18, 1334–1343. [Google Scholar] [CrossRef]
- Hermida, L.; Bernardo, L.; Martín, J.; Alvarez, M.; Prado, I.; López, C.; Sierra Bde, L.; Martínez, R.; Rodríguez, R.; Zulueta, A.; et al. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 2006, 24, 3165–3171. [Google Scholar] [CrossRef] [PubMed]
- Carmenate, T.; Guirola, M.; Alvarez, A.; Canaán, L.; González, S.; Caballero, E.; Menéndez, T.; Guillén, G. Memory immune response generated in Cercopithecus aethiops against meningococcal polysaccharide serogroup C conjugate vaccine. FEMS Immunol. Med. Microbiol. 2005, 43, 133–140. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, R.F.; Gale, J.L.; Holmes, K.K.; Buchanan, T.M. Genital inoculation of male baboons with Neisseria gonorrhoeae. Infect. Immun. 1977, 15, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Reuss, B.; Asif, A.R.; Almamy, A.; Schwerk, C.; Schroten, H.; Ishikawa, H.; Drummer, C.; Behr, R. Antisera against Neisseria gonorrhoeae cross-react with specific brain proteins of the common marmoset monkey and other nonhuman primate species. Brain Res. 2016, 1653, 23–38. [Google Scholar] [CrossRef]
- Weyand, N.J.; Ma, M.; Phifer-Rixey, M.; Taku, N.A.; Rendon, M.A.; Hockenberry, A.M.; Kim, W.J.; Agellon, A.B.; Biais, N.; Suzuki, T.A.; et al. Isolation and characterization of Neisseria musculi sp. nov., from the wild house mouse. Int. J. Syst. Evol. Microbiol. 2016, 66, 3585–3593. [Google Scholar] [CrossRef]
- Ma, M.; Powell, D.A.; Weyand, N.J.; Rhodes, K.A.; Rendon, M.A.; Frelinger, J.A.; So, M. A Natural Mouse Model for Neisseria Colonization. Infect. Immun. 2018, 86, e00839-17. [Google Scholar] [CrossRef]
- Arko, R.J. Neisseria gonorrhoeae: Experimental infection of laboratory animals. Science 1972, 177, 1200–1201. [Google Scholar] [CrossRef]
- Waitkins, S.A. Effect of tissue culture cells in promoting prolonged survival of N. Gonorrhoeae in artificial subcutaneous cavities of mice. Br. J. Vener. Dis. 1975, 51, 376–381. [Google Scholar] [CrossRef]
- Chandler, F.W.; Kraus, S.J.; Watts, J.C. Pathological features of experimental gonococcal infection in mice and guinea pigs. Infect. Immun. 1976, 13, 909–914. [Google Scholar] [CrossRef]
- Arko, R.J.; Wong, K.H.; Steurer, F.J.; Schalla, W.O. Complement-enhanced immunity to infection with Neisseria gonorrhoeae in mice. J. Infect. Dis. 1979, 139, 569–574. [Google Scholar] [CrossRef]
- Diena, B.B.; Ryan, A.; Ashton, F.E.; Wallace, R.; Perry, M.B.; Daoust, V. A mouse intracerebral infection with Neisseria gonorrhoeae. Br. J. Vener. Dis. 1975, 51, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.P.; Taylor-Robinson, D.; Slavin, G. Pneumonia in mice produced by Neisseria gonorrhoeae. Br. J. Vener. Dis. 1977, 53, 26–30. [Google Scholar] [CrossRef]
- Clark, J.M.; Weinhold, K.J. Infection of artificial air pouches in the connective tissue of mice with Neisseria gonorrhoeae. J. Med. Microbiol. 1979, 12, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, L.B.; Wunderlich, A.C.; Corbeil, R.R.; McCutchan, J.A.; Ito, J.I., Jr.; Braude, A.I. Disseminated gonococcal infection in mice. Infect. Immun. 1979, 26, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Streeter, P.R.; Corbeil, L.B. Gonococcal infection in endotoxin-resistant and endotoxin-susceptible mice. Infect. Immun. 1981, 32, 105–110. [Google Scholar] [CrossRef]
- Streeter, P.R.; Wunderlich, A.C.; Corbeil, R.R.; Corbeil, L.B. Natural immunity to murine gonococcal bacteremia: Roles of complement, leucocytes, and sex. Can. J. Microbiol. 1983, 29, 331–337. [Google Scholar] [CrossRef]
- Sintsova, A.; Sarantis, H.; Islam, E.A.; Sun, C.X.; Amin, M.; Chan, C.H.; Stanners, C.P.; Glogauer, M.; Gray-Owen, S.D. Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog. 2014, 10, e1004341. [Google Scholar] [CrossRef]
- Sintsova, A.; Guo, C.X.; Sarantis, H.; Mak, T.W.; Glogauer, M.; Gray-Owen, S.D. Bcl10 synergistically links CEACAM3 and TLR-dependent inflammatory signalling. Cell Microbiol. 2018, 20, e12788. [Google Scholar] [CrossRef]
- Muenzner, P.; Bachmann, V.; Zimmermann, W.; Hentschel, J.; Hauck, C.R. Human-Restricted Bacterial Pathogens Block Shedding of Epithelial Cells by Stimulating Integrin Activation. Science 2010, 329, 1197–1201. [Google Scholar] [CrossRef]
- Muenzner, P.; Hauck, C.R. Neisseria gonorrhoeae Blocks Epithelial Exfoliation by Nitric-Oxide-Mediated Metabolic Cross Talk to Promote Colonization in Mice. Cell Host Microbe 2020, 27, 793–808.e795. [Google Scholar] [CrossRef]
- Currie, E.G.; Coburn, B.; Porfilio, E.A.; Lam, P.; Rojas, O.L.; Novak, J.; Yang, S.; Chowdhury, R.B.; Ward, L.A.; Wang, P.W.; et al. Immunoglobulin A nephropathy is characterized by anticommensal humoral immune responses. JCI Insight 2022, 7, e141289. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.X.; Leontyev, D.; Kaul, R.; Gray-Owen, S.D. Neisseria gonorrhoeae co-infection exacerbates vaginal HIV shedding without affecting systemic viral loads in human CD34+ engrafted mice. PLoS ONE 2018, 13, e0191672. [Google Scholar] [CrossRef] [PubMed]
- Braude, A.I. Maxwell Finland lecture. Resistance to infection with the gonococcus. J. Infect. Dis. 1982, 145, 623–624. [Google Scholar] [CrossRef]
- Kita, E.; Matsuura, H.; Kashiba, S. A Mouse Model for the Study of Gonococcal Genital Infection. J. Infect. Dis. 1981, 143, 67–70. [Google Scholar] [CrossRef]
- Taylor-Robinson, D.; Furr, P.M.; Hetherington, C.M. Neisseria gonorrhoeae colonises the genital tract of oestradiol-treated germ-free female mice. Microb. Pathog. 1990, 9, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Jerse, A.E. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 1999, 67, 5699–5708. [Google Scholar] [CrossRef]
- Raterman, E.L.; Macintyre, A.; Jerse, A. Comparison of use of 17β−estradiol 3-benzoate and premarin for promoting experimental Neisseria gonorrhoeae genital tract infection in female mice. J. Microbiol. Meth. 2018, submitted.
- Raterman, E.L.; Jerse, A.E. Female Mouse Model of Neisseria gonorrhoeae Infection. Methods Mol. Biol. 2019, 1997, 413–429. [Google Scholar] [CrossRef]
- Feinen, B.; Jerse, A.E.; Gaffen, S.l.; Russell, M.W. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010, 3, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feinen, B.; Russell, M.W. New concepts in immunity to Neisseria gonorrhoeae: Innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front. Microbiol. 2011, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Jerse, A.E.; Wu, H.; Packiam, M.; Vonck, R.A.; Begum, A.A.; Garvin, L.E. Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections. Front. Microbiol. 2011, 2, 107. [Google Scholar] [CrossRef]
- Packiam, M.; Yedery, R.D.; Begum, A.A.; Carlson, R.W.; Ganguly, J.; Sempowski, G.D.; Ventevogel, M.S.; Shafer, W.M.; Jerse, A.E. Phosphoethanolamine decoration of Neisseria gonorrhoeae lipid A plays a dual immunostimulatory and protective role during experimental genital tract infection. Infect. Immun. 2014, 82, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Higashi, D.; Goytia, M.; Rendón, M.A.; Pilligua-Lucas, M.; Bronnimann, M.; McLean, J.A.; Duncan, J.; Trees, D.; Jerse, A.E.; et al. Commensal Neisseria Kill Neisseria gonorrhoeae through a DNA-Dependent Mechanism. Cell Host Microbe 2019, 26, 228–239.e228. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.P.; Islam, E.A.; Gower, A.C.; Shaik-Dasthagirisaheb, Y.B.; Gray-Owen, S.D.; Wetzler, L.M. Murine host response to Neisseria gonorrhoeae upper genital tract infection reveals a common transcriptional signature, plus distinct inflammatory responses that vary between reproductive cycle phases. BMC Genom. 2018, 19, 627. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Russo, R.; Westfall, L.; Shrestha, R.; Zimmerman, M.; Dartois, V.; Kurepina, N.; Kreiswirth, B.; Singleton, E.; Li, S.G.; et al. A Novel Oral GyrB/ParE Dual Binding Inhibitor Effective against Multidrug-Resistant Neisseria gonorrhoeae and Other High-Threat Pathogens. Antimicrob. Agents Chemother. 2022, 66, e0041422. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, S.; Yan, J.; Lin, X.; van der Veen, S. Moenomycin is broadly active against multidrug-resistant Neisseria gonorrhoeae and clears an infection from a murine vaginal tract infection model. J. Antimicrob. Chemother. 2022, 77, 2461–2469. [Google Scholar] [CrossRef]
