Inflammatory and Immune Responses during SARS-CoV-2 Infection in Vaccinated and Non-Vaccinated Pregnant Women and Their Newborns
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Quantification of Cytokines
2.3. Antibody Response
2.4. PBMC Isolation
2.5. T-Cell Response (IFNγ Production)
2.6. T-Cell Proliferative Response
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Pregnant Women Analyzed
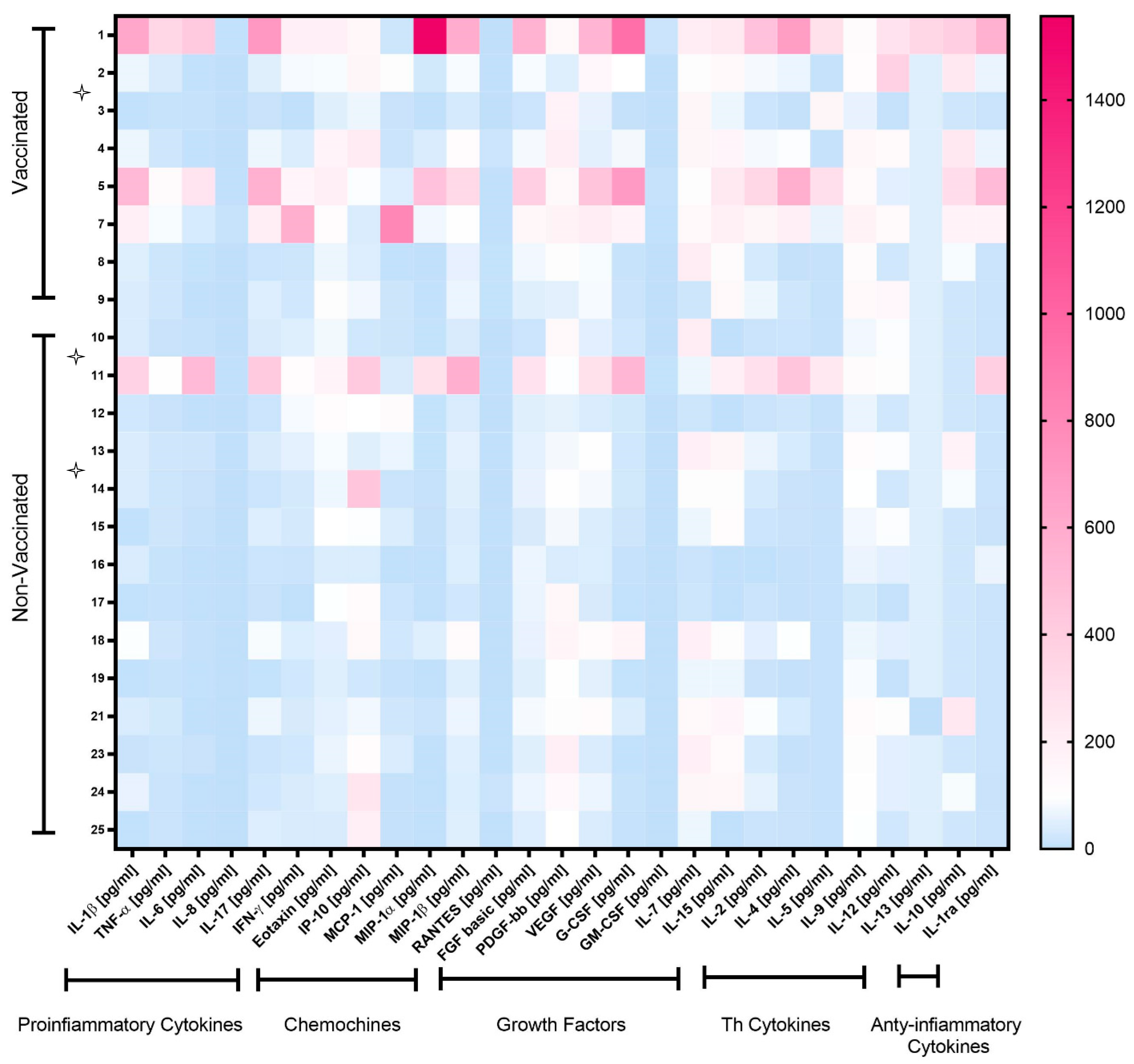
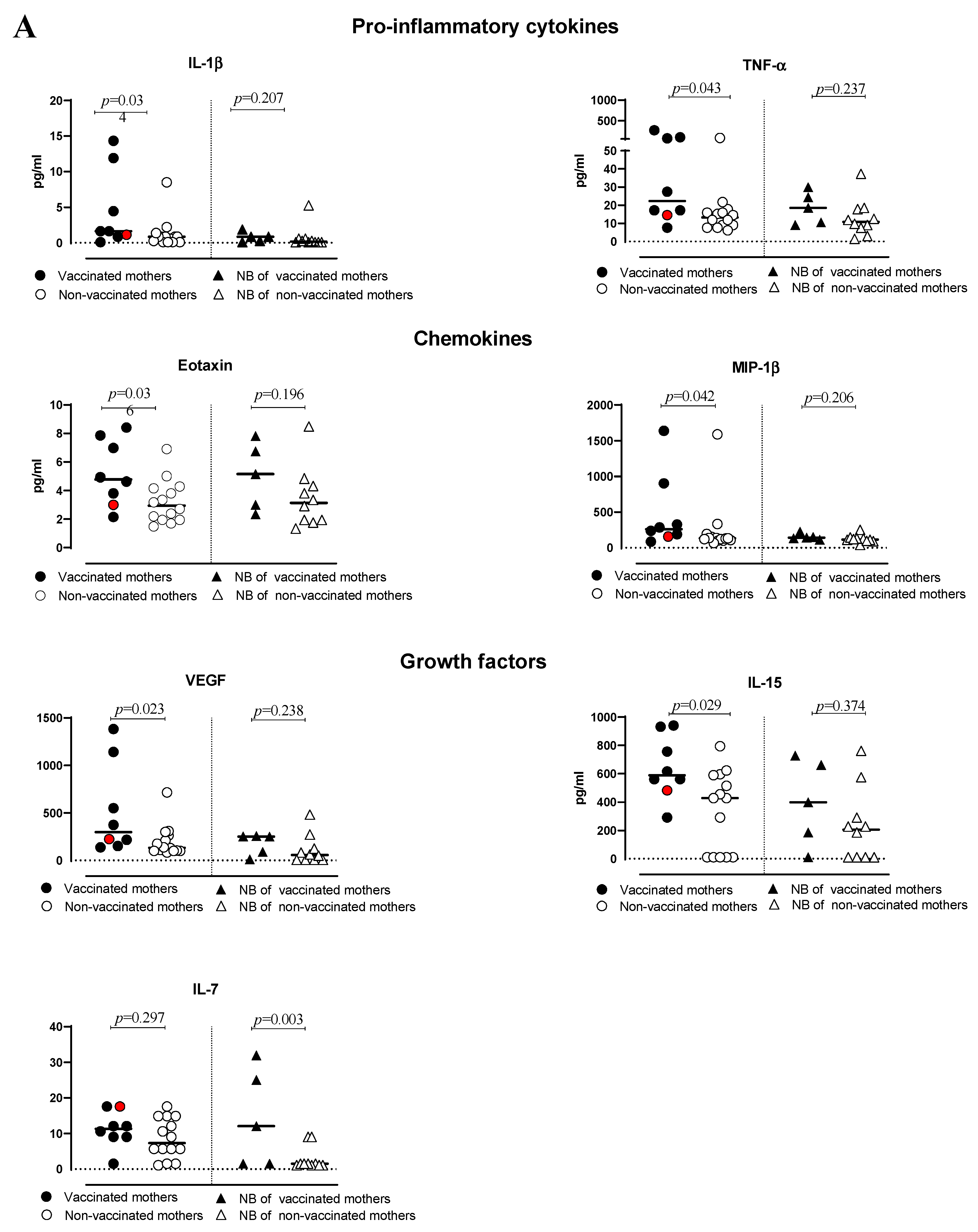
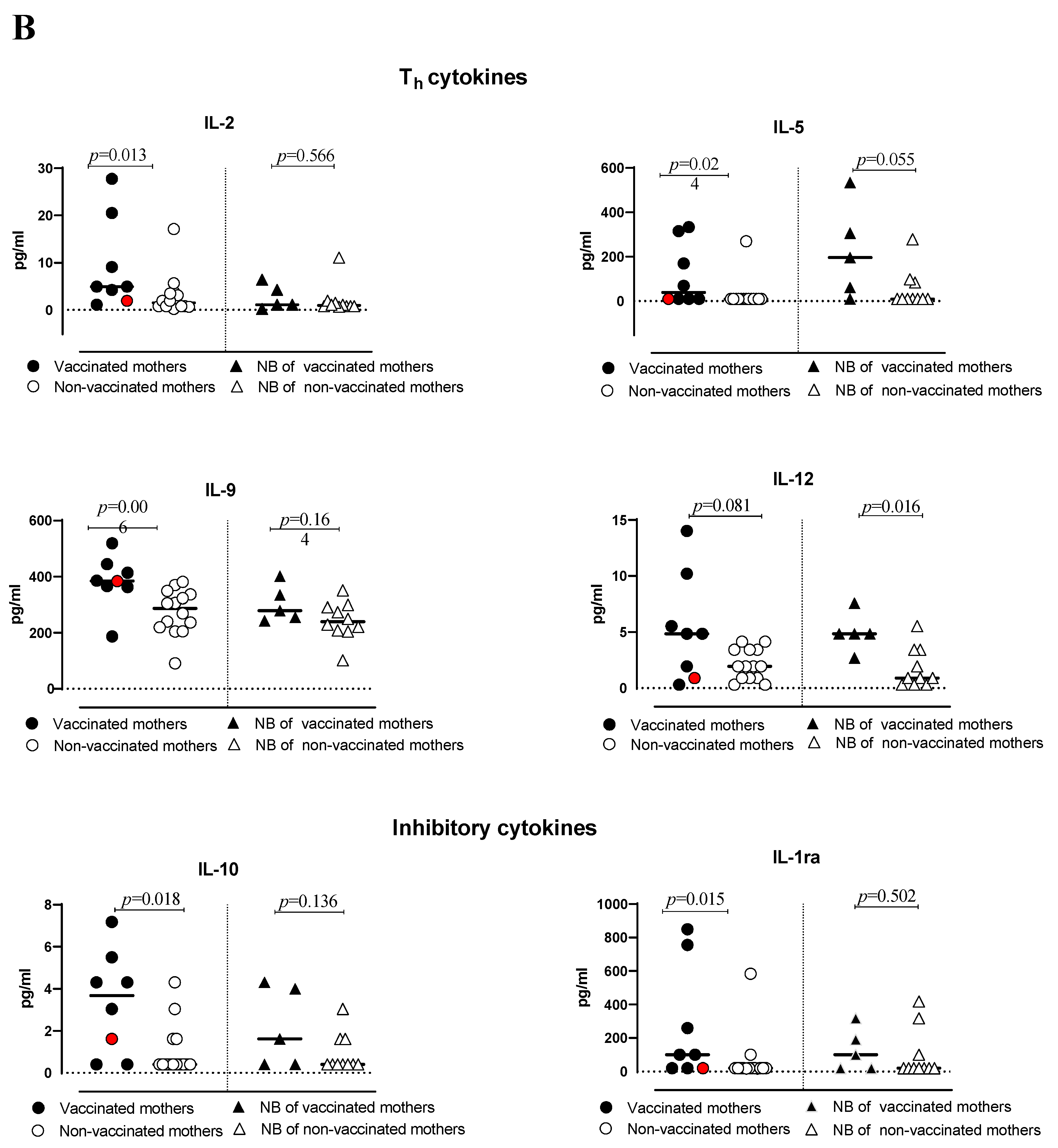
3.2. Cytokine Production
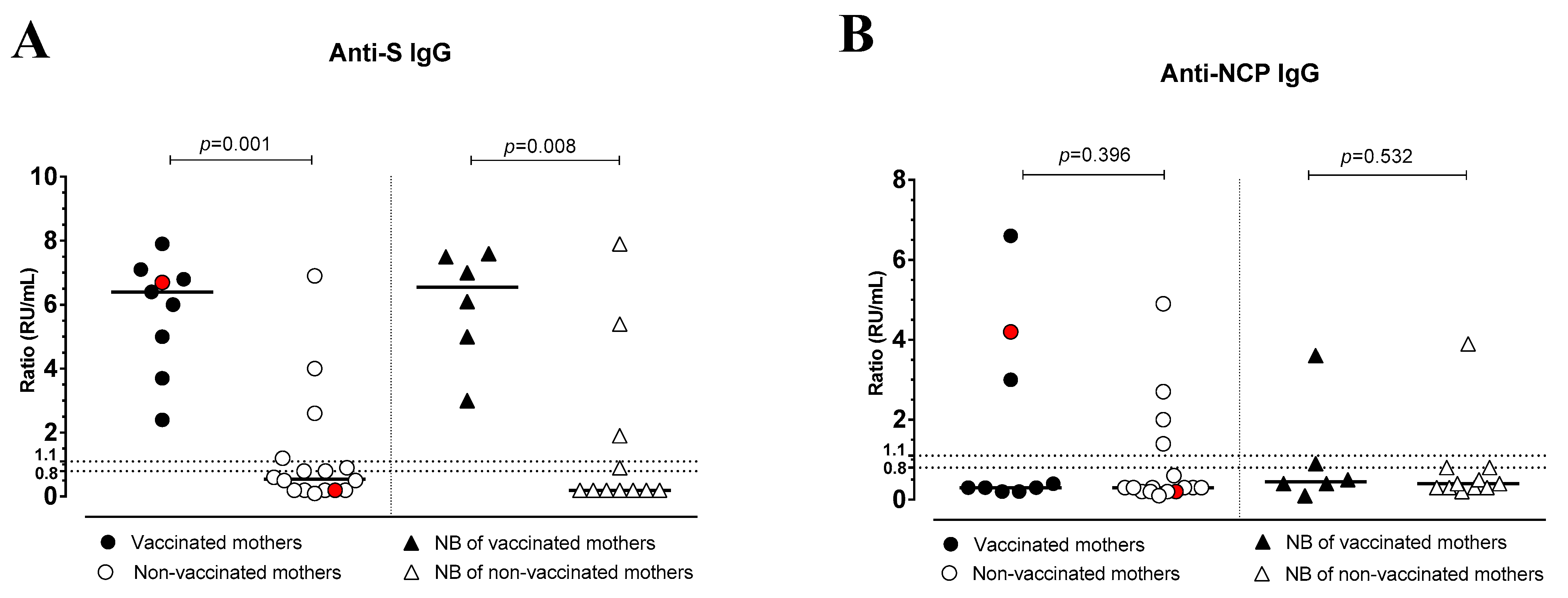
3.3. Antibody Response
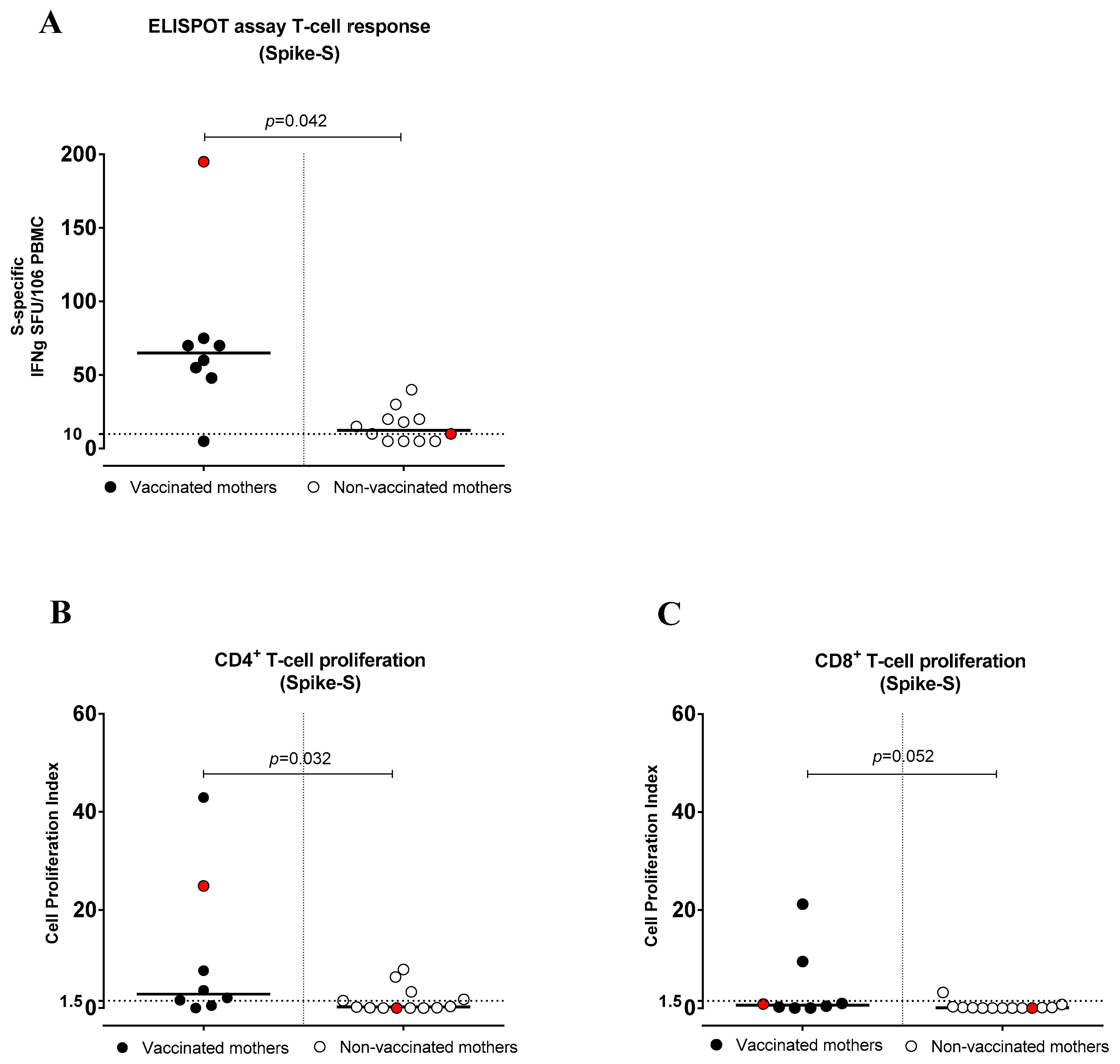
3.4. SARS-CoV-2-Specific T-Cell Response (IFNγ Production)
3.5. SARS-CoV-2-Specific CD4+ and CD8+ T-Cell Proliferative Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rasmussen, S.A.; Jamieson, D.J.; Macfarlane, K.; Cragan, J.D.; Williams, J.; Henderson, Z. Pandemic Influenza and Pregnancy Working Group. Pandemic influenza and pregnant women: Summary of a meeting of experts. Am. J. Public Health 2009, 99 (Suppl. S2), S248–S254. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef] [PubMed]
- Matar, R.; Alrahmani, L.; Monzer, N.; Debiane, L.G.; Berbari, E.; Fares, J.; Fitzpatrick, F.; Murad, M.H. Clinical Presentation and Outcomes of Pregnant Women With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2021, 72, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Liu, Y.Y.; Wu, C.H.; Wang, C.Y.; Wang, C.H.; Long, C.Y. Impact of COVID-19 on Pregnancy. Int. J. Med. Sci. 2021, 18, 763–767. [Google Scholar] [CrossRef]
