Potential Biomarkers for Asymptomatic Visceral Leishmaniasis among Iraq-Deployed U.S. Military Personnel
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Soluble Leishmania Antigen Preparation
2.4. Cell Culture
2.5. Quantification of Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Characteristics of U.S. Military Study Volunteers
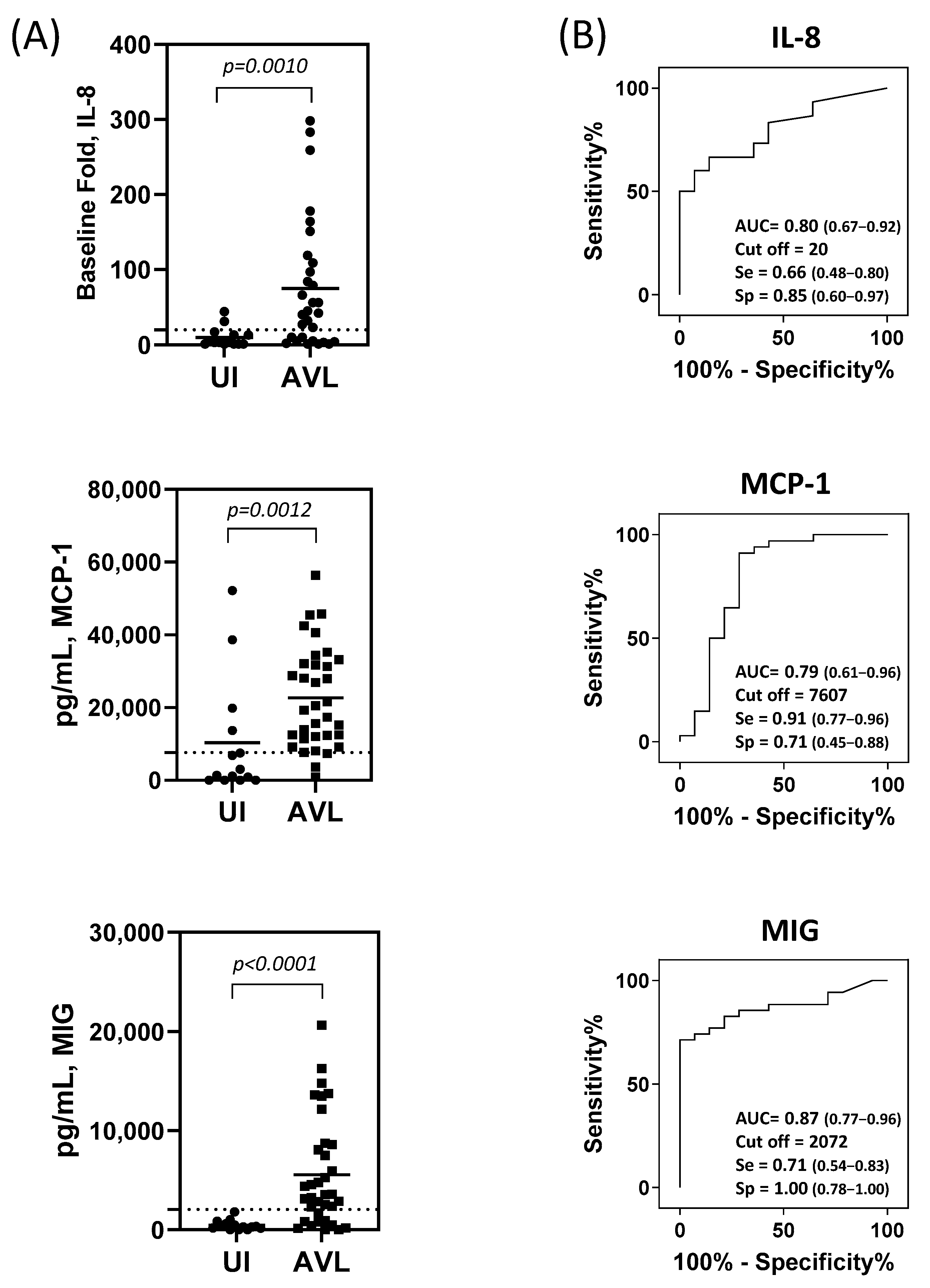