- Narasimhan, J.; Letinski, S.; Jung, S.P.; Gerasyuto, A.; Wang, J.; Arnold, M.; Chen, G.; Hedrick, J.; Dumble, M.; Ravichandran, K.; et al. Ribonucleotide reductase, a novel drug target for gonorrhea. Elife 2022, 11, e67447. [Google Scholar] [CrossRef]
- Elhassanny, A.E.M.; Abutaleb, N.S.; Seleem, M.N. Auranofin exerts antibacterial activity against Neisseria gonorrhoeae in a female mouse model of genital tract infection. PLoS ONE 2022, 17, e0266764. [Google Scholar] [CrossRef] [PubMed]
- Abutaleb, N.S.; Elhassanny, A.E.M.; Seleem, M.N. In vivo efficacy of acetazolamide in a mouse model of Neisseria gonorrhoeae infection. Microb. Pathog. 2022, 164, 105454. [Google Scholar] [CrossRef]
- Masuko, A.; Takata, I.; Fujita, K.; Okumura, H.; Ushiyama, F.; Amada, H.; Sugiyama, H. In Vitro and In Vivo Activities of TP0480066, a Novel Topoisomerase Inhibitor, against Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2021, 65, e02145-20. [Google Scholar] [CrossRef]
- Martin, J.K., 2nd; Sheehan, J.P.; Bratton, B.P.; Moore, G.M.; Mateus, A.; Li, S.H.; Kim, H.; Rabinowitz, J.D.; Typas, A.; Savitski, M.M.; et al. A Dual-Mechanism Antibiotic Kills Gram-Negative Bacteria and Avoids Drug Resistance. Cell 2020, 181, 1518–1532.e1514. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.J.; Charrier, C.; Salisbury, A.M.; Box, H.; Chaffer-Malam, N.; Huxley, A.; Kirk, R.; Noonan, G.M.; Mohmed, S.; Craighead, M.W.; et al. Efficacy of a Novel Tricyclic Topoisomerase Inhibitor in a Murine Model of Neisseria gonorrhoeae Infection. Antimicrob. Agents Chemother. 2016, 60, 5592–5594. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.M.; Connolly, K.L.; Jerse, A.E.; Detrick, M.S.; Horzempa, J. Antibacterial activity of resazurin-based compounds against Neisseria gonorrhoeae in vitro and in vivo. Int. J. Antimicrob. Agents 2016, 48, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Aron, Z.D.; Mehrani, A.; Hoffer, E.D.; Connolly, K.L.; Srinivas, P.; Torhan, M.C.; Alumasa, J.N.; Cabrera, M.; Hosangadi, D.; Barbor, J.S.; et al. trans-Translation inhibitors bind to a novel site on the ribosome and clear Neisseria gonorrhoeae in vivo. Nat. Commun. 2021, 12, 1799. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Connolly, K.L.; Aberman, K.E.; Fonseca, J.C., 2nd; Singh, A.; Jerse, A.E.; Nicholas, R.A.; Davies, C. Molecular Features of Cephalosporins Important for Activity against Antimicrobial-Resistant Neisseria gonorrhoeae. ACS Infect. Dis. 2021, 7, 293–308. [Google Scholar] [CrossRef]
- Vincent, L.R.; Kerr, S.R.; Tan, Y.; Tomberg, J.; Raterman, E.L.; Dunning Hotopp, J.C.; Unemo, M.; Nicholas, R.A.; Jerse, A.E. In vivo-selected compensatory mutations restore the fitness cost of mosaic penA alleles that confer ceftriaxone resistance in Neisseria gonorrhoeae. mBio 2018, 9, e01905-17. [Google Scholar] [CrossRef]
- Connolly, K.L.; Eakin, A.E.; Gomez, C.; Osborn, B.L.; Unemo, M.; Jerse, A.E. Pharmacokinetic Data Are Predictive of In Vivo Efficacy for Cefixime and Ceftriaxone against Susceptible and Resistant Neisseria gonorrhoeae Strains in the Gonorrhea Mouse Model. Antimicrob. Agents Chemother. 2019, 63, e01644-18. [Google Scholar] [CrossRef]
- Kunz, A.N.; Begum, A.A.; Wu, H.; D’Ambrozio, J.A.; Robinson, J.M.; Shafer, W.M.; Bash, M.C.; Jerse, A.E. Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J. Infect. Dis. 2012, 205, 1821–1829. [Google Scholar] [CrossRef]
- Easterhoff, D.; Ontiveros, F.; Brooks, L.R.; Kim, Y.; Ross, B.; Silva, J.N.; Olsen, J.S.; Feng, C.; Hardy, D.J.; Dunman, P.M.; et al. Semen-derived enhancer of viral infection (SEVI) binds bacteria, enhances bacterial phagocytosis by macrophages, and can protect against vaginal infection by a sexually transmitted bacterial pathogen. Antimicrob. Agents Chemother. 2013, 57, 2443–2450. [Google Scholar] [CrossRef]
- Muench, D.F.; Kuch, D.J.; Wu, H.; Begum, A.A.; Veit, S.J.; Pelletier, M.E.; Soler-Garcia, A.A.; Jerse, A.E. Hydrogen peroxide-producing lactobacilli inhibit gonococci in vitro but not during experimental genital tract infection. J. Infect. Dis. 2009, 199, 1369–1378. [Google Scholar] [CrossRef]
- Bozja, J.; Yi, K.; Shafer, W.M.; Stojiljkovic, I. Porphyrin-based compounds exert antibacterial action against the sexually transmitted pathogens Neisseria gonorrhoeae and Haemophilus ducreyi. Int. J. Antimicrob. Agents 2004, 24, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.E.; Valentin-Bon, I.E.; Whaley, K.; Jerse, A.E. Inhibition of Neisseria gonorrhoeae genital tract infection by leading-candidate topical microbicides in a mouse model. J. Infect. Dis. 2004, 189, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Shaughnessy, J.; DeOliveira, R.B.; Lewis, L.A.; Gulati, S.; Rice, P.A. Utilizing complement evasion strategies to design complement-based antibacterial immunotherapeutics: Lessons from the pathogenic Neisseriae. Immunobiology 2016, 221, 1110–1123. [Google Scholar] [CrossRef]
- Shaughnessy, J.; Gulati, S.; Agarwal, S.; Unemo, M.; Ohnishi, M.; Su, X.H.; Monks, B.G.; Visintin, A.; Madico, G.; Lewis, L.A.; et al. A Novel Factor H-Fc Chimeric Immunotherapeutic Molecule against Neisseria gonorrhoeae. J. Immunol. 2016, 196, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, J.; Tran, Y.; Zheng, B.; DeOliveira, R.B.; Gulati, S.; Song, W.C.; Maclean, J.M.; Wycoff, K.L.; Ram, S. Development of Complement Factor H-Based Immunotherapeutic Molecules in Tobacco Plants Against Multidrug-Resistant Neisseria gonorrhoeae. Front. Immunol. 2020, 11, 583305. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, S.; Shaughnessy, J.; Maziarz, K.; Ermert, D.; Gulati, S.; Zheng, B.; Mörgelin, M.; Jacobsson, S.; Riesbeck, K.; Unemo, M.; et al. C4BP-IgM protein as a therapeutic approach to treat Neisseria gonorrhoeae infections. JCI Insight 2019, 4, e131886. [Google Scholar] [CrossRef]
- Butler, M.M.; Waidyarachchi, S.L.; Connolly, K.L.; Jerse, A.E.; Chai, W.; Lee, R.E.; Kohlhoff, S.A.; Shinabarger, D.L.; Bowlin, T.L. Aminomethyl Spectinomycins as Therapeutics for Drug-Resistant Gonorrhea and Chlamydia Coinfections. Antimicrob. Agents Chemother. 2018, 62, e00325-18. [Google Scholar] [CrossRef]
- Liu, Y.; Russell, M.W. Diversion of the immune response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by treatment with anti-transforming growth factor beta antibody generates immunological memory and protective immunity. mBio 2011, 2, e00095-11. [Google Scholar] [CrossRef]
- Leduc, I.; Connolly, K.L.; Begum, A.; Underwood, K.; Darnell, S.; Shafer, W.M.; Balthazar, J.T.; Macintyre, A.N.; Sempowski, G.D.; Duncan, J.A.; et al. The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae. PLoS Pathog. 2020, 16, e1008602. [Google Scholar] [CrossRef]
- Connolly, K.L.; Pilligua-Lucas, M.; Gomez, C.; Costenoble-Caherty, A.C.; Soc, A.; Underwood, K.; Macintyre, A.N.; Sempowski, G.D.; Jerse, A.E. Preclinical Testing of Vaccines and Therapeutics for Gonorrhea in Female Mouse Models of Lower and Upper Reproductive Tract Infection. J. Infect. Dis. 2021, 224, S152–S160. [Google Scholar] [CrossRef]
- Gulati, S.; Pennington, M.W.; Czerwinski, A.; Carter, D.; Zheng, B.; Nowak, N.A.; DeOliveira, R.B.; Shaughnessy, J.; Reed, G.W.; Ram, S.; et al. Preclinical Efficacy of a Lipooligosaccharide Peptide Mimic Candidate Gonococcal Vaccine. mBio 2019, 10, e02552-19. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.A.; Gulati, S.; Zelek, W.M.; Morgan, B.P.; Song, W.C.; Zheng, B.; Nowak, N.; DeOliveira, R.B.; Sanchez, B.; DeSouza Silva, L.; et al. Efficacy of an Experimental Gonococcal Lipooligosaccharide Mimitope Vaccine Requires Terminal Complement. J. Infect. Dis. 2022, 225, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Matthias, K.A.; Connolly, K.L.; Begum, A.A.; Jerse, A.E.; Macintyre, A.N.; Sempowski, G.D.; Bash, M.C. Meningococcal Detoxified Outer Membrane Vesicle Vaccines Enhance Gonococcal Clearance in a Murine Infection Model. J. Infect. Dis. 2022, 225, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.E.; Gomez, C.; Le Van, A.; Baarda, B.I.; Darnell, S.; Martinez, F.G.; Zielke, R.A.; Bonventre, J.A.; Jerse, A.E. A novel gonorrhea vaccine composed of MetQ lipoprotein formulated with CpG shortens experimental murine infection. Vaccine 2020, 38, 8175–8184. [Google Scholar] [CrossRef] [PubMed]
