Abstract
Allogeneic stem cell transplantation is a lifesaving treatment for many malignancies. Post-transplant patients may suffer from graft versus host disease in the acute and/or the chronic form(s). Post-transplantation immune deficiency due to a variety of factors is a major cause of morbidity and mortality. Furthermore, immunosuppression can lead to alterations in host factors that predisposes these patients to infections. Although patients who receive stem cell transplant are at an increased risk of opportunistic pathogens, which include fungi and viruses, bacterial infections remain the most common cause of morbidity. Here, we review bacterial pathogens that lead to pneumonias specifically in the chronic GVHD population.
1. Introduction
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a lifesaving treatment for a multitude of benign and malignant diseases [1]. Annually, more than 50,000 alloHSCTs are performed worldwide [1,2]. Pulmonary complications remain a major contributor to morbidity and mortality following alloHSCT. Etiologies include non-infectious diseases, as well as lung infections caused by bacteria, fungi, and viruses [1].
Despite the utilization of antimicrobial prophylaxis and healthcare infection prevention measures, bacterial infections continue to cause significant morbidity and mortality in alloHSCT recipients [1,3]. Recent data report that up to 20–30% of alloHSCT recipients develop at least one episode of pneumonia, with bacteria being the predominant causative pathogen [3,4,5]. Increased susceptibility to bacterial organisms occurs due to alterations in the immune system, disruption of the microbial flora, lung architectural derangements, and malnutrition [3]. In addition, the frequent and prolonged exposures to healthcare systems increase the risk of acquiring nosocomial pathogens, including resistant bacteria [3].
The immunocompromised status following alloHSCT is multifactorial and affects multiple pathways of immune function. High-intensity cytotoxic conditioning chemotherapy is generally administered in the days prior to the infusion of allogeneic donor stem cells. This preparatory regimen functions both to eradicate any residual malignancy and to prevent native lymphocytes from attacking donor cells to optimize chances for successful engraftment. The preparative cytotoxic chemotherapy regimen targets rapidly dividing cells and, therefore, destroys hematopoietic cells and causes damage to mucosal barriers [6]. This disruption of oral, respiratory, and gut mucosa allow organisms to invade or relocate into underlying tissues. Recipients of alloHSCT are generally neutropenic for more than 10–14 days until engraftment of donor neutrophils, which places them at high risk for infections caused by bacteria and other pathogens. Furthermore, the recovery of lymphocyte cells and function can take months to years depending on cell source and iatrogenic immunosuppression post alloHSCT, causing prolonged deficiencies in cellular and humoral immunity [7]. Graft-versus-host-disease (GVHD) is a multisystem alloreactive inflammatory process by which donor lymphocytes recognize recipient tissue as “non-self” and can lead to significant multi-organ dysfunction. GVHD is a leading cause of morbidity and mortality in alloHSCT recipients and generally requires immunosuppression both prophylactically in the early months post alloHSCT, as well as for treatment of acute GVHD flares [8]. The depth and duration of this immunosuppression directly influences risk for opportunistic infections [9]. Risk for infection and GVHD post alloHSCT varies according to conditioning regimens, donor type (related versus unrelated), recipient traits (gender, age, and CMV serostatus), HLA match (matched, haploidentical, and mismatch), and cell source (peripheral blood, bone marrow, and umbilical cord), among other factors [10,11].
2. Chronic GVHD
Chronic graft versus host disease (cGVHD) is defined based on standard criteria defined by the National Institute of Health and is divided into a limited and extensive form [12]. The cGVHD population usually has dysfunctional cellular and humoral immunity, which is compounded by immunosuppressive agents used for its treatment [12,13]. The incidence of pneumonia declines by 100 days post alloHSCT with the exception of patients with cGVHD [1]. Nearly 28% of patients with cGVHD have three or more infections by 6 months post transplant [14]. Chronic GVHD causes inflammation, tissue injury, lymphoid organ dysfunction (including spleen, and thymus), dysregulated T and B cell responses, and abnormal tissue repair, often leading to fibrosis [14,15]. These complex processes result in cGVHD manifestations such as bronchiolitis or sclerodema and induce prolonged cellular and humoral immune deficits. Encapsulated bacteria, such as Streptococcus pneumoniae and Haemophilus influenzae, have been dominantly seen in this population [1,12,16,17,18]. Pneumonia in the chronic GVHD patient carries a fivefold risk for mortality [12]. We hereby present all other cases reported in the literature of bacterial pathogens causing pneumonia in the chronic GVHD population.
3. Pathogenesis of Bacterial Pneumonia
Bacteria reach the lung through inhalation, aspiration, migration from the proximal airway, or hematogenous spread [3]. The majority of the pathogens are generally expelled via the mucociliary process along with other particulates trapped in the viscous and elastic fluid that lines the airways. Bacteria need to breach normal barrier defenses to reach the lung periphery [3].