- WAPM (World Association of Perinatal Medicine) Working Group on COVID-19. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet. Gynecol. 2021, 57, 232–241. [Google Scholar] [CrossRef]
- Dubey, P.; Reddy, S.Y.; Manuel, S.; Dwivedi, A.K. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: An updated systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 490–501. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826, Erratum in: JAMA Pediatr. 2022, 176, 104. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Romero, R.; Xu, Y.; Theis, K.R.; Arenas-Hernandez, M.; Miller, D.; Peyvandipour, A.; Bhatti, G.; Galaz, J.; Gershater, M.; et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022, 13, 320. [Google Scholar] [CrossRef]
- Gee, S.; Chandiramani, M.; Seow, J.; Pollock, E.; Modestini, C.; Das, A.; Tree, T.; Doores, K.J.; Tribe, R.M.; Gibbons, D.L. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 2021, 22, 1490–1502. [Google Scholar] [CrossRef]
- Joubert, E.; Kekeh, A.C.; Amin, C.N. COVID-19 and novel mRNA vaccines in pregnancy: An updated literature review. BJOG 2022, 129, 21–28. [Google Scholar] [CrossRef]
- Wainstock, T.; Yoles, I.; Sergienko, R.; Sheiner, E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 2021, 39, 6037–6040. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Hernán, M.A.; Lipsitch, M.; Hernandez-Diaz, S.; Reism, B.Y.; et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef]
- Dugas, C.; Slane, V.H. Miscarriage. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ferré, C.; Callaghan, W.; Olson, C.; Sharma, A.; Barfield, W. Effects of Maternal Age and Age-Specific Preterm Birth Rates on Overall Preterm Birth Rates—United States, 2007 and 2014. MMWR Morb. Mortal Wkly. Rep. 2016, 65, 1181–1184. [Google Scholar] [CrossRef]
- Boghossian, N.S.; Geraci, M.; Edwards, E.M.; Horbar, J.D. Morbidity and Mortality in Small for Gestational Age Infants at 22 to 29 Weeks’ Gestation. Pediatrics 2018, 141, e20172533. [Google Scholar] [CrossRef]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e154834. [Google Scholar] [CrossRef]
- Burd, I.; Kino, T.; Segars, J. The Israeli study of Pfizer BNT162b2 vaccine in pregnancy: Considering maternal and neonatal benefits. J. Clin. Investig. 2021, 131, e150790. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, e1–e303. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
- Baird, J.K.; Jensen, S.M.; Urba, W.J.; Fox, B.A.; Baird, J.R. SARS-CoV-2 Antibodies detected in mother’s milk post-vaccination. J. Hum. Lact. 2021, 37, 492–498. [Google Scholar] [CrossRef]