3.2. IL-8, MCP-1, and MIG produced by SLA-stimulated PBMC Are Useful Biomarkers for the Identification of People with AVL
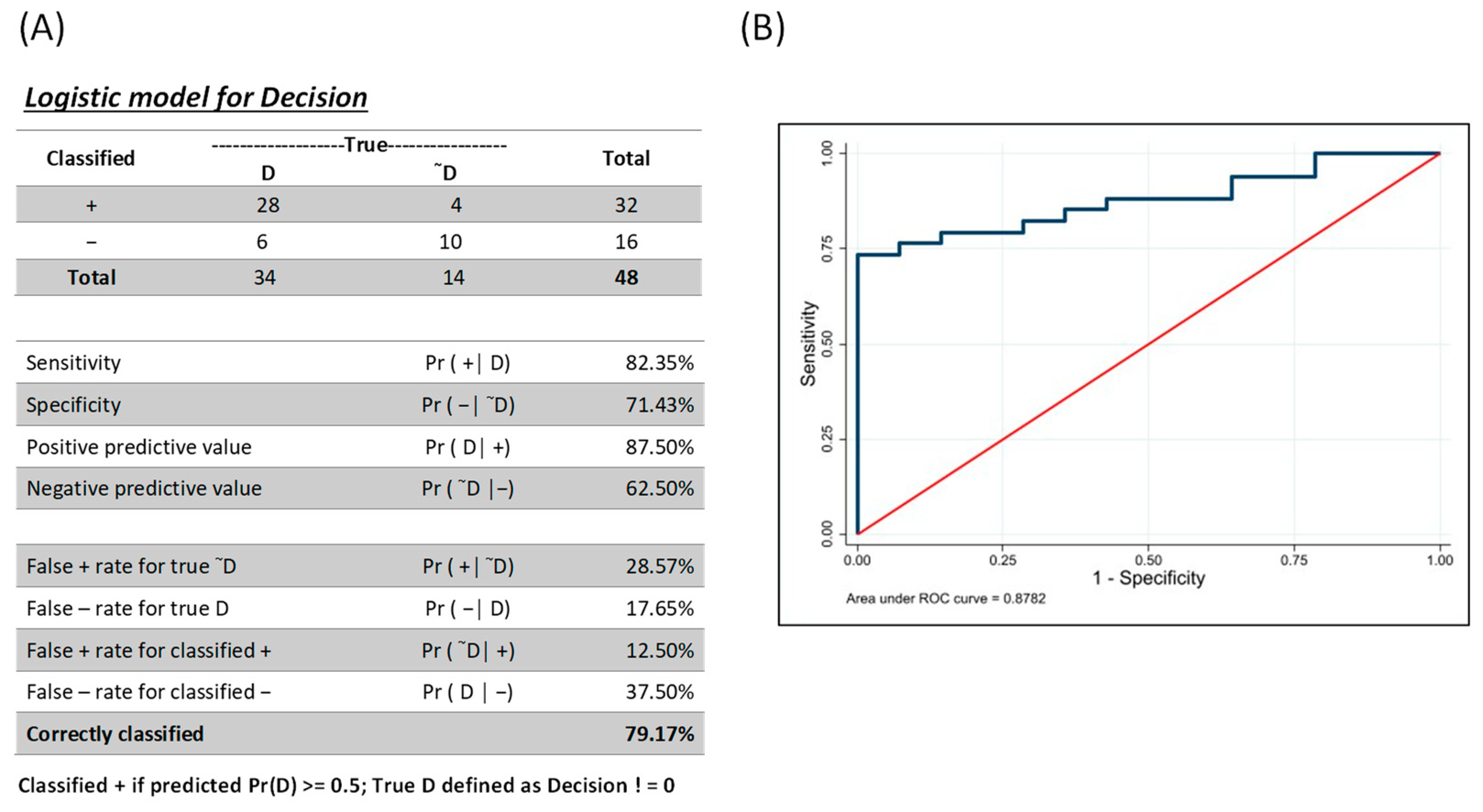
3.3. Combinatorial Analysis for Identification of Individuals with AVL
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Leishmania. 2023. Available online: https://www.who.int/health-topics/leishmaniasis (accessed on 15 April 2023).