Both structural and immunologic barriers protect the lungs from entry of invading pathogens. In an immunocompetent host, these barriers are often effective in eliminating most infections [3]. However, the resulting immune reaction in response to infection causes tissue injury and systemic inflammation [3]. Pneumonia, as a syndrome, is a culmination of these responses. It constitutes the radiographic findings that happen as a result of airspace filled by edema, debris, and white blood cells along with the systemic response fever and leukocyte elevation along with a productive cough [3].
Cancer, along with its treatments, leads to changes in both the innate and adaptive responses to a bacterial pathogen [3]. In addition, functional and anatomical defects may arise either directly related to the underlying neoplasm or its associated therapy. Complications related to therapy may result in a need for hospitalization and invasive procedures, which increases risk for acquiring nosocomial pathogens [3]. Furthermore, due to impaired immune function, the clinical presentation or radiographic findings of pneumonia may be blunted, sometimes leading to delayed diagnosis [3].
Patients with GHVD can be uniquely susceptible to bacterial pneumonias secondary to chronic inflammation and damage to tissue within airways and lungs along with the chronic deficits in cell-mediated and humoral immunity [3]. Immunosuppression for active GVHD will only increase the risk of infection and susceptibility to bacteria [3].
4. Methods
We performed a systematized review with EMBASE (via embase.com, 1974-present version) and simultaneous MEDLINE and CINAHL searches (via EBSCOhost). Search strategies included subject headings and keywords for the three search concepts: (1) hematopoietic transplant, (2) chronic GVHD, and (3) bacterial pneumonia (see complete search strategies in the appendix).
Filters were used to remove conference abstracts from the EMBASE results and to split all search results into three groups: (1) articles indexed as case reports, (2) review articles not indexed as case reports, and (3) all articles not retrieved by the case report or review search. No language or publication date filters were applied. All result groups were added to the project’s EndNote database. Both EndNote and Zotero duplicate detection tools were used to identify duplicates. Results concerning pediatric cases were then separated by searching the EndNote database for records containing words beginning with “pediatric”, “paediatric”, “infan”, “neonat”, “newborn”, or “adolescen”, but not containing “adult”.
The 182 total EMBASE search results and the 125 total results of the simultaneous MEDLINE and CINAHL searches were imported into our review’s EndNote database. After removal of the 82 duplicate records identified by the EndNote and Zotero duplicate detection tools, 225 records for unique articles remained for the title abstract review. We chose the 40 that were most relevant and with no redundancy of information for inclusion in this review; cases of pneumonia due to Streptococcus pneumoniae or Haemophilus influenzae were excluded (Figure 1).
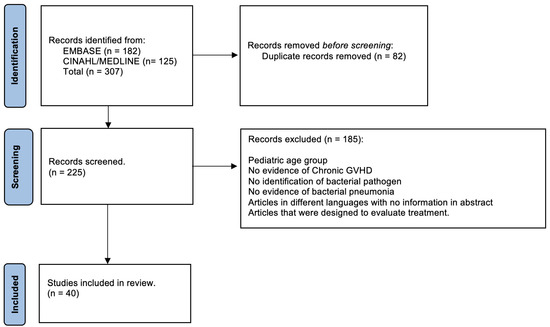
Figure 1.
Flow chart diagram highlighting methodology.
5. Mycobacterium Tuberculosis
The incidence of tuberculosis (TB) varies from 0.001% to more than 10% in highly endemic countries [19]. The incidence of active disease amongst alloHSCT recipients is nearly triple compared with autologous HSCT recipients, with the lungs being the most affected organ [1,19]. Patients with cGVHD are particularly susceptible given prolonged cellular immune dysfunction. Use of specific agents to treat GVHD, including corticosteroids, ruxolitinib, and anti-CD52 therapies augment the risk for active TB [20]. Mortality secondary to TB pneumonia can reach up to 50%; hence, early recognition and intervention is important [19]. Findings on imaging vary from infiltrates, miliary pattern, nodules, pleural effusions, or cavitary lesions [19]. While nucleic acid testing has a sensitivity of 84% and a specificity of 99%, false-negative results may occur in the setting of recent TB exposure and low burden of mycobacteria within a specimen [19]. Culture continues to be the gold standard for diagnosis [19]. In order to prevent reactivation of TB post alloHSCT, it is important to treat for latent TB in patients with abnormal interferon-gamma release assays or tuberculin skin test with ≥5 mm induration.
The cases below (Table 1) describe the characteristics of patients with pulmonary TB along with geographic distribution. Erdstein et al. described two cases of pulmonary TB associated with cGVHD. One presented as a pleural effusion, while the other (case 1) presented as pneumonia [21]. The reported cases were from Burma, Portugal, Taiwan, Turkey, Spain, and Hong Kong.

Table 1.
Characteristics of patients extrapolated from the literature who suffered from mycobacterium tuberculosis.
6. Nontuberculous Mycobacterial Infections
Nontuberculous mycobacterial (NTM) infections are more frequent in alloHSCT recipients compared with the general population, particularly among patients with pulmonary cGVHD [1,29] Recent data report that pulmonary NTM occurred in up to 2.9% of patients who received alloHSCT [30]. Treatment and clinical appearance are typical of the general population [1].