- Ricciardi, A.; Zelini, P.; Cassaniti, I.; Avanzini, M.A.; Colaneri, M.; De Silvestri, A.; Baldanti, F.; Bruno, R. Serum and breastmilk SARS-CoV-2 specific antibodies following BNT162b2 vaccine: Prolonged protection from SARS-CoV-2 in newborns and older children. Int. J. Infect. Dis. 2022, 122, 905–909. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, L.; Zhang, P.; Li, K.; Liang, L.; Sun, J.; Zhang, Y. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 2020, 5, e139834. [Google Scholar] [CrossRef]
- Buszko, M.; Nita-Lazar, A.; Park, J.H.; Schwartzberg, P.L.; Verthelyi, D.; Young, H.A.; Rosenberg, A.S. Lessons learned: New insights on the role of cytokines in COVID-19. Nat. Immunol. 2021, 22, 404–411. [Google Scholar] [CrossRef]
- Nossent, E.J.; Schuurman, A.R.; Reijnders, T.D.Y.; Saris, A.; Jongerius, I.; Blok, S.G.; Van der Poll, T. Pulmonary Procoagulant and Innate Immune Responses in Critically Ill COVID-19 Patients. Front. Immunol. 2021, 12, 1. [Google Scholar] [CrossRef]
- Shimizu, S.; Gabazza, E.C.; Hayashi, T.; Ido, M.; Adachi, Y.; Suzuki, K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L503–L510. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Dhar, S.K.; K, V.; Damodar, S.; Gujar, S.; Das, M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression. Heliyon 2021, 7, e06155. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C. Innate and adaptive immune responses to SARS-CoV-2 in humans: Relevance to acquired immunity and vaccine responses. Clin. Exp. Immunol. 