- Singh, S.; Kumari, V.; Singh, N. Predicting kala-azar disease manifestations in asymptomatic patients with latent Leishmania donovani infection by detection of antibody against recombinant K39 antigen. Clin. Diagn. Lab. Immunol. 2002, 9, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Bimal, S.; Singh, S.K.; Das, V.N.R.; Sinha, P.K.; Gupta, A.K.; Bhattacharya, S.K.; Das, P. Leishmania donovani: Effect of therapy on expression of CD2 antigen and secretion of macrophage migration inhibition factor by T-cells in patients with visceral leishmaniasis. Exp. Parasitol. 2005, 111, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Kalani, M.; Choopanizadeh, M.; Pourabbas, B.; Pouladfar, G.; Asaee, S.; Ghalati, E.G.; Moravej, A. Dynamic alterations and durability of T helper 22 and its corresponding cytokines following treatment in pediatric visceral leishmaniasis. Cytokine 2021, 144, 155579. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Bacellar, O.; Barral, A.; Badaro, R.; Johnson, W.D., Jr. Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J. Clin. Investig. 1989, 83, 860–864. [Google Scholar] [CrossRef]
- Kharazmi, A.; Kemp, K.; Ismail, A.; Gasim, S.; Gaafar, A.; Kurtzhals, J.A.; Hassan, A.M.E.; Theander, T.G.; Kemp, M. T-cell response in human leishmaniasis. Immunol. Lett. 1999, 65, 105–108. [Google Scholar] [CrossRef]
- Ferrua, B.; Luci, C.; Fichoux, Y.L.; Paul, A.; Marty, P. Imprinting of BALB/c mice with low Leishmania infantum parasite dose markedly protects spleen against high-dose challenge. Vaccine 2006, 24, 589–596. [Google Scholar] [CrossRef]
- Gouvea, V.L.; de Assis, T.S.M.; Orsini, M.; da Silva, A.R.; de Souza, G.F.; Caligiorne, R.; da Silva, A.C.L.; Peruhype-Magalhães, V.; Marciano, A.P.V.; Martins-Filho, O.A.; et al. Combined diagnostic methods identify a remarkable proportion of asymptomatic Leishmania (Leishmania) chagasi carriers who present modulated cytokine profiles. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 548–555. [Google Scholar] [CrossRef]
- Peruhype-Magalhaes, V.; Martins-Filho, O.A.; Prata, A.; Silva, L.A.; Rabello, A.; Teixeira-Carvalho, A.; Figueiredo, R.M.; Guimarães-Carvalho, S.F.; Ferrari, T.C.A.; Correa-Oliveira, R. Immune response in human visceral leishmaniasis: Analysis of the correlation between innate immunity cytokine profile and disease outcome. Scand. J. Immunol. 2005, 62, 487–495. [Google Scholar] [CrossRef]
- Peruhype-Magalhaes, V.; Martins-Filho, O.A.; Prata, A.; Silva, L.A.; Rabello, A.; Teixeira-Carvalho, A.; Figueiredo, R.M.; Guimarães-Carvalho, S.F.; Ferrari, T.C.A.; Van Weyenbergh, J.; et al. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferongamma and interleukin-10 and low frequency of tumour necrosis factoralpha(+) monocytes are hallmarks of active human visceral Leishmaniasis due to Leishmania chagasi infection. Clin. Exp. Immunol. 2006, 146, 124–132. [Google Scholar] [CrossRef]