The use of macrolides in the treatment of post-alloHSCT patients who develop bronchiolitis obliterans syndrome (BOS) is controversial [29]. More recent data suggest an association with negative outcomes, especially worse airflow-free survival and stimulation of immune cells that increase the risk of relapse [31,32]. The chronic immunocompromised state following alloHSCT, including the use of numerous immunosuppressants, is linked to a significantly greater incidence rate of NTM infection in patients receiving alloHSCT than in the general population [29]. BOS appears to be a further risk factor for the development of NTM infection, presumably reflecting an immunological condition brought on by GVHD [29].
Table 2 below summarizes all the cases noted in the literature that were able to identify the species of NTM along with patient characteristics. Cases 1, 2, and 3 were identified as part of the M. abscessus complex [29]. Differentiation between the subspecies of the M. abscessus complex, M. abscessus and M. massiliense, is important as it may affect treatment outcomes [29].
Liue et al. studied the incidence, risk factors, and survival post alloHSCT in an Asian academic center in a high endemic area. They performed a retrospective review over an 11-year span and identified 17 patients with chronic GVHD who had NTM infection. Nearly 60% of the patients had extensive GVHD with limited disease in the rest. The identification of the NTM species was performed in three patients and included M. kansasii, M. avium complex, and M. chelonae, while the rest were unclassified. In the M. kansaii case, the pathogen was isolated from spinal biopsy and a knee joint. The characteristics of the other two patients are noted below (cases 9 and 10) [24].
Cases 11 through 20 show the characteristics of patients who were treated for NTM in a University hospital in South Korea [33]. The immunosuppressants used where unknown, and it was also unknown if patients were on a macrolide part of BO management [33].

Table 2.
Characteristics of patients who suffered from non-tuberculous mycobacterial infections.
Table 2.
Characteristics of patients who suffered from non-tuberculous mycobacterial infections.
| Patient | Age/Gender | Cancer | cGHVD/Organ Involved | Immunosuppressive | Macrolide Use | Lung Radiographic Features | NTM | Ref |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 27/M | ALL | Yes/Lung (BOS) | CST + Tacrolimus | Yes | Fibrocavitary | M. abscessus | [29] |
| Case 2 | 47/M | Lymphoma | Yes/Lung (BOS) | CST + Tacrolimus | Yes | Nodular and Bronchiectatic | M. abscessus | [29] |
| Case 3 | 48/M | Lymphoma | Yes/Lung (BOS) | CST + Tacrolimus + ICS | Yes | Nodular and Bronchiectatic | M. massiliense | [29] |
| Case 4 | 34/M | ALL | Yes/Lung (BOS) + skin + sclera | CST | No | Cavitary nodules | M. chelonae | [30] |
| Case 5 | 29/M | AML | Yes/Skin | CST + Cyclosporine + Azathioprine | No | Bronchiectasisand cavitary nodules | M. chelonae | [34] |
| Case 6 | 33/M | CML | Yes/Skin + Liver | CST + Cyclosporine | No | Miliary pattern | Mycobacterium fortuitum chelonaecomplex | [35] |
| Case 7 | 40/F | CML | Yes/Unknown | Unknown | Unknown | Cavitary nodule | Mycobacterium avium complex | [36] |
| Case 8 | 66/F | MDS | Yes/Skin | CST + cyclosporin A + methotrexate + ECP + Ruxolitinib | Yes | Ground-glass opacities + pulmonary infiltrate | Mycobacterium abscessus | [37] |
| Case 9 | 49/F | AML | Yes/Skin + Mucosae | Unknown | Unknown | Unknown | Mycobacterium avium complex | [24] |
| Case 10 | 55/F | AML | Yes/Skin + Mucosa + Lung | Unknown | Unknown | Unknown | Mycobacterium chelonae | [24] |
| Case 11 | 31/F | ALL | Yes/Lung (BOS) | Yes (Unknown) | Unknown | Normal | M. abscessus | [33] |
| Case 12 | 34/M | Lymphoma | Yes/Lung | Yes (Unknown) | Unknown | Nonspecific pneumonia | M. abscessus | [33] |
| Case 13 | 21/M | AML | Yes/Lung | Yes (Unknown) | Unknown | Nonspecific pneumonia | M. intracellulare | [33] |
| Case 14 | 52/M | AML | Yes/Lung | Yes (Unknown) | Unknown | Bronchiectasis + nodules or infiltrate or tree-in-bud | M. avium | [33] |
| Case 15 | 49/M | CML | Yes/Lung (BOS) | Yes (Unknown) | Unknown | Cavitary pneumonia | M. abscessus | [33] |
| Case 16 | 43/M | CML | Yes/Lung (BOS) | No | Unknown | Nonspecific pneumonia | M. avium | [33] |
| Case 17 | 19/M | ALL | Yes/Lung (BOS) | Yes (Unknown) | Unknown | Cavitary pneumonia | M. fortuitum | [33] |
| Case 18 | 28/F | AML | Yes/Lung (BOS) | Yes (Unknown) | Unknown | Cavitary pneumonia | M. intracellulare | [33] |
| Case 19 | 40/M | AML | Yes/Lung | Yes (Unknown) | Unknown | Bronchiectasis + nodules or infiltrate or tree in bud | M. intracellulare | [33] |
| Case 20 | 48/M | AML | Yes/Lung (BOS) | Yes (Unknown) | Unknown | Cavitary pneumonia | M. intracellulare | [33] |
CST = Corticosteroids.