- Fegan, J.E.; Calmettes, C.; Islam, E.A.; Ahn, S.K.; Chaudhuri, S.; Yu, R.H.; Gray-Owen, S.D.; Moraes, T.F.; Schryvers, A.B. Utility of Hybrid Transferrin Binding Protein Antigens for Protection Against Pathogenic Neisseria Species. Front. Immunol. 2019, 10, 247. [Google Scholar] [CrossRef]
- Parzych, E.M.; Gulati, S.; Zheng, B.; Bah, M.A.; Elliott, S.T.C.; Chu, J.D.; Nowak, N.; Reed, G.W.; Beurskens, F.J.; Schuurman, J.; et al. Synthetic DNA Delivery of an Optimized and Engineered Monoclonal Antibody Provides Rapid and Prolonged Protection against Experimental Gonococcal Infection. mBio 2021, 12, e00242-21. [Google Scholar] [CrossRef]
- Gulati, S.; Beurskens, F.J.; de Kreuk, B.J.; Roza, M.; Zheng, B.; DeOliveira, R.B.; Shaughnessy, J.; Nowak, N.A.; Taylor, R.P.; Botto, M.; et al. Complement alone drives efficacy of a chimeric antigonococcal monoclonal antibody. PLoS Biol. 2019, 17, e3000323. [Google Scholar] [CrossRef]
- Ram, S.; Gulati, S.; Lewis, L.A.; Chakraborti, S.; Zheng, B.; DeOliveira, R.B.; Reed, G.W.; Cox, A.D.; Li, J.; St Michael, F.; et al. A Novel Sialylation Site on Neisseria gonorrhoeae Lipooligosaccharide Links Heptose II Lactose Expression with Pathogenicity. Infect. Immun. 2018, 86, e00285-18. [Google Scholar] [CrossRef]
- Gulati, S.; Zheng, B.; Reed, G.W.; Su, X.; Cox, A.D.; St, M.F.; Stupak, J.; Lewis, L.A.; Ram, S.; Rice, P.A. Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLoS Pathog. 2013, 9, e1003559. [Google Scholar] [CrossRef]
- Liu, Y.; Hammer, L.A.; Daamen, J.; Stork, M.; Egilmez, N.K.; Russell, M.W. Microencapsulated IL-12 Drives Genital Tract Immune Responses to Intranasal Gonococcal Outer Membrane Vesicle Vaccine and Induces Resistance to Vaginal Infection with Diverse Strains of Neisseria gonorrhoeae. mSphere 2023, 8, e0038822. [Google Scholar] [CrossRef]
- Liu, Y.; Hammer, L.A.; Liu, W.; Hobbs, M.M.; Zielke, R.A.; Sikora, A.E.; Jerse, A.E.; Egilmez, N.K.; Russell, M.W. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017, 10, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Jerse, A.; Hamel, J.; Couture, F.; Rioux, C.R.; Brodeur, B.R.; Martin, D. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J. Infect. Dis. 2000, 182, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Arko, R.J. An immunologic model in laboratory animals for the study of neisseria gonorrhoeae. J. Infect. Dis. 1974, 129, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Thomas, C.E.; Chen, C.J.; Van Dam, C.N.; Johnston, R.E.; Davis, N.L.; Sparling, P.F. Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles. Infect. Immun. 2005, 73, 7558–7568. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.G.; Jerse, A.E. Functional characterization of antibodies against Neisseria gonorrhoeae opacity protein loops. PLoS ONE 2009, 4, e8108. [Google Scholar] [CrossRef]
- Miller, C.P. Experimental meningococcal infection in mice. Science 1933, 78, 340–341. [Google Scholar] [CrossRef]
- Calver, G.A.; Kenny, C.P.; Lavergne, G. Iron As A Replacement for Mucin in Establishment of Meningococcal Infection in Mice. Can. J. Microbiol. 1976, 22, 832–838. [Google Scholar] [CrossRef]
- Kolbe, K.R.; Sanches, T.R.; Fanelli, C.; Garnica, M.R.; Urbano de Castro, L.; Gooch, K.; Thomas, S.; Taylor, S.; Gorringe, A.; Noronha, I.L.; et al. Acute kidney injury in a mouse model of meningococcal disease. Int. J. Immunopathol. Pharm. 2021, 35, 20587384211056507. [Google Scholar] [CrossRef]
- Quakyi, E.K.; Hochstein, H.D.; Tsai, C.M. Modulation of the biological activities of meningococcal endotoxins by association with outer membrane proteins is not inevitably linked to toxicity. Infect. Immun. 1997, 65, 1972–1979. [Google Scholar] [CrossRef]
- Wilks, K.E.; Dunn, K.L.R.; Farrant, J.L.; Reddin, K.M.; Gorringe, A.R.; Langford, P.R.; Kroll, J.S. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect. Immun. 1998, 66, 213–217. [Google Scholar] [CrossRef]
- Newcombe, J.; Eales-Reynolds, L.J.; Wootton, L.; Gorringe, A.R.; Funnell, S.G.; Taylor, S.C.; McFadden, J.J. Infection with an avirulent phoP mutant of Neisseria meningitidis confers broad cross-reactive immunity. Infect. Immun. 2004, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Renauld-Mongénie, G.; Poncet, D.; Mignon, M.; Fraysse, S.; Chabanel, C.; Danve, B.; Krell, T.; Quentin-Millet, M.J. Role of transferrin receptor from a Neisseria meningitidis tbpB isotype II strain in human transferrin binding and virulence. Infect. Immun. 2004, 72, 3461–3470. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Chow, N.Y.; Sinha, S.; Halliwell, D.; Finney, M.; Gorringe, A.R.; Watson, M.W.; Kroll, J.S.; Langford, P.R.; Webb, S.A. A Neisseria meningitidis NMB1966 mutant is impaired for invasion of respiratory epithelial cells, survival in human blood and for virulence in vivo. Med. Microbiol. Immunol. 2009, 198, 57–67. [Google Scholar] [CrossRef]
- Kuwae, A.; Sjölinder, H.; Eriksson, J.; Eriksson, S.; Chen, Y.; Jonsson, A.B. NafA negatively controls Neisseria meningitidis piliation. PLoS ONE 2011, 6, e21749. [Google Scholar] [CrossRef] [PubMed]
- Scribner, R.K.; Wedro, B.C.; Weber, A.H.; Marks, M.I. Activities of eight new beta-lactam antibiotics and seven antibiotic combinations against Neisseria meningitidis. Antimicrob. Agents Chemother. 1982, 21, 678–680. [Google Scholar] [CrossRef]
- Salit, I.E.; Van Melle, E.; Tomalty, L. Experimental meningococcal infection in neonatal animals: Models for mucosal invasiveness. Can. J. Microbiol. 1984, 30, 1022–1029. [Google Scholar] [CrossRef]
- Holbein, B.E. Differences in virulence between disease and carrier strains of neisseria meningitidis. Can. J. Microbiol. 1981, 27, 738–741. [Google Scholar] [CrossRef]
- Salit, I.E.; Tomalty, L. Experimental meningococcal infection in neonatal mice: Differences in virulence between strains isolated from human cases and carriers. Can. J. Microbiol. 1984, 30, 1042–1045. [Google Scholar] [CrossRef]
- Mackinnon, F.G.; Gorringe, A.R.; Funnell, S.G.P.; Robinson, A. Intranasal infection of infant mice with neisseria-meningitidis. Microb. Path. 1992, 12, 415–420. [Google Scholar] [CrossRef]
- Mackinnon, F.G.; Borrow, R.; Gorringe, A.R.; Fox, A.J.; Jones, D.M.; Robinson, A. Demonstration of lipooligosaccharide immunotype and capsule as virulence factors for neisseria-meningitidis using an infant mouse intranasal infection model. Microb. Path. 1993, 15, 359–366. [Google Scholar] [CrossRef]
- Yi, K.; Stephens, D.S.; Stojiljkovic, I. Development and evaluation of an improved mouse model of meningococcal colonization. Infect. Immun. 2003, 71, 1849–1855. [Google Scholar] [CrossRef]
- Sjölinder, H.; Eriksson, J.; Maudsdotter, L.; Aro, H.; Jonsson, A.B. Meningococcal outer membrane protein NhhA is essential for colonization and disease by preventing phagocytosis and complement attack. Infect. Immun. 2008, 76, 5412–5420. [Google Scholar] [CrossRef]
- Zarantonelli, M.L.; Huerre, M.; Taha, M.K.; Alonso, J.M. Differential role of lipooligosaccharide of Neisseria meningitidis in virulence and inflammatory response during respiratory infection in mice. Infect. Immun. 2006, 74, 5506–5512. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Guiyoule, A.; Zarantonelli, M.L.; Ramisse, F.; Pires, R.; Antignac, A.; Deghmane, A.E.; Huerre, M.; van der Werf, S.; Taha, M.K. A model of meningococcal bacteremia after respiratory superinfection in influenza A virus-infected mice. FEMS Microbiol. Lett. 2003, 222, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zarantonelli, M.L.; Skoczynska, A.; Antignac, A.; El Ghachi, M.; Deghmane, A.E.; Szatanik, M.; Mulet, C.; Werts, C.; Peduto, L.; d’Andon, M.F.; et al. Penicillin resistance compromises Nod1-dependent proinflammatory activity and virulence fitness of neisseria meningitidis. Cell Host Microbe 2013, 13, 735–745. [Google Scholar] [CrossRef]