- Machado, C.M.; Martins, T.C.; Colturato, I.; Leite, M.S.; Simione, A.J.; Souza, M.P.; Mauad, M.A.; Colturato, V.R. Epidemiology of neglected tropical diseases in transplant recipients. Review of the literature and experience of a Brazilian HSCT center. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 309–324. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cordeiro-da-Silva, A.; Laforge, M.; Silvestre, R.; Estaquier, J. Regulation of immunity during visceral Leishmania infection. Parasit. Vectors 2016, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Saporito, L.; Giammanco, G.M.; De Grazia, S.; Colomba, C. Visceral leishmaniasis: Host-parasite interactions and clinical presentation in the immunocompetent and in the immunocompromised host. Int. J. Infect. Dis. 2013, 17, e572–e576. [Google Scholar] [CrossRef] [PubMed]
- Oghumu, S.; Lezama-Dávila, C.M.; Isaac-Márquez, A.P.; Satoskar, A.R. Role of chemokines in regulation of immunity against leishmaniasis. Exp. Parasitol. 2010, 126, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, R.J.; Kumar, R.; Hafner, L.M.; Engwerda, C.R. Immune regulation during chronic visceral leishmaniasis. PLoS Negl. Trop. Dis. 2014, 8, e2914. [Google Scholar] [CrossRef]
- Singh, O.P.; Gidwani, K.; Kumar, R.; Nylén, S.; Jones, S.L.; Boelaert, M.; Sacks, D.; Sundara, S. Reassessment of immune correlates in human visceral leishmaniasis as defined by cytokine release in whole blood. Clin. Vac. Immunol. 2012, 19, 961–966. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Carrillo, E.; Sanchez, C.; García-Martínez, J.; López Lacomba, D.; San Martin, J.V.; Alves, F.; Alvar, J.; Moreno, J. Interleukin-2 as a marker for detecting asymptomatic individuals in areas where Leishmania infantum is endemic. Clin. Microbiol. Infect. 2016, 22, 739. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Ghosh, P.; Hossain, F.; Chowdhury, R.; Mondal, D.; Alvar, J.; Moreno, J.; Carrillo, E. IFN-γ, IL-2, IP-10, and MIG as biomarkers of exposure to Leishmania spp., and of cure in human visceral leishmaniasis. Front. Cell. Infect. Microbiol. 2017, 7, 200. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Sanchez, C.; Alvar, J.; Moreno, J.; Carrillo, E. Monocyte chemotactic protein 1 in plasma from soluble Leishmania antigen-stimulated whole blood as a potential biomarker of the cellular immune response to Leishmania infantum. Front. Immunol. 2017, 8, 1208. [Google Scholar] [CrossRef]
- Adem, E.; Tajebe, F.; Getahun, M.; Kiflie, A.; Diro, E.; Hailu, A.; Shkedy, Z.; Mengesha, B.; Mulaw, T.; Atnafu, S. Successful treatment of human visceral leishmaniasis restores antigen-specific IFN-gamma, but not IL-10 production. PLoS Negl. Trop. Dis. 2016, 10, e0004468. [Google Scholar] [CrossRef]
- Schaefer, K.U.; Kurtzhals, J.A.; Gachihi, G.S.; Muller, A.S.; Kager, P.A. A prospective sero-epidemiological study of visceral leishmaniasis in Baringo District, Rift Valley Province, Kenya. Trans. R Soc. Trop. Med. Hyg. 1995, 89, 471–475. [Google Scholar] [CrossRef]
- Sinha, P.K.; Bimal, S.; Pandey, K.; Singh, S.K.; Ranjan, A.; Kumar, N.; Lal, C.S.; Barman, S.B.; Verma, R.B.; Jeyakumar, A.; et al. A community-based, comparative evaluation of direct agglutination and rK39 strip tests in the early detection of subclinical Leishmania donovani infection. Ann. Trop. Med. Parasitol. 2008, 102, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.P.; Bairagi, B.; Kundu, P.K. rK39 strip test-easy, reliable and cost-effective field diagnosis for visceral leishmaniasis in India. J. Assoc. Physicians India 2003, 51, 759–761. [Google Scholar] [PubMed]