7. Legionnaires’ Disease
Legionella is an intracellular Gram-negative bacterium of environmental origin (particularly water sources) that most commonly presents as pneumonia in an entity termed Legionnaires’ disease (LD) [38,39]. It was first described in 1976 after a fatal outbreak of respiratory illness following a American Legion convention and was attributed to contamination within the hotel’s air conditioning system [40]. More than 50 species are recognized, and the most common to cause disease in humans is Legionella pneumophilia serogroup 1 [40]. Legionellosis is becoming more widely acknowledged as a cause of pneumonia due to the development of more accurate diagnostic testing techniques; in the US, its prevalence increased 217% from 2000 (n = 1110) to 2009 (n = 3522) [40]. While LD can affect immunocompetent hosts, immunocompromised patients with solid tumors or hematological malignancies; solid organ transplants; or immunosuppressive medications such as tumor necrosis factor (TNF) inhibitors, corticosteroids, or antirejection medications are at increased risk [39]. Most importantly, impaired cellular immunity increases risk for severe illness due to Legionella [38]. Legionella is often acquired via community exposure either by aerosolization or aspiration of freshwater reservoirs. In addition, Legionella has been associated with nosocomial outbreaks, including within transplant centers [41]. Table 3 describes the cases of LD that have been found in the literature affecting the chronic GVHD population. Case 1 and case 2 were cases that relapsed after initial therapy and progressed to a lung infection [39]. Case 3 was nonresponsive to initial therapy and developed a lung abscess [42]. Case number 4 had a progressive case of legionellosis with skin involvement [40].

Table 3.
Characteristics of patients who suffered from Legionnaires’ disease.
8. Nocardia
Nocardia is an abundant Gram-positive, aerobic bacterium found worldwide in soil, water, and decaying vegetation [43]. Pulmonary infection, generally acquired via inhalation, can present as an acute, subacute, or chronic illness. Most common clinical symptoms are fever and cough but can also manifest as non-specific night sweats, fatigue, and malaise. Radiographically, it can present as pulmonary nodules, mass-like consolidations, infiltrates, or pleural effusion [43]. Infection of the central nervous system via hematogenous spread occurs in up to 20–50% of nocardiosis [43]. Nocardiosis is rare among alloHSCT recipients, with incidence being between 0.3 and 1.7% [43]. Trimethoprim-sulfamethoxazole is commonly used as a prophylaxis against Pneumocystis jiroveci pneumonia (PJP) in alloHSCT recipients and is often effective in preventing infection due to Nocardia sp. [43]. However, despite intermittent prophylactic TMP-SMX administration, some transplant recipients develop nocardiosis, demonstrating that infection risk likely depends on both the dosage of prophylactic TMP-SMX and other factors [43]. The use of atovaquone or other alternatives to PJP prophylaxis is associated with an increased risk of nocardiosis [43]. The majority of the cases reported were not receiving TMP-SMX for PJP prophylaxis but on inhaled pentamidine, atovaquone, and dapsone (Table 4) [43,44,45,46]. Cases 3, 9, 13, 14, and 15 were on TMP-SMX [43,47,48,49]. Case 3 was on TMP-SMX prophylaxis, 80–400 mg; case 9 was on TMP-SMX, 80/400 mg; case 14 was on TMP-SMX, 20 mg/kg; and case 15 was on TMP-SMX, 160/800 mg [43,47,48,49].

Table 4.
Characteristics of patients who suffered from Nocardiosis.
9. Pseudomonas Aeruginosa
Pseudomonas aeruginosa is a Gram-negative, aerobic, rod-shaped bacterium that can be isolated from environmental reservoirs including soil, plants, and animal tissue [51,52]. Using its potent binding components, including as flagella, pili, and biofilms, this bacteria can survive on water, various surfaces, and medical equipment [51]. P. aeruginosa is therefore prevalent in both natural and artificial settings, such as lakes, hospitals, and domestic sink drains [51]. A variety of diseases in humans are brought on by the opportunistic bacterium Pseudomonas aeruginosa [51]. It is now a significant contributor to antibiotic resistance and nosocomial infections [51]. Pseudomonas aeruginosa is a type of opportunistic bacteria that has been linked to a number of healthcare-associated infections, such as ventilator-associated pneumonia (VAP), bloodstream infections from central lines, surgical site infections, urinary tract infections, burn wound infections, keratitis, and otitis media [51]. It is a bacterium that can quickly acquire antibiotic resistance, adapt to environmental changes, and produce a wide range of virulence factors [51]. Due in part to the infection’s capacity to defy both innate and acquired immune defenses through adhesion, colonization, and biofilm formation, as well as the production of different virulence factors that cause severe tissue damage, this pathogen can impact immunocompromised people [51]. Moreover, it contributes to illnesses with high death rates in people with cystic fibrosis, infections in newborns, cancer, and severe burns [51]. The most significant risk factors include structural lung illnesses, hematological neoplasms, transplantation, skin burns, recently used antibiotics, the presence of implants, prolonged hospitalization, and mechanical ventilation [51]. Eleven cases were found to have infections due to P. aeruginosa (Table 5) [36,53,54,55]. All patients received an allogeneic transplant and one patient had bronchiectasis [36,53,54,55].