- Belkacem, N.; Bourdet-Sicard, R.; Taha, M.K. Lactobacillus paracasei feeding improves the control of secondary experimental meningococcal infection in flu-infected mice. BMC Infect. Dis. 2018, 18, 167. [Google Scholar] [CrossRef]
- Colicchio, R.; Ricci, S.; Lamberti, F.; Pagliarulo, C.; Pagliuca, C.; Braione, V.; Braccini, T.; Talà, A.; Montanaro, D.; Tripodi, S.; et al. The meningococcal ABC-Type L-glutamate transporter GltT is necessary for the development of experimental meningitis in mice. Infect. Immun. 2009, 77, 3578–3587. [Google Scholar] [CrossRef]
- Colicchio, R.; Pagliuca, C.; Ricci, S.; Scaglione, E.; Grandgirard, D.; Masouris, I.; Farina, F.; Pagliarulo, C.; Mantova, G.; Paragliola, L.; et al. Virulence Traits of a Serogroup C Meningococcus and Isogenic cssA Mutant, Defective in Surface-Exposed Sialic Acid, in a Murine Model of Meningitis. Infect. Immun. 2019, 87, e00688-18. [Google Scholar] [CrossRef]
- Ricci, S.; Grandgirard, D.; Wenzel, M.; Braccini, T.; Salvatore, P.; Oggioni, M.R.; Leib, S.L.; Koedel, U. Inhibition of matrix metalloproteinases attenuates brain damage in experimental meningococcal meningitis. BMC Infect. Dis. 2014, 14, 726. [Google Scholar] [CrossRef]
- Sjölinder, H.; Mogensen, T.H.; Kilian, M.; Jonsson, A.B.; Paludan, S.R. Important role for Toll-like receptor 9 in host defense against meningococcal sepsis. Infect. Immun. 2008, 76, 5421–5428. [Google Scholar] [CrossRef] [PubMed]
- Quattroni, P.; Li, Y.; Lucchesi, D.; Lucas, S.; Hood, D.W.; Herrmann, M.; Gabius, H.J.; Tang, C.M.; Exley, R.M. Galectin-3 binds Neisseria meningitidis and increases interaction with phagocytic cells. Cell Microbiol. 2012, 14, 1657–1675. [Google Scholar] [CrossRef]
- Pluddemann, A.; Hoe, J.C.; Makepeace, K.; Moxon, E.R.; Gordon, S. The macrophage scavenger receptor A is host-protective in experimental meningococcal septicaemia. PLoS Pathog. 2009, 5, e1000297. [Google Scholar] [CrossRef]
- Johansson, L.; Rytkonen, A.; Bergman, P.; Albiger, B.; Källström, H.; Hökfelt, T.; Agerberth, B.; Cattaneo, R.; Jonsson, A.B. CD46 in meningococcal disease. Science 2003, 301, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Rytkönen, A.; Wan, H.; Bergman, P.; Plant, L.; Agerberth, B.; Hökfelt, T.; Jonsson, A.B. Human-like immune responses in CD46 transgenic mice. J. Immunol. 2005, 175, 433–440. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Sjölinder, M.; Wan, Y.; Sjölinder, H. CD46 accelerates macrophage-mediated host susceptibility to meningococcal sepsis in a murine model. Eur J. Immunol. 2017, 47, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Sjölinder, H.; Jonsson, A.B. Imaging of disease dynamics during meningococcal sepsis. PLoS ONE 2007, 2, e241. [Google Scholar] [CrossRef] [PubMed]
- Sjolinder, H.; Jonsson, A.B. Olfactory nerve--a novel invasion route of Neisseria meningitidis to reach the meninges. PLoS ONE 2010, 5, e14034. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Engström, Å.; Merényi, G.; Hagner, M.; Yang, H.; Kuwae, A.; Wan, Y.; Sjölinder, M.; Sjölinder, H. Dynamic niche-specific adaptations in Neisseria meningitidis during infection. Microbes Infect. 2016, 18, 109–117. [Google Scholar] [CrossRef]
- Engman, J.; Negrea, A.; Sigurlásdóttir, S.; Geörg, M.; Eriksson, J.; Eriksson, O.S.; Kuwae, A.; Sjölinder, H.; Jonsson, A.B. Neisseria meningitidis Polynucleotide Phosphorylase Affects Aggregation, Adhesion, and Virulence. Infect. Immun. 2016, 84, 1501–1513. [Google Scholar] [CrossRef]
- Khairalla, A.S.; Omer, S.A.; Mahdavi, J.; Aslam, A.; Dufailu, O.A.; Self, T.; Jonsson, A.B.; Georg, M.; Sjolinder, H.; Royer, P.J.; et al. Nuclear trafficking, histone cleavage and induction of apoptosis by the meningococcal App and MspA autotransporters. Cell Microbiol. 2015, 17, 1008–1020. [Google Scholar] [CrossRef]
- Eriksson, J.; Eriksson, O.S.; Jonsson, A.B. Loss of meningococcal PilU delays microcolony formation and attenuates virulence in vivo. Infect. Immun. 2012, 80, 2538–2547. [Google Scholar] [CrossRef]
- Mahdavi, J.; Royer, P.J.; Sjölinder, H.S.; Azimi, S.; Self, T.; Stoof, J.; Wheldon, L.M.; Brännström, K.; Wilson, R.; Moreton, J.; et al. Pro-inflammatory cytokines can act as intracellular modulators of commensal bacterial virulence. Open Biol. 2013, 3, 130048. [Google Scholar] [CrossRef]
- Levy, M.; Aouiti Trabelsi, M.; Taha, M.K. Evidence for Multi-Organ Infection During Experimental Meningococcal Sepsis due to ST-11 Isolates in Human Transferrin-Transgenic Mice. Microorganisms 2020, 8, 1456. [Google Scholar] [CrossRef]
- Szatanik, M.; Hong, E.; Ruckly, C.; Ledroit, M.; Giorgini, D.; Jopek, K.; Nicola, M.A.; Deghmane, A.E.; Taha, M.K. Experimental meningococcal sepsis in congenic transgenic mice expressing human transferrin. PLoS ONE 2011, 6, e22210. [Google Scholar] [CrossRef]
- Sevestre, J.; Diene, S.M.; Aouiti-Trabelsi, M.; Deghmane, A.E.; Tournier, I.; François, P.; Caron, F.; Taha, M.K. Differential expression of hemoglobin receptor, HmbR, between carriage and invasive isolates of Neisseria meningitidis contributes to virulence: Lessons from a clonal outbreak. Virulence 2018, 9, 923–929. [Google Scholar] [CrossRef]
- Eriksson, L.; Stenmark, B.; Deghmane, A.E.; Thulin Hedberg, S.; Säll, O.; Fredlund, H.; Mölling, P.; Taha, M.K. Difference in virulence between Neisseria meningitidis serogroups W and Y in transgenic mice. BMC Microbiol. 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Antunes, A.; Fiette, L.; Deghmane, A.E.; Taha, M.K. Impact of corticosteroids on experimental meningococcal sepsis in mice. Steroids 2015, 101, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Johswich, K.O.; McCaw, S.E.; Islam, E.; Sintsova, A.; Gu, A.; Shively, J.E.; Gray-Owen, S.D. In vivo adaptation and persistence of Neisseria meningitidis within the nasopharyngeal mucosa. PLoS Pathog. 2013, 9, e1003509. [Google Scholar] [CrossRef]
- Melican, K.; Michea, V.P.; Martin, T.; Bruneval, P.; Dumenil, G. Adhesion of Neisseria meningitidis to dermal vessels leads to local vascular damage and purpura in a humanized mouse model. PLoS Pathog. 2013, 9, e1003139. [Google Scholar] [CrossRef] [PubMed]
- Join-Lambert, O.; Lecuyer, H.; Miller, F.; Lelievre, L.; Jamet, A.; Furio, L.; Schmitt, A.; Pelissier, P.; Fraitag, S.; Coureuil, M.; et al. Meningococcal Interaction to Microvasculature Triggers the Tissular Lesions of Purpura Fulminans. J. Infect. Dis. 2013, 208, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Bonazzi, D.; Lo Schiavo, V.; Machata, S.; Djafer-Cherif, I.; Nivoit, P.; Manriquez, V.; Tanimoto, H.; Husson, J.; Henry, N.; Chaté, H.; et al. Intermittent Pili-Mediated Forces Fluidize Neisseria meningitidis Aggregates Promoting Vascular Colonization. Cell 2018, 174, 143–155.e116. [Google Scholar] [CrossRef] [PubMed]
- Barnier, J.P.; Euphrasie, D.; Join-Lambert, O.; Audry, M.; Schonherr-Hellec, S.; Schmitt, T.; Bourdoulous, S.; Coureuil, M.; Nassif, X.; El Behi, M. Type IV pilus retraction enables sustained bacteremia and plays a key role in the outcome of meningococcal sepsis in a humanized mouse model. PLoS Pathog. 2021, 17, e1009299. [Google Scholar] [CrossRef]
- Manriquez, V.; Nivoit, P.; Urbina, T.; Echenique-Rivera, H.; Melican, K.; Fernandez-Gerlinger, M.P.; Flamant, P.; Schmitt, T.; Bruneval, P.; Obino, D.; et al. Colonization of dermal arterioles by Neisseria meningitidis provides a safe haven from neutrophils. Nat. Commun. 2021, 12, 4547. [Google Scholar] [CrossRef] [PubMed]
- Barnier, J.P.; Meyer, J.; Kolappan, S.; Bouzinba-Ségard, H.; Gesbert, G.; Jamet, A.; Frapy, E.; Schönherr-Hellec, S.; Capel, E.; Virion, Z.; et al. The minor pilin PilV provides a conserved adhesion site throughout the antigenically variable meningococcal type IV pilus. Proc. Natl. Acad. Sci. USA 2021, 118, e2109364118. [Google Scholar] [CrossRef] [PubMed]