- Mody, R.M.; Lakhal-Naouar, I.; Sherwood, J.E.; Koles, N.L.; Shaw, D.; Bigley, D.P.; Co, E.-M.A.; Copeland, N.K.; Jagodzinski, L.L.; Mukbel, R.M. Asymptomatic Visceral Leishmania infantum Infection in US Soldiers Deployed to Iraq. Clin. Infect. Dis. 2019, 68, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Lakhal-Naouar, I.; Mukbel, R.; DeFraites, R.F.; Mody, R.M.; Massoud, L.N.; Shaw, D.; Co, E.M.; Sherwood, J.E.; Kamhawi, S.; Aronson, N.E. The human immune response to saliva of Phlebotomus alexandri, the vector of visceral leishmaniasis in Iraq, and its relationship to sand fly exposure and infection. PLoS Negl. Trop. Dis. 2021, 15, e0009378. [Google Scholar] [CrossRef]
- Beasley, E.A.; Mahachi, K.G.; Petersen, C.A. Possibility of Leishmania transmission via Lutzomyia spp. sand flies within the USA and implications for human and canine autochthonous Infection. Curr. Trop. Med. Rep. 2022, 9, 160–168. [Google Scholar] [CrossRef]
- Curtin, J.M.; Aronson, N.E. Leishmaniasis in the United States: Emerging Issues in a Region of Low Endemicity. Microorganisms 2021, 9, 578. [Google Scholar] [CrossRef]
- Vallur, A.C.; Reinhart, C.; Mohamath, R.; Goto, Y.; Ghosh, P.; Mondal, D.; Duthie, M.S.; Reed, S.G. Accurate Serodetection of Asymptomatic Leishmania donovani Infection by Use of Defined Antigens. J. Clin. Microbiol. 2016, 54, 1025–1030. [Google Scholar] [CrossRef]
- Vallur, A.C.; Duthie, M.S.; Reinhart, C.; Tutterrow, Y.; Hamano, S.; Bhaskar, K.R.H.; Coler, R.; Mondal, D.; Reed, S. Biomarkers for intracellular pathogens: Establishing tools as vaccine and therapeutic endpoints for visceral leishmaniasis. Clin. Microbiol. Infect. 2014, 20, O374–O383. [Google Scholar] [CrossRef]
- Berens, R.L.; Brun, R.; Krassner, S.M. A simple monophasic medium for axenic culture of hemoflagellates. J. Parasitol. 1976, 62, 360–365. [Google Scholar] [CrossRef]
- Ramer-Tait, A.E.; Lei, S.M.; Bellaire, B.H.; Beetham, J.K. Differential surface deposition of complement proteins on logarithmic and stationary phase Leishmania chagasi promastigotes. J. Parasitol. 2012, 98, 1109–1116. [Google Scholar] [CrossRef]
- Scott, P.; Pearce, E.; Natovitz, P.; Sher, A. Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of soluble promastigote extract. J. Immunol. 1987, 139, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Marovich, M.A.; Mascola, J.R.; Eller, M.; Louder, M.K.; Caudrelier, P.A.; El-Habib, R.; Ratto-Kim, S.; Cox, J.H.; Currier, J.R.; Levine, B.L.; et al. Preparation of Clinical-Grade Recombinant Canarypox–Human Immunodeficiency Virus Vaccine–Loaded Human Dendritic Cells. J. Infect. Dis. 2002, 186, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software; Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Mazzara, S.; Rossi, R.L.; Grifantini, R.; Donizetti, S.; Abrignani, S.; Bombaci, M. CombiROC: An interactive web tool for selecting accurate marker combinations of omics data. Sci. Rep. 2017, 30, 45477. [Google Scholar] [CrossRef]
- Nylén, S.; Sacks, D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007, 28, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J. Cytokines orchestrating the immune response. Rev. Sci. Tech. 1998, 17, 84–94. [Google Scholar] [CrossRef]