Table 5.
Characteristics of patients who suffered from Pseudomonas aeruginosa infection.
10. Preventative and Mitigation Measures
Multiple mitigation and preventative strategies can be utilized to help decrease the risk of infection [3]. Optimized hand hygiene, avoidance of sick contacts, and development of protected hospital environments have been shown to be effective [3,12]. Regular dental care is vital as well.
Vaccination has been studied extensively in this population [3,56]. The type of HSCT, the timing of immunization after transplantation, the age at transplantation, and the presence or absence of chronic GVHD all affect immune responses and development of long-term immunity [56]. Even after receiving vaccinations, patients may still have a compromised immune system, necessitating additional safety measures to reduce the risk of contracting infections [56]. Active GVHD, its treatment, and the use of rituximab within 6 months of immunization attenuate the immunological response to vaccines [56]. Recipients of alloHSCT are recommended to receive vaccine series for Streptococcus pneumoniae, Haemophilus influenzae type b, SARS-CoV-2, and seasonal influenza among other others [1,3]. IVIG can be considered in alloHSCT recipients in the first 200 days post-transplant if there was profound hypogammaglobulinemia, Ig levels <400 mg/dL [1].
Author Contributions
Conceptualization, S.C. and R.T.S.; Methodology, S.C. and C.S.; writing—original draft preparation, S.C.; writing—review and editing, S.C., A.Z., V.R.B. and R.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Merit Review Award (BX001786), received by R.T.S., from the Department of Veterans Affairs and by RO1 funding (HL144478), received by R.T.S., from NIH/NHLBI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Vijaya Raj Bhatt reports participating in Safety Monitoring Committee for Protagonist, receiving consulting fees from Imugene, research funding (institutional) from Abbvie, Pfizer, Incyte, Jazz, and National Marrow Donor Program, and drug support (institutional) from Chimerix for a trial. All other authors declare no conflict of interest.
References
- Diab, M.; ZazaDitYafawi, J.; Soubani, A.O. Major pulmonary complications after hematopoietic stem cell transplant. Exp. Clin. Transplant. 2016, 14, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Aljurf, M.; Weisdorf, D.; Alfraih, F.; Szer, J.; Müller, C.; Confer, D.; Hashmi, S.; Kröger, N.; Shaw, B.E.; Greinix, H. Worldwide Network for Blood & Marrow Transplantation (WBMT) special article, challenges facing emerging alternate donor registries. Bone Marrow Transplant. 2019, 54, 1179–1188. [Google Scholar] [PubMed]
- Wong, J.L.; Evans, S.E. Bacterial Pneumonia in Patients with Cancer: Novel Risk Factors and Management. Clin. Chest Med. 2017, 38, 263–277. [Google Scholar] [CrossRef]
- Schuster, M.G.; Cleveland, A.A.; Dubberke, E.R.; Kauffman, C.A.; Avery, R.K.; Husain, S.; Paterson, D.L.; Silveira, F.P.; Chiller, T.M.; Benedict, K. Infections in hematopoietic cell transplant recipients: Results from the organ transplant infection project, a multicenter, prospective, cohort study. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2017; p. ofx050. [Google Scholar]
- Aguilar-Guisado, M.; Jiménez-Jambrina, M.; Espigado, I.; Rovira, M.; Martino, R.; Oriol, A.; Borrell, N.; Ruiz, I.; Martín-Dávila, P.; de la Cámara, R. Pneumonia in allogeneic stem cell transplantation recipients: A multicenter prospective study. Clin. Transplant. 2011, 25, E629–E638. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.J.; Freifeld, A.G. Optimal management of neutropenic fever in patients with cancer. J. Oncol. Pract. 2019, 15, 19–24. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.; Boeckh, M.J. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: A global perspective. Bone Marrow Transplant. 2009, 44, 453–455. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute graft-versus-host disease—Biologic process, prevention, and therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Robin, M.; Porcher, R.; Araujo, R.D.C.; De Latour, R.P.; Devergie, A.; Rocha, V.; Larghero, J.; Adès, L.; Ribaud, P.; Mary, J.-Y. Risk factors for late infections after allogeneic hematopoietic stem cell transplantation from a matched related donor. Biol. Blood Marrow Transplant. 2007, 13, 1304–1312. [Google Scholar] [CrossRef]