2021, 204, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tak-Yin Tsang, O.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Rizzi, M.; Costanzo, M.; Tonello, S.; Matino, E.; Casciaro, F.G.; Croce, A.; Rizzi, E.; Zecca, E.; Pedrinelli, A.; Vassia, V.; et al. Prognostic Markers in Hospitalized COVID-19 Patients: The Role of IP-10 and C-Reactive Protein. Dis. Mark. 2022, 2022, 3528312. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell. Rep. 2021, 36, 109504. [Google Scholar] [CrossRef]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef]
- Schlondorff, D. The glomerular mesangial cell: An expanding role for a specialized pericyte. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1987, 1, 272–281. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Zhang, K. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, S.; Su, S.; Ye, H.; Shu, H. Interactions Between Specific Immune Status of Pregnant Women and SARS-CoV-2 Infection. Front. Cell. Infect. Microbiol. 2021, 11, 753. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini Bellinazzi, C.; Borella, R.; Fidanza, L. Marked T cell activation, senescence, exhaustion, and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Skelly, D.T.; Harding, A.C.; Gilbert-Jaramillo, J.; Knight, M.L.; Longet, S.; Brown, A.; Adele, S.; Adland, E.; Brown, H.; Medawar Laboratory Team; et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat. Commun. 2021, 12, 5061. [Google Scholar] [CrossRef]