- Denis, K.; Le Bris, M.; Le Guennec, L.; Barnier, J.P.; Faure, C.; Gouge, A.; Bouzinba-Ségard, H.; Jamet, A.; Euphrasie, D.; Durel, B.; et al. Targeting Type IV pili as an antivirulence strategy against invasive meningococcal disease. Nat. Microbiol. 2019, 4, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.I., Jr.; Corbeil, L.B.; Wunderlich, A.C.; McCutchan, J.A.; Braude, A.I. Common heat stable protective antigen in gonococci. Trans. Assoc. Am. Physicians 1979, 92, 160–168. [Google Scholar]
- Diena, B.B.; Ashton, F.E.; Ryan, A.; Wallace, R. The lipopolysaccharide (R type) as a common antigen of Neisseria gonorrhoeae. I. Immunizing properties. Can. J. Microbiol. 1978, 24, 117–123. [Google Scholar] [CrossRef]
- Bernet, E.; Lebughe, M.; Vincent, A.T.; Haghdoost, M.M.; Golbaghi, G.; Laplante, S.; Castonguay, A.; Veyrier, F.J. Sodium Tetraphenylborate Displays Selective Bactericidal Activity against Neisseria meningitidis and N. gonorrhoeae and Is Effective at Reducing Bacterial Infection Load. Antimicrob. Agents Chemother. 2021, 65, e00254-20. [Google Scholar] [CrossRef]
- Van De Verg, L.L.; Hartman, A.B.; Bhattacharjee, A.K.; Tall, B.D.; Yuan, L.; Sasala, K.; Hadfield, T.L.; Zollinger, W.D.; Hoover, D.L.; Warren, R.L. Outer membrane protein of Neisseria meningitidis as a mucosal adjuvant for lipopolysaccharide of Brucella melitensis in mouse and guinea pig intranasal immunization models. Infect. Immun. 1996, 64, 5263–5268. [Google Scholar] [CrossRef]
- Branham, S.E.; Lillie, R.D. Experimental meningitis in guinea pigs. J. Bact. 1933, 25, 90. [Google Scholar] [CrossRef]
- Nairn, K.; Shepherd, G.L.; Edwards, J.R. Efficacy of meropenem in experimental meningitis. J. Antimicrob. Chemother. 1995, 36 (Suppl. A), 73–84. [Google Scholar] [CrossRef]
- Siadat, S.D.; Kheirandish, M.; Norouzian, D.; Behzadiyannejad, Q.; Najar Peerayeh, S.; Zangeneh, M.; Nejati, M. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after immunization with outer membrane vesicle of Neisseria meningitidis serogroup B. Pak. J. Biol. Sci. 2007, 10, 3578–3584. [Google Scholar] [CrossRef]
- Finney, M.; Vaughan, T.; Taylor, S.; Hudson, M.J.; Pratt, C.; Wheeler, J.X.; Vipond, C.; Feavers, I.; Jones, C.; Findlow, J.; et al. Characterization of the key antigenic components and pre-clinical immune responses to a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Hum. Vaccines 2008, 4, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.P. Experimental gonococcal infection of the rabbit’s eye. Am. J. Syph. Gonorrhea Vener. Dis. 1948, 32, 437–444. [Google Scholar]
- Kaspar, R.L.; Drutz, D.J. Perihepatitis and hepatitis as complications of experimental endocarditis due to Neisseria gonorrhoeae in the rabbit. J. Infect. Dis. 1977, 136, 37–42. [Google Scholar] [CrossRef]
- Goldenberg, D.L.; Chisholm, P.L.; Rice, P.A. Experimental models of bacterial arthritis: A microbiologic and histopathologic characterization of the arthritis after the intraarticular injections of Neisseria gonorrhoeae, Staphylococcus aureus, group A streptococci, and Escherichia coli. J. Rheumatol. 1983, 10, 5–11. [Google Scholar]
- Campagnari, A.A.; Wild, L.M.; Griffiths, G.E.; Karalus, R.J.; Wirth, M.A.; Spinola, S.M. Role of lipooligosaccharides in experimental dermal lesions caused by haemophilus-ducreyi. Infect. Immun. 1991, 59, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Marcovici, I.; Brill, A.I.; Scommegna, A. Effects of colchicine on pelvic adhesions associated with the intrauterine inoculation of neisseria-gonorrhoeae in rabbits. Obstet. Gynecol. 1993, 81, 118–121. [Google Scholar] [PubMed]
- Roberts, L.M.; Sanfilippo, J.S.; Raab, S. Effects of laparoscopic lavage on adhesion formation and peritoneum in an animal model of pelvic inflammatory disease. J. Am. Assoc. Gynecol. Laparosc. 2002, 9, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Grasso, R.J.; Heller, R.; Cooley, J.C.; Haller, E.M. Electrofusion of individual animal cells directly to intact corneal epithelial tissue. Biochim. Biophys. Acta 1989, 980, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.; Grasso, R.J. Transfer of human membrane surface components by incorporating human cells into intact animal tissue by cell-tissue electrofusion in vivo. Biochim. Biophys. Acta 1990, 1024, 185–188. [Google Scholar] [CrossRef]
- Caputo, G.L.; Baldwin, G.; Alpert, G.; Parsonnet, J.; Gillis, Z.A.; Siber, G.; Fleisher, G. Effect of meningococcal endotoxin in a rabbit model of shock. Circ. Shock 1992, 36, 104–112. [Google Scholar] [PubMed]
- Saladino, R.; Alpert, G.; Baldwin, G.; Caputo, G.; Parsonnet, J.; Gillis, Z.; Fleisher, G.; Siber, G. Effect of intravenous immunoglobulin in a rabbit model of meningococcal endotoxic-shock. Pediatr. Res. 1990, 27, A34. [Google Scholar]
- Kartalija, M.; Kim, Y.; White, M.L.; Nau, R.; Tureen, J.H.; Täuber, M.G. Effect of a recombinant N-terminal fragment of bactericidal/permeability-increasing protein (rBPI23) on cerebrospinal fluid inflammation induced by endotoxin. J. Infect. Dis. 1995, 171, 948–953. [Google Scholar] [CrossRef]
- Flynn, J. Resistance of rats to experimental infection with Neisseria gonorrhoeae despite attempts to alter cellular and humoral defences. Br. J. Vener. Dis. 1972, 48, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.M.; Faro, S.; Dodson, M.G.; Phillips, L.E.; Aamodt, L.; Riddle, G. Role of Neisseria gonorrhoeae and Chlamydia trachomatis in intraabdominal abscess formation in the rat. J. Reprod. Med. 1991, 36, 202–205. [Google Scholar]
- Nowicki, S.; Martens, M.G.; Nowicki, B.J. Gonococcal infection in a nonhuman host is determined by human complement C1q. Infect. Immun. 1995, 63, 4790–4794. [Google Scholar] [CrossRef]
- Nowicki, S.; Selvarangan, R.; Anderson, G. Experimental transmission of Neisseria gonorrhoeae from pregnant rat to fetus. Infect. Immun. 1999, 67, 4974–4976. [Google Scholar] [CrossRef]
- Nowicki, S.; Ram, P.; Pham, T.; Goluszko, P.; Morse, S.; Anderson, G.D.; Nowicki, B. Pelvic inflammatory disease isolates of Neisseria gonorrhoeae are distinguished by C1q-dependent virulence for newborn rats and by the sac-4 region. Infect. Immun. 1997, 65, 2094–2099. [Google Scholar] [CrossRef]
- Fleming, T.J.; Wallsmith, D.E.; Rosenthal, R.S. Arthropathic properties of gonococcal peptidoglycan fragments: Implications for the pathogenesis of disseminated gonococcal disease. Infect. Immun. 1986, 52, 600–608. [Google Scholar] [CrossRef]
- Stimpson, S.A.; Esser, R.E.; Carter, P.B.; Sartor, R.B.; Cromartie, W.J.; Schwab, J.H. Lipopolysaccharide induces recurrence of arthritis in rat joints previously injured by peptidoglycan-polysaccharide. J. Exp. Med. 1987, 165, 1688–1702. [Google Scholar] [CrossRef]
- Biberstine, K.J.; Darr, D.S.; Rosenthal, R.S. Tolerance to appetite suppression induced by peptidoglycan. Infect. Immun. 1996, 64, 3641–3645. [Google Scholar] [CrossRef]
- Jeurissen, S.H.; Sminia, T.; Beuvery, E.C. Induction of mucosal immunoglobulin A immune response by preparations of Neisseria gonorrhoeae porin proteins. Infect. Immun. 1987, 55, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Hwa, V.; De Saint Martin, L.; Ogaora, P.; Nassif, X.; Heffron, F.; So, M. The Neisseria meningitidis haemoglobin receptor: Its role in iron utilization and virulence. Mol. Microbiol. 1995, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, E.; Frigimelica, E.; Giovinazzi, S.; Galli, G.; Shaik, Y.; Genco, C.; Welsch, J.A.; Granoff, D.M.; Grandi, G.; Grifantini, R. Role of FNR and FNR-regulated, sugar fermentation genes in Neisseria meningitidis infection. Mol. Microbiol. 2006, 60, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Dove, J.E.; Yasukawa, K.; Tinsley, C.R.; Nassif, X. Production of the signalling molecule, autoinducer-2, by Neisseria meningitidis: Lack of evidence for a concerted transcriptional response. Microbiology 2003, 149, 1859–1869. [Google Scholar] [CrossRef]