- Bhatt, V.R.; Wang, T.; Chen, K.; Kitko, C.L.; MacMillan, M.L.; Pidala, J.A.; Al Malki, M.M.; Badawy, S.M.; Beitinjaneh, A.; Ganguly, S. Chronic graft-versus-host disease, nonrelapse mortality, and disease relapse in older versus younger adults undergoing matched allogeneic peripheral blood hematopoietic cell transplantation: A Center for International Blood and Marrow Transplant Research Analysis. Transplant. Cell. Ther. 2022, 28, 34–42. [Google Scholar]
- Rashidi, A.; Hamadani, M.; Zhang, M.-J.; Wang, H.-L.; Abdel-Azim, H.; Aljurf, M.; Assal, A.; Bajel, A.; Bashey, A.; Battiwalla, M. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019, 3, 1826–1836. [Google Scholar] [CrossRef]
- Styczyński, J. Pneumonia in patients after hematopoietic stem cell transplantation. OnCOReview 2017, 7, A126–A138. [Google Scholar] [CrossRef]
- Soiffer, R.J.; LeRademacher, J.; Ho, V.; Kan, F.; Artz, A.; Champlin, R.E.; Devine, S.; Isola, L.; Lazarus, H.M.; Marks, D.I. Impact of immune modulation with anti–T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood J. Am. Soc. Hematol. 2011, 117, 6963–6970. [Google Scholar] [CrossRef]
- Soubani, A.O.; Miller, K.B.; Hassoun, P.M. Pulmonary complications of bone marrow transplantation. Chest 1996, 109, 1066–1077. [Google Scholar] [CrossRef]
- Hamilton, B.K. Updates in chronic graft-versus-host disease. Hematology 2021, 2021, 648–654. [Google Scholar] [CrossRef]
- Wollenberg, B.; Riera-Knorrenschild, J.; Neubauer, A.; Görg, C. Functional hyposplenia after allogenic bone marrow transplantation: A case report. Ultraschall Med. 2001, 22, 289–291. [Google Scholar] [CrossRef]
- Youssef, S.; Rodriguez, G.; Rolston, K.V.; Champlin, R.E.; Raad, I.I.; Safdar, A. Streptococcus pneumoniae infections in 47 hematopoietic stem cell transplantation recipients: Clinical characteristics of infections and vaccine-breakthrough infections, 1989–2005. Medicine 2007, 86, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Rege, K.; Mehta, J.; Treleaven, J.; Jameson, B.; Meller, S.; Mitchell, P.; Milan, S.; Powles, R. Fatal pneumococcal infections following allogeneic bone marrow transplant. Bone Marrow Transplant. 1994, 14, 903–906. [Google Scholar] [PubMed]
- Saj, F.; Reddy, V.N.; Kayal, S.; Dubashi, B.; Singh, R.; Joseph, N.M.; Ganesan, P. Double Infection in a Patient with Chronic GVHD Post Allogeneic Transplant: Hickam’s Dictum Trumps Occam’s Razor!—A Case Report with Review of Literature. Indian J. Med. Paediatr. Oncol. 2022. [Google Scholar] [CrossRef]
- Bergeron, A.; Mikulska, M.; De Greef, J.; Bondeelle, L.; Franquet, T.; Herrmann, J.-L.; Lange, C.; Spriet, I.; Akova, M.; Donnelly, J.P. Mycobacterial infections in adults with haematological malignancies and haematopoietic stem cell transplants: Guidelines from the 8th European Conference on Infections in Leukaemia. Lancet Infect. Dis. 2022, 22, e359–e369. [Google Scholar] [CrossRef]
- Erdstein, A.A.; Daas, P.; Bradstock, K.F.; Robinson, T.; Hertzberg, M.S. Tuberculosis in allogeneic stem cell transplant recipients: Still a problem in the 21st century. Transpl. Infect. Dis. 2004, 6, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Vaz, C.P.; Campilho, F.; Morais, A.; Guimarães, M.A.; Lopes, C.; Portal, A.; Carvalhais, A.; Pimentel, P. Central nervous system (CNS) tuberculosis following allogeneic stem cell transplantation. Bone Marrow Transplant. 2000, 25, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.C.; Tang, J.L.; Hsueh, P.R.; Luh, K.T.; Yu, C.J.; Yang, P.C. Pulmonary tuberculosis in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 27, 1293–1297. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wu, C.-J.; Ko, P.-S.; Chien, S.-H.; Fan, N.-W.; Wang, H.-Y.; Gau, J.-P.; Liu, C.-J.; Hsiao, L.-T.; Chiou, T.-J.; et al. Mycobacterial infections in adult recipients of allogeneic hematopoietic stem cell transplantation: A cohort study in a high endemic area. J. Microbiol. Immunol. Infect. = Wei Mian Yu Gan Ran Za Zhi 2020, 53, 274–282. [Google Scholar] [CrossRef]