- Zelini, P.; Perotti, F.; Scatigno, A.L.; Dominoni, M.; Zavaglio, F.; Arossa, A.; Piccini, S.; Angelini, M.; Ghirardello, S.; Lilleri, D.; et al. Impact of SARS-CoV-2 infection during pregnancy and persistence of antibody response. New Microbiol. 2022, 45, 181–189. [Google Scholar]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Bergami, F.; Arena, F.; Sammartino, J.C.; Ferrari, A.; Zavaglio, F.; Zelini, P.; Paolucci, S.; Comolli, G.; Percivalle, E.; Lilleri, D.; et al. Differential Kinetics of Effector and Memory Responses Induced by Three Doses of SARS-CoV-2 mRNA Vaccine in a Cohort of Healthcare Workers. Vaccines 2022, 10, 1809. [Google Scholar] [CrossRef]
- Zavaglio, F.; Cassaniti, I.; Sammartino, J.C.; Tonello, S.; Sainaghi, P.P.; Novelli, V.; Meloni, F.; Lilleri, D.; Baldanti, F. mRNA BNT162b Vaccine Elicited Higher Antibody and CD4+ T-Cell Responses than Patients with Mild COVID-19. Microorganisms 2022, 10, 1250. [Google Scholar] [CrossRef]
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.; Rai, D.; Huang, A.; Flores, C.; Lin, C.Y.; Jigmeddagva, U.; Wu, A.H.B.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642.e10. [Google Scholar] [CrossRef]