- Antunes, A.; Golfieri, G.; Ferlicca, F.; Giuliani, M.M.; Scarlato, V.; Delany, I. HexR Controls Glucose-Responsive Genes and Central Carbon Metabolism in Neisseria meningitidis. J. Bacteriol. 2015, 198, 644–654. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Salvatore, P.; De Vitis, L.R.; Colicchio, R.; Monaco, C.; Tredici, M.; Talà, A.; Bardaro, M.; Lavitola, A.; Bruni, C.B.; et al. Regulation and differential expression of gdhA encoding NADP-specific glutamate dehydrogenase in Neisseria meningitidis clinical isolates. Mol. Microbiol. 2004, 51, 1757–1772. [Google Scholar] [CrossRef]
- Fantappie, L.; Metruccio, M.M.; Seib, K.L.; Oriente, F.; Cartocci, E.; Ferlicca, F.; Giuliani, M.M.; Scarlato, V.; Delany, I. The RNA Chaperone Hfq is involved in stress response and virulence in Neisseria meningitidis and is a pleiotropic regulator of protein expression. Infect. Immun. 2009, 77, 1842–1853. [Google Scholar] [CrossRef]
- Sun, Y.H.; Bakshi, S.; Chalmers, R.; Tang, C.M. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 2000, 6, 1269–1273. [Google Scholar] [CrossRef]
- Fagnocchi, L.; Bottini, S.; Golfieri, G.; Fantappiè, L.; Ferlicca, F.; Antunes, A.; Guadagnuolo, S.; Del Tordello, E.; Siena, E.; Serruto, D.; et al. Global transcriptome analysis reveals small RNAs affecting Neisseria meningitidis bacteremia. PLoS ONE 2015, 10, e0126325. [Google Scholar] [CrossRef] [PubMed]
- Vogel, U.; Hammerschmidt, S.; Frosch, M. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med. Microbiol. Immunol. 1996, 185, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Seib, K.L.; Oriente, F.; Adu-Bobie, J.; Montanari, P.; Ferlicca, F.; Giuliani, M.M.; Rappuoli, R.; Pizza, M.; Delany, I. Influence of serogroup B meningococcal vaccine antigens on growth and survival of the meningococcus in vitro and in ex vivo and in vivo models of infection. Vaccine 2010, 28, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.A.; Vu, D.M.; Granoff, D.M.; Ram, S. Inhibition of the alternative pathway of nonhuman infant complement by porin B2 contributes to virulence of Neisseria meningitidis in the infant rat model. Infect. Immun. 2014, 82, 2574–2584. [Google Scholar] [CrossRef]
- Seib, K.L.; Haag, A.F.; Oriente, F.; Fantappiè, L.; Borghi, S.; Semchenko, E.A.; Schulz, B.L.; Ferlicca, F.; Taddei, A.R.; Giuliani, M.M.; et al. The meningococcal vaccine antigen GNA2091 is an analogue of YraP and plays key roles in outer membrane stability and virulence. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 12324–12335. [Google Scholar] [CrossRef]
- Salvi, A.; Bussinello, E.; Bajocchi, E. Experimental thyropathy by inoculation of Neisseria sicca into the apex of lingual V in rat. Boll Ist Sieroter Milan 1954, 33, 500–504. [Google Scholar] [PubMed]
- Salit, I.E.; Tomalty, L. Experimental meningococcal infection in mice-a model for mucosal invasion. Infect. Immun. 1986, 51, 648–652. [Google Scholar] [CrossRef]
- Saukkonen, K.; Abdillahi, H.; Poolman, J.T.; Leinonen, M. Protective efficacy of monoclonal antibodies to class 1 and class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model-new prospects for vaccine development. Microb. Path. 1987, 3, 261–267. [Google Scholar] [CrossRef]
- Welsch, J.A.; Moe, G.R.; Rossi, R.; Adu-Bobie, J.; Rappuoli, R.; Granoff, D.M. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 2003, 188, 1730–1740. [Google Scholar] [CrossRef]
- Vu, D.M.; Shaughnessy, J.; Lewis, L.A.; Ram, S.; Rice, P.A.; Granoff, D.M. Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis. Infect. Immun. 2012, 80, 643–650. [Google Scholar] [CrossRef]
- Vu, D.M.; Pajon, R.; Reason, D.C.; Granoff, D.M. A broadly cross-reactive monoclonal antibody against an epitope on the n-terminus of meningococcal fHbp. Sci. Rep. 2012, 2, 341. [Google Scholar] [CrossRef]
- Shaughnessy, J.; Lewis, L.A.; Zheng, B.; Carr, C.; Bass, I.; Gulati, S.; DeOliveira, R.B.; Gose, S.; Reed, G.W.; Botto, M.; et al. Human Factor H Domains 6 and 7 Fused to IgG1 Fc Are Immunotherapeutic against Neisseria gonorrhoeae. J. Immunol. 2018, 201, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.Z.; Glode, M.; Schneerson, R.; Robbins, J.B. Identification of immunoglobulin heavy-chain isotypes of specific antibodies of horse 46 group B meningococcal antiserum. J. Clin. Microbiol. 1982, 15, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Bumgarner, L.R.; Finkelstein, R.A. Pathogenesis and immunology of experimental gonococcal infection: Virulence of colony types of Neisseria gonorrhoeae for chicken embryos. Infect. Immun. 1973, 8, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.M.; Finkelstein, R.A. Pathogenesis and immunology of experimental gonococcal infection: Role of iron in virulence. Infect. Immun. 1975, 12, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.M.; Finkelstein, R.A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect. Immun. 1977, 18, 94–98. [Google Scholar] [CrossRef]
- Salit, I.E.; Gotschlich, E.C. Gonococcal color and opacity variants: Virulence for chicken embryos. Infect. Immun. 1978, 22, 359–364. [Google Scholar] [CrossRef]
- Baron, E.S.; Saz, A.K. Genetic transformation of pilation and virulence into Neisseria gonorrhoeae T4. J. Bacteriol. 1978, 133, 972–986. [Google Scholar] [CrossRef]
- Hafiz, S. Role of endotoxin in the pathogenicity of Neisseria gonorrhoeae colonial types 1, 4 and 5 determined by chicken embryo model. J. Med. Microbiol. 1986, 22, 63–67. [Google Scholar] [CrossRef]
- Foster, R.S.; Vinson, J.W. Chicken embryo as an animal model for gonorrhea. Infect. Immun. 1977, 16, 568–574. [Google Scholar] [CrossRef]
- Diena, B.B.; Lavergne, G.; Ryan, A.; Ashton, F.; Wallace, R. Transmission of immunity to Neisseria gonorrhoeae from vaccinated hens to embryos. Immunol. Commun. 1976, 5, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.N. Protection by monospecific gonococcal antisera of the chick embryo challenged with neisseria gonorrhoeae. J. Med. Microbiol. 1979, 12, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ashton, F.E.; Ryan, J.A.; Diena, B.B.; Frasch, C.E. Immunogenic and protective properties of meningococcal serotype 2a protein in the hen-embryo model. J. Med. Microbiol. 1983, 16, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M.; Meixell, B.W.; Van Hemert, C.; Hare, R.F.; Hueffer, K. Microbial Infections Are Associated with Embryo Mortality in Arctic-Nesting Geese. Appl. Env. Microbiol 2015, 81, 5583–5592. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.W.; Hellerud, B.C.; Thorgersen, E.B.; Castellheim, A.; Pharo, A.; Lindstad, J.; Tønnessen, T.I.; Brandtzaeg, P.; Mollnes, T.E. A new dynamic porcine model of meningococcal shock. Shock 2009, 32, 302–309. [Google Scholar] [CrossRef]
- Hellerud, B.C.; Nielsen, E.W.; Thorgersen, E.B.; Lindstad, J.K.; Pharo, A.; Tønnessen, T.I.; Castellheim, A.; Mollnes, T.E.; Brandtzaeg, P. Dissecting the effects of lipopolysaccharides from nonlipopolysaccharide molecules in experimental porcine meningococcal sepsis. Crit. Care Med. 2010, 38, 1467–1474. [Google Scholar] [CrossRef]
- Hellerud, B.C.; Olstad, O.K.; Nielsen, E.W.; Trøseid, A.M.; Skadberg, Ø.; Thorgersen, E.B.; Vege, Å.; Mollnes, T.E.; Brandtzæg, P. Massive Organ Inflammation in Experimental and in Clinical Meningococcal Septic Shock. Shock 2015, 44, 458–469. [Google Scholar] [CrossRef]
- Brusletto, B.S.; Hellerud, B.C.; Olstad, O.K.; Øvstebø, R.; Brandtzaeg, P. Transcriptomic changes in the large organs in lethal meningococcal shock are reflected in a porcine shock model. Front. Cell Infect. Microbiol. 2022, 12, 908204. [Google Scholar] [CrossRef]
- Wester, T.; Häggblad, E.; Awan, Z.A.; Barratt-Due, A.; Kvernebo, M.; Halvorsen, P.S.; Mollnes, T.E.; Kvernebo, K. Assessments of skin and tongue microcirculation reveals major changes in porcine sepsis. Clin. Physiol. Funct. Imaging 2011, 31, 151–158. [Google Scholar] [CrossRef]
- Barratt-Due, A.; Johansen, H.T.; Sokolov, A.; Thorgersen, E.B.; Hellerud, B.C.; Reubsaet, J.L.; Seip, K.F.; Tønnessen, T.I.; Lindstad, J.K.; Pharo, A.; et al. The role of bradykinin and the effect of the bradykinin receptor antagonist icatibant in porcine sepsis. Shock 2011, 36, 517–523. [Google Scholar] [CrossRef]