- Arslan, O.; Gürman, G.; Dilek, I.; Ozcan, M.; Koç, H.; Ilhan, O.; Akan, H.; Konuk, N.; Uysal, A.; Beksaç, M. Incidence of tuberculosis after bone marrow transplantation in a single center from Turkey. Haematologia 1998, 29, 59–62. [Google Scholar] [PubMed]
- Martino, R.; Martínez, C.; Brunet, S.; Sureda, A.; López, R.; Domingo-Albós, A. Tuberculosis in bone marrow transplant recipients: Report of two eases and review of the literature. Bone Marrow Transplant. 1996, 18, 809–812. [Google Scholar] [PubMed]
- Ip, M.S.; Yuen, K.Y.; Woo, P.C.; Luk, W.K.; Tsang, K.W.; Lam, W.K.; Liang, R.H. Risk factors for pulmonary tuberculosis in bone marrow transplant recipients. Am. J. Respir. Crit. Care Med. 1998, 158, 1173–1177. [Google Scholar] [CrossRef]
- De La Camara, R.; Martino, R.; Granados, E.; Rodriguez-Salvanes, F.; Rovira, M.; Cabrera, R.; López, J.; Parody, R.; Sierra, J.; Fernández-Rañada, J. Tuberculosis after hematopoietic stem cell transplantation: Incidence, clinical characteristics and outcome. Bone Marrow Transplant. 2000, 26, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Miyake, N.; Chong, Y.; Nishida, R.; Takenaka, K.; Kato, K.; Miyamoto, T.; Aono, A.; Takaki, A.; Mitarai, S.; Shimoda, S.; et al. Mycobacterium abscessus and massiliense lung infection during macrolide treatment for bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. J. Infect. Chemother. 2018, 24, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Peres, E.; Khaled, Y.; Krijanovski, O.I.; Mineishi, S.; Levine, J.E.; Kaul, D.R.; Riddell Iv, J. Mycobacterium chelonae necrotizing pneumonia after allogeneic hematopoietic stem cell transplant: Report of clinical response to treatment with tigecycline. Transpl. Infect. Dis. 2009, 11, 57–63. [Google Scholar] [CrossRef]
- Bergeron, A.; Chevret, S.; Granata, A.; Chevallier, P.; Vincent, L.; Huynh, A.; Tabrizi, R.; Labussiere-Wallet, H.; Bernard, M.; Chantepie, S. Effect of azithromycin on airflow decline–free survival after allogeneic hematopoietic stem cell transplant: The allozithro randomized clinical trial. JAMA 2017, 318, 557–566. [Google Scholar] [CrossRef]
- Vallet, N.; Le Grand, S.; Bondeelle, L.; Hoareau, B.; Corneau, A.; Bouteiller, D.; Tournier, S.; Derivry, L.; Bohineust, A.; Tourret, M. Azithromycin promotes relapse by disrupting immune and metabolic networks after allogeneic stem cell transplantation. Blood J. Am. Soc. Hematol. 2022, 140, 2500–2513. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Ha, J.H.; Kang, H.S.; Yoon, H.K.; Kim, H.J.; Lee, S.; Lee, D.G.; Jung, J.I.; Kim, S.C.; Kim, Y.K. Clinical significance of nontuberculous mycobacteria from respiratory specimens in stem cell transplantation recipients. Int. J. Hematol. 2015, 101, 505–513. [Google Scholar] [CrossRef]
- Au, W.Y.; Lie, A.K.; Cheng, V.C.C.; Cheng, L.C.; Wang, E.P.; Wong, C.F. Successful Lung Transplantation for Post-BMT Bronchiolitis Obliterans and Lipoid Pneumonia Associated with Atypical Mycobacterium and Aspergillosis Infection. J. Heart Lung Transplant. 2007, 26, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Mohite, U.; Das, M.; Saikia, T.; Parikh, P.; Gopal, R.; Kelkar, R.; Advani, S. Mycobacterial pulmonary infection post allogeneic bone marrow transplantation. Leuk. Lymphoma 2001, 40, 675–678. [Google Scholar] [CrossRef]
- Alangaden, G.J.; Brown, W.J.; Chandrasekar, P.H. Recognition of misdiagnosed tuberculosis in a bone marrow transplant recipient leads to identification of “pseudotuberculosis” due to laboratory contamination. Infect. Dis. Clin. Pract. 2004, 12, 328–333. [Google Scholar] [CrossRef]
- Salvator, H.; Berti, E.; Catherinot, E.; Rivaud, E.; Chabrol, A.; Nguyen, S.; Zemoura, L.; Cardot, E.; Tcherakian, C.; Couderc, L.-J. Pulmonary alveolar proteinosis and Mycobacterium abscessus lung infection related to ruxolitinib after allogeneic stem cell transplantation. Eur. Respir. J. 2018, 51, 1701960. [Google Scholar] [CrossRef] [PubMed]
- Sivagnanam, S.; Podczervinski, S.; Butler-Wu, S.M.; Hawkins, V.; Stednick, Z.; Helbert, L.A.; Glover, W.A.; Whimbey, E.; Duchin, J.; Cheng, G.S.; et al. Legionnaires’ disease in transplant recipients: A 15-year retrospective study in a tertiary referral center. Transpl. Infect. Dis. 2017, 19, e12745. [Google Scholar] [CrossRef]