- Enengl, S.; Pecks, U.; Oppelt, P.; Stelzl, P.; Trautner, P.S.; Shebl, O.; Lamprecht, B.; Longardt, A.C.; Eckmann-Scholz, C.; Keil, C.; et al. Antibody Response and Maternofetal Antibody Transfer in SARS-CoV-2-Positive Pregnant Women: A Multicenter Observational Study. Geburtshilfe Frauenheilkd 2022, 82, 501–509. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef] [PubMed]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The Impact of IgG transplacental transfer on early life immunity. Immunohorizons 2018, 2, 14–25. [Google Scholar] [CrossRef] [PubMed]
- De Rose, D.U.; Salvatori, G.; Dotta, A.; Auriti, C. SARS-CoV-2 Vaccines during Pregnancy and Breastfeeding: A Systematic Review of Maternal and Neonatal Outcomes. Viruses 2022, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, I.; Percivalle, E.; Zelini, P.; Ngaradoumbe Nanhorngue, K.; Parolo, A.; Bernardi, V.; Jorizzo, G.; Santer, P.; Perotti, F.; Spinillo, A.; et al. Both SARS-CoV-2 infection and vaccination in pregnancy elicited neutralizing antibodies in pregnant women and newborns. Clin. Microbiol. Infect. 2021, 27, 1708–1709. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Vorontsov, O.; Zigron, R.; Kleinstern, G.; Wolf, D.G.; Porat, S. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 419–425. [Google Scholar] [CrossRef]
| Vaccinated (n = 9) | No Vaccinated (n = 16) | |
|---|---|---|
| Age at delivery, median [range] | 33.0 [26.0–40.0] | 32.0 [19.0–43.0] |
| Caucasian n. (%) | 5 (55.5) | 9 (56.2) |
| Co-morbidities n. (%) | 5 (55.5) | 7 (43.7) |