- Kaser, T.; Renois, F.; Wilson, H.L.; Cnudde, T.; Gerdts, V.; Dillon, J.R.; Jungersen, G.; Agerholm, J.S.; Meurens, F. Contribution of the swine model in the study of human sexually transmitted infections. Infect. Genet. Evol. 2018, 66, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Breshears, L.M.; Edwards, V.L.; Ravel, J.; Peterson, M.L. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.C.; Mostowy, S. The Case for Modeling Human Infection in Zebrafish. Trends. Microbiol. 2020, 28, 10–18. [Google Scholar] [CrossRef]
- Stream, A.; Madigan, C.A. Zebrafish: An underutilized tool for discovery in host-microbe interactions. Trends. Immunol. 2022, 43, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Schipper, K.; Preusting, L.C.; van Sorge, N.M.; Pannekoek, Y.; van der Ende, A. Meningococcal virulence in zebrafish embryos depends on capsule polysaccharide structure. Front. Cell Infect. Microbiol. 2022, 12, 1020201. [Google Scholar] [CrossRef]
- Dijokaite, A.; Humbert, M.V.; Borkowski, E.; La Ragione, R.M.; Christodoulides, M. Establishing an invertebrate Galleria mellonella greater wax moth larval model of Neisseria gonorrhoeae infection. Virulence 2021, 12, 1900–1920. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Ribeiro, F.C.; Dos Santos, H.F.S.; Dos Santos, J.D.; Oliveira, N.S.; Dutra, M.; de Lapena, S.A.B.; Junqueira, J.C. Galleria mellonella as an experimental model to study human oral pathogens. Arch. Oral. Biol. 2019, 101, 13–22. [Google Scholar] [CrossRef]
- Vilmos, P.; Kurucz, E. Insect immunity: Evolutionary roots of the mammalian innate immune system. Immunol. Lett. 1998, 62, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cloud, K.A.; Dillard, J.P. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 2002, 70, 2752–2757. [Google Scholar] [CrossRef]
- Weinstein, P. The Australian bushfly (Musca vetustissima Walker) as a vector of Neisseria gonorrhoeae conjunctivitis. Med. J. Aust. 1991, 155, 717. [Google Scholar] [CrossRef]
- Mikru, F.S.; Molla, T.; Ersumo, M.; Henriksen, T.H.; Klungseyr, P.; Hudson, P.J.; Kindan, T.T. Community-wide outbreak of Neisseria gonorrhoeae conjunctivitis in Konso district, North Omo administrative region. Ethiop. Med. J. 1991, 29, 27–35. [Google Scholar] [PubMed]
- Říhová, J.; Batani, G.; Rodríguez-Ruano, S.M.; Martinů, J.; Vácha, F.; Nováková, E.; Hypša, V. A new symbiotic lineage related to Neisseria and Snodgrassella arises from the dynamic and diverse microbiomes in sucking lice. Mol. Ecol. 2021, 30, 2178–2196. [Google Scholar] [CrossRef] [PubMed]
- Pierzchalski, J.L.; Bretl, D.A.; Matson, S.C. Phthirus pubis as a predictor for chlamydia infections in adolescents. Sex. Transm. Dis. 2002, 29, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakash, A.; Hoy, M.A.; Allsopp, M.H. Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J. Invertebr. Pathol. 2003, 84, 96–103. [Google Scholar] [CrossRef]
- Wei, T.; Hu, J.; Miyanaga, K.; Tanji, Y. Comparative analysis of bacterial community and antibiotic-resistant strains in different developmental stages of the housefly (Musca domestica). Appl. Microbiol. Biotechnol. 2013, 97, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Ssepuuya, G.; Wynants, E.; Verreth, C.; Crauwels, S.; Lievens, B.; Claes, J.; Nakimbugwe, D.; Van Campenhout, L. Microbial characterisation of the edible grasshopper Ruspolia differens in raw condition after wild-harvesting in Uganda. Food Microbiol. 2019, 77, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Nones, S.; Simões, F.; Trindade, C.S.; Matos, J.; Sousa, E. Microbiome Associated with the Mycangia of Female and Male Adults of the Ambrosia Beetle Platypus cylindrus Fab. (Coleoptera: Curculionidae). Insects 2021, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Clark, J.M. The 3Rs in research: A contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 2018, 120, S1–S7. [Google Scholar] [CrossRef]
- Heydarian, M.; Rühl, E.; Rawal, R.; Kozjak-Pavlovic, V. Tissue Models for Neisseria gonorrhoeae Research-From 2D to 3D. Front. Cell Infect. Microbiol. 2022, 12, 840122. [Google Scholar] [CrossRef]
- Patrício, D.; Santiago, J.; Mano, J.F.; Fardilha, M. Organoids of the male reproductive system: Challenges, opportunities, and their potential use in fertility research. WIREs Mech. Dis. 2022, 15, e1590. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ronfard, V. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): A mini review. Int. J. Burn. Trauma 2013, 3, 173. [Google Scholar]
- Wang, F.; Song, Q.; Du, L.; Wu, X. Development and characterization of an acellular porcine small intestine submucosa scaffold for use in corneal epithelium tissue engineering. Curr. Eye Res. 2020, 45, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Heydarian, M.; Schweinlin, M.; Schwarz, T.; Rawal, R.; Walles, H.; Metzger, M.; Rudel, T.; Kozjak-Pavlovic, V. Triple co-culture and perfusion bioreactor for studying the interaction between Neisseria gonorrhoeae and neutrophils: A novel 3D tissue model for bacterial infection and immunity. J. Tissue Eng. 2021, 12, 2041731420988802. [Google Scholar] [CrossRef]
- Chang, C.C.; Krishnan, L.; Nunes, S.S.; Church, K.H.; Edgar, L.T.; Boland, E.D.; Weiss, J.A.; Williams, S.K.; Hoying, J.B. Determinants of microvascular network topologies in implanted neovasculatures. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 5–14. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Nakamura, M.; Henmi, C.; Yamaguchi, K.; Mochizuki, S.; Nakagawa, H.; Takiura, K. Development of a Three-Dimensional Bioprinter: Construction of Cell Supporting Structures Using Hydrogel and State-of-the-Art Inkjet Technology. J. Biomech. Eng. 2009, 131, 035001. [Google Scholar] [CrossRef]
- Köpf, M.; Campos, D.F.D.; Blaeser, A.; Sen, K.S.; Fischer, H. A tailored three-dimensionally printable agarose–collagen blend allows encapsulation, spreading, and attachment of human umbilical artery smooth muscle cells. Biofabrication 2016, 8, 025011. [Google Scholar] [CrossRef]
- Kucukgul, C.; Ozler, B.; Karakas, H.E.; Gozuacik, D.; Koc, B. 3D hybrid bioprinting of macrovascular structures. Procedia Eng. 2013, 59, 183–192. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Blaeser, A.; Weber, M.; Jäkel, J.; Neuss, S.; Jahnen-Dechent, W.; Fischer, H. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 2012, 5, 015003. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Pensabene, V.; Markov, D.A.; Allwardt, V.; Neely, M.D.; Shi, M.; Britt, C.M.; Hoilett, O.S.; Yang, Q.; Brewer, B.M. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015, 9, 054124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Griep, L.M.; Wolbers, F.; de Wagenaar, B.; ter Braak, P.M.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Vermes, I.; van der Meer, A.D.; van den Berg, A. BBB on chip: Microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed. Microdevices 2013, 15, 145–150. [Google Scholar] [CrossRef]
- Taylor-Robinson, D.; Whytock, S.; Green, C.; Carney, F., Jr. Effect of Neisseria gonorrhoeae on human and rabbit oviducts. Br. J. Vener. Dis. 1974, 50, 279. [Google Scholar] [CrossRef]
- Johnson, A.P.; Clark, J.B.; Osborn, M.F.; Taylor-Robinson, D. A comparison of the association of Neisseria gonorrhoeae with human and guinea-pig genital mucosa maintained in organ culture. Br. J. Exp. Pathol. 1980, 61, 521–527. [Google Scholar]
- Churchward, C.P.; Snyder, L.A.S. In Vitro Models of Eye Infection with Neisseria gonorrhoeae. Methods Mol. Biol. 2019, 1997, 363–376. [Google Scholar] [CrossRef]
- Stephens, D.S. Gonococcal and meningococcal pathogenesis as defined by human cell, cell culture and organ culture assays. Clin. Microbiol. Rev. 1989, 2, S104–S111. [Google Scholar] [CrossRef]
- Jacobsson, S.; Golparian, D.; Oxelbark, J.; Wicha, W.W.; da Costa, R.M.A.; Franceschi, F.; Brown, D.; Louie, A.; Gelone, S.P.; Drusano, G.; et al. Pharmacodynamic evaluation of lefamulin in the treatment of gonorrhea using a hollow fiber infection model simulating Neisseria gonorrhoeae infections. Front. Pharm. 2022, 13, 1035841. [Google Scholar] [CrossRef]