- Pouderoux, C.C.; Ginevra, C.; Descours, G.; Lle Ranc, A.G.; Beraud, L.; Boisset, S.; Magand, N.; Conrad, A.; Bergeron-Lafaurie, A.; Jarraud, S.; et al. Slowly or nonresolving legionnaires’ disease: Case series and literature review. Clin. Infect. Dis. 2020, 70, 1933–1940. [Google Scholar] [CrossRef]
- Padrnos, L.J.; Blair, J.E.; Kusne, S.; Dicaudo, D.J.; Mikhael, J.R. Cutaneous legionellosis: Case report and review of the medical literature. Transpl. Infect. Dis. 2014, 16, 307–314. [Google Scholar] [CrossRef]
- Sivagnanam, S.; Pergam, S.A. Legionellosis in transplantation. Curr. Infect. Dis. Rep. 2016, 18, 9. [Google Scholar] [CrossRef]
- Schindel, C.; Siepmann, U.; Han, S.-R.; Ullmann, A.; Mayer, E.; Fischer, T.; Maeurer, M. Persistent Legionella infection in a patient after bone marrow transplantation. J. Clin. Microbiol. 2000, 38, 4294–4295. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Winston, D.J.; Pan, D.; Schiller, G.J. Increased Incidence of Nocardial Infections in an Era of Atovaquone Prophylaxis in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol. Blood Marrow Transplant. 2018, 24, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Yoshida, M.; Morikawa, N.; Fujimoto, S.; Ishikawa, T.; Sano, K.; Nishiwaki, K.; Takagi, M.; Hayashi, M.; Kuwano, K.; et al. Pulmonary Nocardia nova Infection after allogeneic hematopoietic stem cell transplantation. Intern. Med. 2014, 53, 1391–1395. [Google Scholar] [CrossRef]
- Hamadani, M.; Benson Jr, D.M.; Blum, W.; Garzon, R.; Devine, S.M. Pulmonary Nocardia and Aspergillus co-infection in a patient with chronic graft-versus-host disease: Images in transplant infectious disease. Transpl. Infect. Dis. 2008, 10, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.A.; Durrant, S. Nocardia infection following bone marrow transplantation. Intern. Med. J. 2006, 36, 402. [Google Scholar] [CrossRef]
- Nishida, R.; Mori, Y.; Iwasaki, H.; Tokuyama, T.; Kamezaki, K.; Nagasaki, Y.; Oka, H.; Miyawaki, K.; Saito, N.; Takenaka, K.; et al. Pulmonary nocardiosis developed in a hematopoietic stem cell transplant recipient with bronchiolitis obliterans. Intern. Med. 2010, 49, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Lee, M.-Y.; Hsiao, L.-T.; Yang, M.-H.; Chao, T.-C.; Chen, P.-M.; Chiou, T.-J. Pulmonary nocardiosis in a patient with CML relapse undergoing imatinib therapy after bone marrow transplantation. Ann. Hematol. 2004, 83, 444–446. [Google Scholar] [CrossRef]
- Shearer, C.; Chandrasekar, P.H.; Sensenbrenner, L.; Ratanatharathorn, V.; Karanes, C.; Momin, F.; Abella, S.; Lum, L.; Uberti, J. Pulmonary nocardiosis in a patient with a bone marrow transplant. Bone Marrow Transplant. 1995, 15, 479–481. [Google Scholar]
- Kakihana, K.; Ohashi, K.; Iguchi, M.; Negishi, K.; Suzuki, T.; Shitara, M.; Honma, M.; Akiyama, H.; Sakamaki, H. Frequent exacerbation of pulmonary nocardiosis during maintenance antibiotic therapies in a hematopoietic stem cell transplant recipient. Int. J. Hematol. 2007, 86, 455–458. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa biofilm: A review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Blackwell, T.S.; Christman, J.W.; Prince, A.S. Pathogen–host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Suanzes, P.; Aguilar-Company, J.; Rodríguez-González, E.; Martín-Gómez, M.T.; Gómez-Domingo, M.R.; Ruiz-Camps, I. Long-term treatment with nebulized colistin in oncological patients with recurrent respiratory infections. Med. Clin. 2022, 159, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Zander, A.; Kanojia, M. Obstructive lung disease after allogeneic bone marrow transplantation. Transplantation 1984, 37, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Link, H.; Reinhard, U.; Walter, E. Lung diseases after bone marrow transplantation. Klin. Wochenschr. 1986, 64, 595–614. [Google Scholar] [CrossRef]
- Issa, N.C.; Baden, L.R. Current issues in vaccines for adult patients with hematologic malignancies. J. Natl. Compr. Cancer Netw. JNCCN 2012, 10, 1447–1454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).