- Pitta, M.G.R.; Romano, A.; Cabantous, S.; Henri, S.; Hammad, A.; Kouriba, B.; Argiro, L.; El Kheir, M.; Bucheton, B.; Mary, C.; et al. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J. Clin. Investig. 2009, 119, 2379–2387. [Google Scholar] [CrossRef]
- Mary, C.; Auriault, V.; Faugère, B.; Dessein, A.J. Control of Leishmania infantum infection is associated with CD8(+) and gamma interferon- and interleukin-5-producing CD4(+) antigen-specific T cells. Infect. Immun. 1999, 67, 5559–5566. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Corbeil, A.; Wagner, V.; Onwuchekwa, C.; Fernandez-Prada, C. Identification of asymptomatic Leishmania infections: A scoping review. Parasit. Vectors 2022, 15, 5. [Google Scholar] [CrossRef]
- Ritter, U.; Korner, H. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol. 2002, 24, 295–301. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Mondal, D.; Alvar, J.; Moreno, J.; Carrillo, E. Cytokines and chemokines measured in dried SLA-stimulated whole blood spots for asymptomatic Leishmania infantum and Leishmania donovani infection. Sci. Rep. 2017, 7, 17266. [Google Scholar] [CrossRef]
- Porcino, G.N.; Carvalho, K.S.S.; Braz, D.C.; Silva, V.C.; Costa, C.H.N.; Santos, I.K.F.M. Evaluation of methods for detection of asymptomatic individuals infected with Leishmania infantum in the state of Piauí, Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007493. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Pacheco, G.; Loría-Cervera, E.N.; Sosa-Bibiano, E.I.; Canché-Pool, E.B.; Vargas-Gonzalez, A.; Melby, P.C.; Andrade-Narvaez, F.J. In situ cytokines (IL-4, IL-10, IL-12, IFN-γ) and chemokines (MCP-1, MIP-1α) gene expression in human Leishmania (Leishmania) mexicana infection. Cytokine 2014, 69, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Moll, H. The role of chemokines and accessory cells in the immunoregulation of cutaneous leishmaniasis. Behring Inst. Mitt. 1997, 99, 73–78. [Google Scholar]
- Arango Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Scorza, B.M.; Wacker, M.A.; Messingham, K.; Kim, P.; Klingelhutz, A.; Fairley, J.; Wilson, M.E. Differential Activation of Human Keratinocytes by Leishmania Species Causing Localized or Disseminated Disease. J. Investig. Dermatol. 2017, 137, 2149–2156. [Google Scholar] [CrossRef]
- Elshafie, A.I.; Hlin, E.; Håkansson, L.D.; Elghazali, G.; El Safi, S.H.; Rönnelid, J.; Venge, P. Activity and turnover of eosinophil and neutrophil granulocytes are altered in visceral leishmaniasis. Int. J. Parasitol. 2011, 41, 463–469. [Google Scholar] [CrossRef]
- Tasew, G.; Gadisa, E.; Abera, A.; Chanyalew, M.; Abebe, M.; Howe, R.; Ritter, U.; Aseffa, A.; Laskay, T. Whole blood-based in vitro culture reveals diminished secretion of pro-inflammatory cytokines and chemokines in visceral leishmaniasis. Cytokine 2021, 145, 155246. [Google Scholar] [CrossRef]
- Kumar, V.; Bimal, S.; Singh, S.K.; Chaudhary, R.; Das, S.; Lal, C.; Pandey, K.; Das, V.R.; Das, P. Leishmania donovani: Dynamics of L. donovani evasion of innate immune cell attack due to malnutrition in visceral leishmaniasis. Nutrition 2014, 30, 449–458. [Google Scholar] [CrossRef]
- Hajilooi, M.; Abasi, M.; Bazmani, A.; Ahmadi, A.; Matini, M.; Solgi, G.; Sardarian, K. Evaluation of interleukin-8-251 t/a polymorphisms in visceral leishmaniasis. J. Res. Health Sci. 2015, 15, 59–61. [Google Scholar]