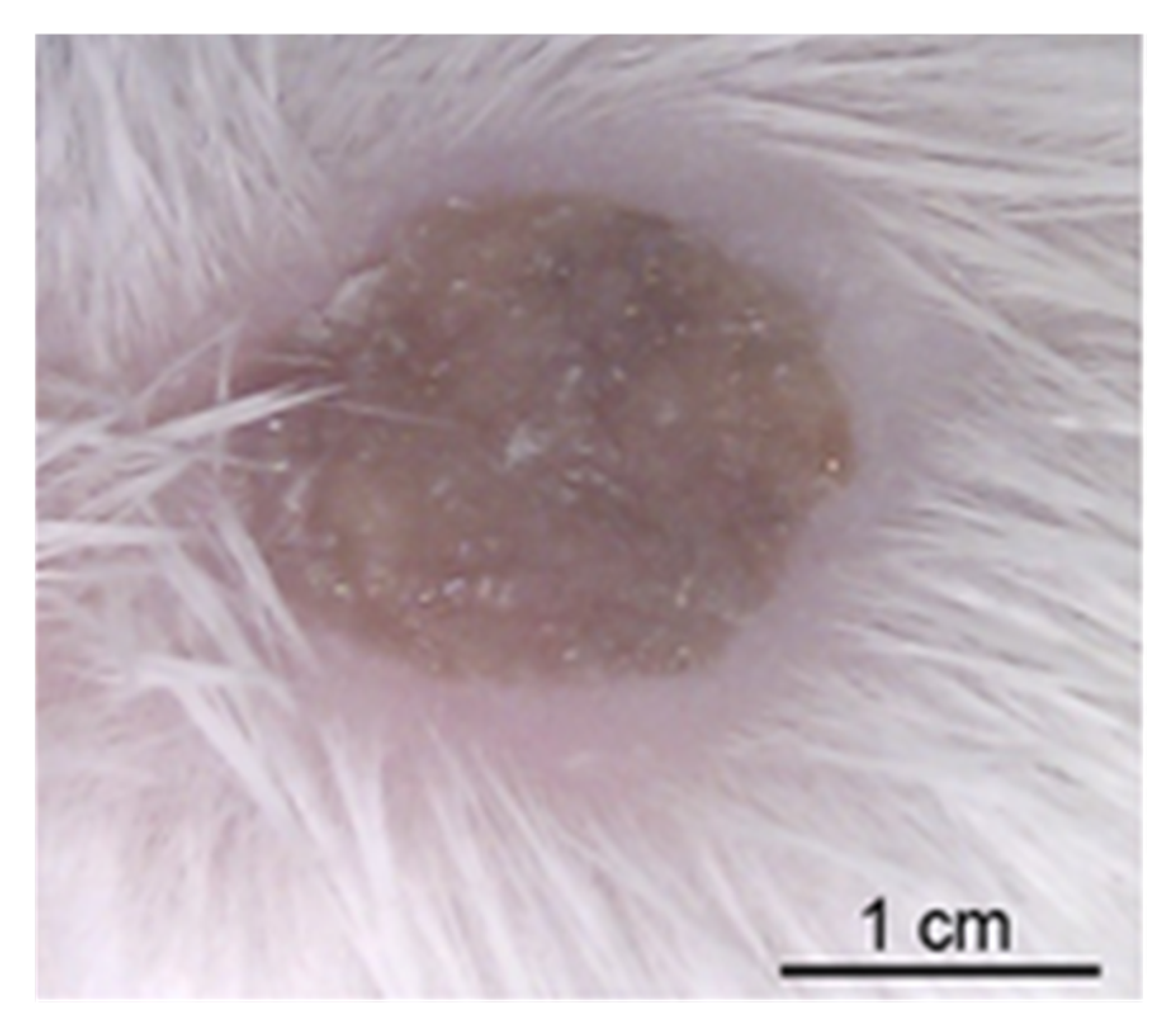
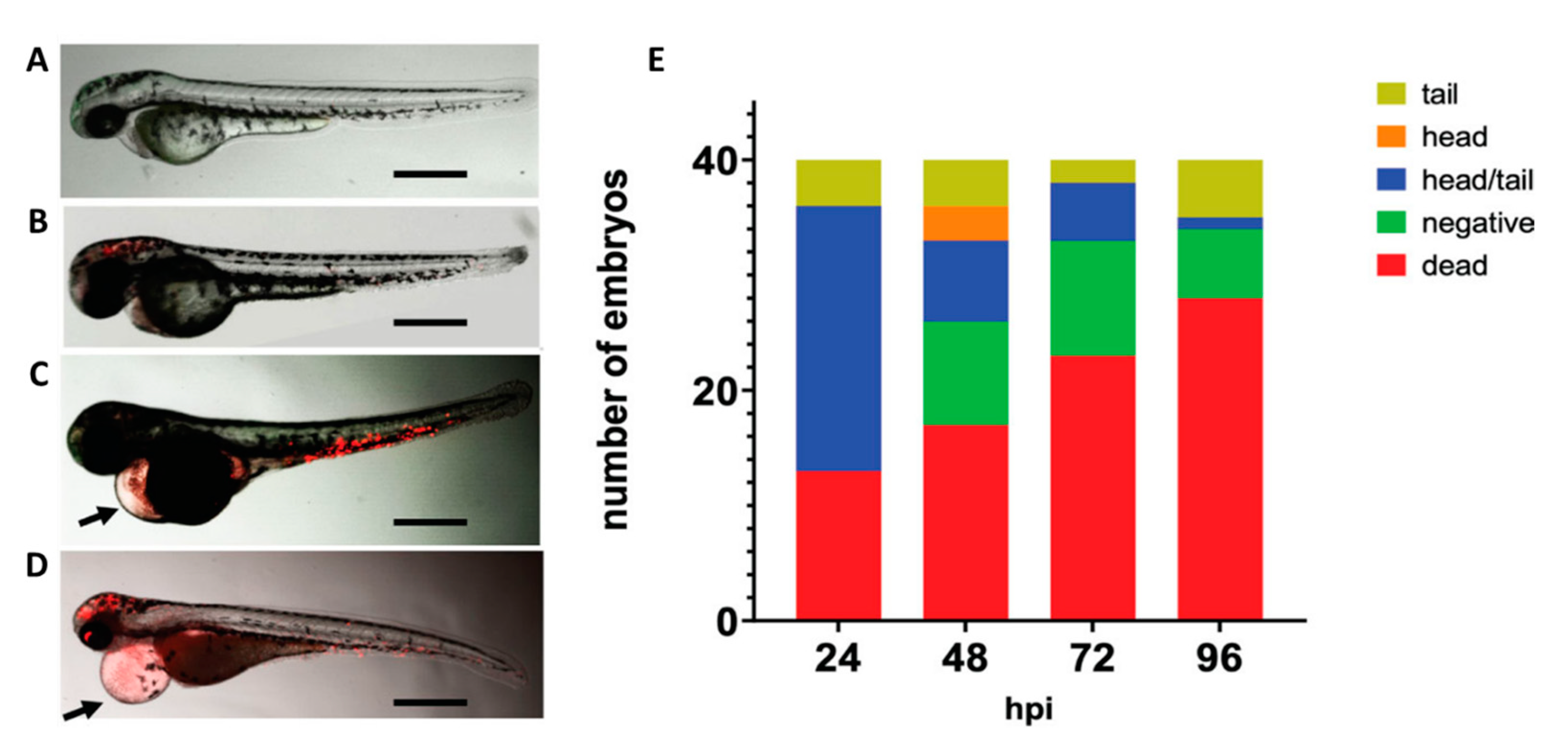
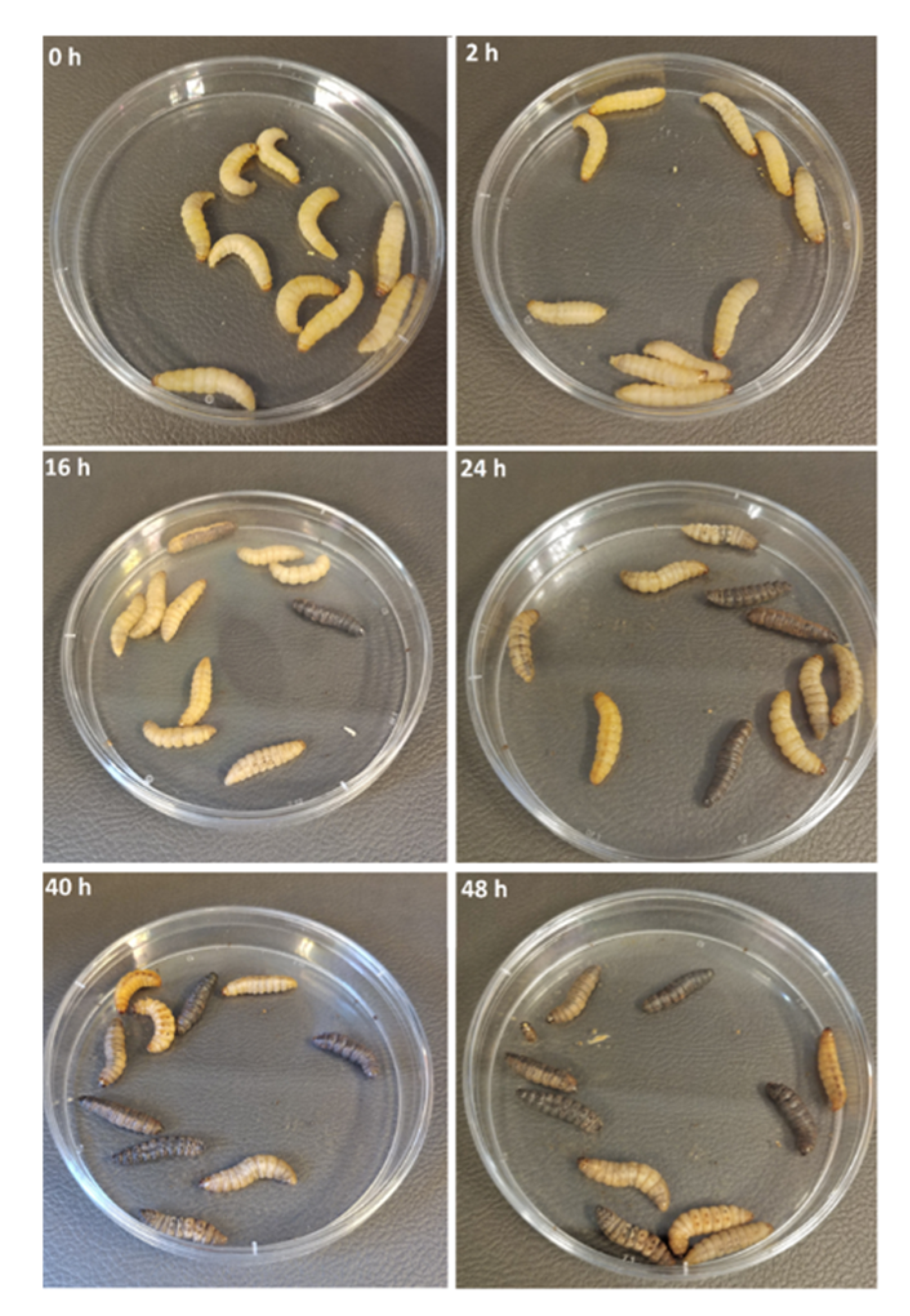
| Rhesus Macaque Monkeys Used in Neisseria Vaccine-Related Research | Reference | |
|---|---|---|
| Vaccine studies | Immunization with meningococcal OMV vaccine with overexpressed mutant factor H binding protein (infant RMs) | [75] |
| Trivalent native OMVs derived from three genetically modified serogroup B meningococci (infant RMs) | [76] | |
| MenB-4C immunization (infant RMs) | [68,77,78] | |
| MenB capsular polysaccharide–protein conjugate vaccines (juvenile RMs) | [79,80] | |
| Chemically modified Escherichia coli K1 N-propionylated polysialic acid coupled to purified recombinant rPorB | [81] | |
| Adjuvant studies | Bacillus anthracis polyglutamic acid capsule covalently conjugated to the N. meningitidis serotype B OM protein complex | [82,83] |
| Recombinant modified human vascular endothelial growth factor (VEGF) combined with proteoliposomes of meningococcal OM | [84] | |
| Plasmodium falciparum circumsporozoite protein NANP6 peptide conjugated to N. meningitidis serotype B OM protein (in adult RM) | [85] | |
| Domain III of the dengue 1 virus fused to meningococcal P64k protein (in adult RM) | [86] | |
| P. falciparum recombinant Pfs25H transmission-blocking protein conjugated to N. meningitidis serotype B OM protein complex | [87] | |
| Influenza virus A M2 peptide-N. meningitidis serotype B OM protein complex conjugate | [88] | |
| Pneumococcal capsular polysaccharide (Ps)–N. meningitidis serotype B OM protein complex conjugate | [89,90] | |
| Haemophilus influenzae type B polysaccharide conjugated to meningococcal Class 2 porin (in infant RMs) | [91] | |
| H. influenzae type B polysaccharide–meningococcal OM protein complex conjugate | [92] | |
| Insect | Species | Reference |
|---|---|---|
| Musca vetustissima Walker (Australian bushfly) | N. gonorrhoeae | [295] |
| Apis mellifera capensis and Apis mellifera scutellata (worker honeybees) | Sequences related to Simonsiella/Neisseria (amongst others) | [299] |
| Musca domestica (housefly) | Neisseria in pupae; denaturing gradient gel electrophoresis analysis identifies N. gonorrhoeae NCTC83785 (X07714) and Neisseria sp.93S1 (EU370420) | [300] |
| Ruspolia differens (edible grasshopper) | Neisseria (operational taxonomic unit) | [301] |
| Platypus cylindrus Fab. (Ambrosia Beetle) | Neisseria spp. | [302] |
| Hoplopleura acanthopus, Polyplax microbiomes (louse) | Neisseriaceae-related bacterium (dominant obligate symbiont) | [297] |
| Pathogen | Animal | Aspect of Human Infection Mimicked by the Animal | Potential of Model | In Use Today |
|---|---|---|---|---|
| Neisseria gonorrhoeae | Ng CHIM | Natural urethral male challenge model; mimics clinical symptoms of pus urethritis | Essential for studying transmission, inflammation, re-infection, changing bacterial population dynamics, antigen variation, immunity and immunosuppression and vaccine trials. | Yes |
| Chimpanzee | Mimics clinical symptoms of pus urethritis and pharyngitis |
| No | |
| Rhesus macaque | Colonization of nasal cavity and epithelium covering the cribiform plate, and persistence | Potential for studying
| Limited | |
| Wild-type mouse | Used to mimic histopathology of DGI, neutrophil infiltration, pneumonia | Most models have fallen into disuse as wild-type mice are not naturally infected with gonococci, and aspects of histopathology are dependent on route of infection and iron supplementation. | No | |
| ‘Humanized’ mouse | Aspects of the human inflammatory and cellular response and neutrophil infiltration | Transgenic mice overcome the resistance to natural colonization by gonococci and are useful for studying the cellular biology of infection. | Yes | |
| Mouse model of intravaginal infection | Hormone-influenced colonization of the lower genital tract mucosa | Important for studying gonococcal
| Yes | |
| Mouse model of upper genital tract infection | Mimics tissue invasion, inflammatory cytokine production and neutrophil infiltration | Useful for studying aspects of disseminated infection, including endometritis, salpingitis (Fallopian tube) and peritonitis, and associated organ damage and potentially infertility. | Yes | |
| Guinea pig | Pathological findings as with mouse | Guinea pigs have the advantages of sensitivity to infection, less variable in response, free of interfering micro-organisms, ready availability. | No | |
| Rabbit | Conjunctivitis, endocarditis, arthritis, pelvic inflammatory disease | Useful for studying aspects of gonococcal pathogenesis and host response, but largely superseded by small rodent models, which can provide animal numbers for better reproducibility. | No | |
| Rat | Disseminated infection and pelvic inflammatory disease; arthritis | Useful for studying induced pelvic inflammatory disease processes. | No | |
| Chicken embryo | Lethality | Useful for studying gonococcal virulence factors, immunity, and for passive protection studies of anti-gonococcal sera. | No | |
| Neisseria meningitidis | Wild-type adult mouse | Peritonitis, bacteraemia, sepsis, meningitis, kidney damage | Useful for studying aspects of systemic meningococcal disease, pathogen virulence factors and host innate immune responses. Vaccine studies—active and passive protection against infection and generation of bactericidal and/or opsonophagocytic antibodies. | Yes |
| Knock-out mice | Peritonitis, bacteraemia, sepsis, meningitis, kidney damage | Useful for studying aspects of systemic meningococcal disease host innate immune responses. Vaccine studies—active and passive protection against infection and generation of bactericidal and/or opsonophagocytic antibodies. | Yes | |
| Neonatal mouse | Intranasal infection leading to colonization, bacteraemia, meningitis and ventriculitis | Useful for studying mucosal invasion and pathogen virulence factors and host innate immune responses. | Yes | |
| Adult mouse human skin xenograft | Replicates purpura fulminans | Useful for deeper understanding of vascular colonization by meningococci and the inflammatory process that generates purpura fulminans, and virulence factors involved. | Limited | |
| Guinea pig | Acute meningitis | As above, but more generally useful for vaccine studies. | No | |
| Rabbit | Meningococcal shock and meningitis | Useful for studying systemic meningococcal disease, but largely superseded by small rodent models. | No | |
| Infant rat | Bacteraemia, peritonitis, meningitis | Used extensively for pathogenesis studies and for active and passive immunization studies. | Yes | |
| Pig | Sepsis | Established model for studying the pathophysiology of endotoxic shock and anti-sepsis therapies. | Limited | |
| Zebrafish | Innate immune responses to infection | Not generally used, yet. Potential for studying bacteria–host interactions, pathogen interactions with the innate immune system, high-resolution imaging of bacterial interactions with cellular barriers, e.g., the blood–cerebrospinal fluid/brain barriers. | Limited | |
| Neisseria lactamica | Nlac CHIM | Neisseria colonization of nasopharynges | Useful for studying Neisseria colonization and carriage, host mucosal immunity, vaccine delivery with genetically modified bacteria. | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girgis, M.M.; Christodoulides, M. Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology. Pathogens 2023, 12, 782. https://doi.org/10.3390/pathogens12060782
Girgis MM, Christodoulides M. Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology. Pathogens. 2023; 12(6):782. https://doi.org/10.3390/pathogens12060782
Chicago/Turabian StyleGirgis, Michael M., and Myron Christodoulides. 2023. "Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology" Pathogens 12, no. 6: 782. https://doi.org/10.3390/pathogens12060782
APA StyleGirgis, M. M., & Christodoulides, M. (2023). Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology. Pathogens, 12(6), 782. https://doi.org/10.3390/pathogens12060782