- Frade, A.F.; de Oliveira, L.C.; Costa, D.L.; Costa, C.H.N.; Aquino, D.; Van Weyenbergh, J.; Barral-Netto, M.; Barral, A.; Kalil, J.; Goldberg, A.C. TGFB1 and IL8 gene polymorphisms and susceptibility to visceral leishmaniasis. Infect. Genet. Evol. 2011, 11, 912–916. [Google Scholar] [CrossRef]
- Kurkjian, K.M.; Mahmutovic, A.J.; Kellar, K.L.; Haque, R.; Bern, C.; Secor, W.E. Multiplex analysis of circulating cytokines in the sera of patients with different clinical forms of visceral leishmaniasis. Cytometry A. 2006, 69, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Stahlman, S.; Williams, V.F.; Taubman, S.B. Incident diagnoses of leishmaniasis, active and reserve components, U.S. Armed Forces, 2001–2016. Med. Surveill. Mon. Rep. (MSMR) 2017, 24, 2–7. [Google Scholar]
- Mazumder, S.A.; Pandey, S.; Brewer, S.C.; Baselski, V.S.; Weina, P.J.; Land, M.A.; Fleckenstein, J.M. Lingual Leishmaniasis Complicating Visceral Disease. J. Travel Med. 2010, 17, 212–214. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource Silver Spring (MD): Food and Drug Administration (US); Monitoring Biomarker; National Institutes of Health (US): Bethesda, MD, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK402282/ (accessed on 25 January 2021).
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource Silver Spring (MD): Food and Drug Administration (US); Susceptibility/Risk Biomarker; National Institutes of Health (US): Bethesda, MD, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK402288/ (accessed on 27 August 2020).
| Population | AVL Negative Controls (n = 14) | AVL+ Participants (n = 35) |
|---|---|---|
| Mean age, years (range) | 35.1 (25–57) | 40.7 (29–58) |
| Sex Female Male | 5 (35.7%) 9 (64.3%) | 3 (8.6%) 32 (91.4%) |
| Race/Ethnicity | ||
| African American | 2 (14.3%) | 5 (14.3%) |
| White | ||
| Hispanic/Latino | 0 | 5 (14.3%) |
| Not Hispanic/Latino | 9 (64.3%) | 22 (62.8%) |
| Other | 3 (21.4%) | 3 (8.6%) |
| Positive Leishmania Assay IGRA+ ELISA+ qPCR+ | negative negative negative | 27 (77.2%) 6 (17.1%) 2 (5.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araujo, F.F.; Lakhal-Naouar, I.; Koles, N.; Raiciulescu, S.; Mody, R.; Aronson, N. Potential Biomarkers for Asymptomatic Visceral Leishmaniasis among Iraq-Deployed U.S. Military Personnel. Pathogens 2023, 12, 705. https://doi.org/10.3390/pathogens12050705
de Araujo FF, Lakhal-Naouar I, Koles N, Raiciulescu S, Mody R, Aronson N. Potential Biomarkers for Asymptomatic Visceral Leishmaniasis among Iraq-Deployed U.S. Military Personnel. Pathogens. 2023; 12(5):705. https://doi.org/10.3390/pathogens12050705
Chicago/Turabian Stylede Araujo, Fernanda Fortes, Ines Lakhal-Naouar, Nancy Koles, Sorana Raiciulescu, Rupal Mody, and Naomi Aronson. 2023. "Potential Biomarkers for Asymptomatic Visceral Leishmaniasis among Iraq-Deployed U.S. Military Personnel" Pathogens 12, no. 5: 705. https://doi.org/10.3390/pathogens12050705
APA Stylede Araujo, F. F., Lakhal-Naouar, I., Koles, N., Raiciulescu, S., Mody, R., & Aronson, N. (2023). Potential Biomarkers for Asymptomatic Visceral Leishmaniasis among Iraq-Deployed U.S. Military Personnel. Pathogens, 12(5), 705. https://doi.org/10.3390/pathogens12050705