| Gestational weeks at infection, median [range] | 39.0 [21.0–41.0] | 38.5 [16.0–42.0] |
| Haematological and biochemical parameters | ||
| Lymphocytes, 106 cells/µL, median [range] | 0.20 [0.3–7.26] | |
| C-reactive protein, median [range] | 0.09 [1.28–7.04] | |
| AST/ALT, median [range] | 26.0/19.0 [14.0–89.0/9.0–146.0] | |
| Symptoms: | ||
| Fever n. (%) | 1 (11.1) | 3 (18.7) |
| Cough n. (%) | 1 (11.1) | 1 (6.2) |
| Pharyngitis n. (%) | 0 (0.0) | 1 (6.2) |
| Rhinitis n. (%) | 1 (11.1) | 0 (0.0) |
| Mode of delivery: | ||
| Vaginal n. (%) | 6 (66.6) | 12 (75.0) |
| Cesarean section n. (%) | 3 (33.3) | 4 (25.0) |
| Therapy: | ||
| Azitromicin | 1 (11.1) | 0 (0.0) |
| Steroid | 0 (0.0) | 1 (11.1) |
| Vaccine | 2 dose | \ |
| Vaccinated Mothers (Median, Range) | Non-Vaccinated Mothers (Median, Range) | p Value | New Borns of Vaccinated Mothers (Median, Range) | New Borns of Non-Vaccinated Mothers (Median, Range) | p Value | Vaccinated Mothers/New Borns (p Value; r Spearman) | Non-Vaccinated Mothers/New Borns (p Value; r Spearman) | |
|---|---|---|---|---|---|---|---|---|
| Pro-Inflammatory Cytokines | ||||||||
| IL-1β | 1.63 (0.10–14–34) | 0.86 (0.10–8.5) | 0.034 | 0.86 (0.10–1.87) | 0.18 (0.10–5.27) | 0.207 | 0.366; 0.564 | 0.862; −0.068 |
| TNF-α | 22.35 (7.63–266.10) | 13.22 (6.16–78.03) | 0.043 | 18.54 (9.060–29.94) | 10.82 (1.38–37.25) | 0.237 | 0.300; 0.615 | 0.046; −0.652 |
| IL-6 | 14.94 (6.11–826.50) | 9.835 (1250.00–1016.00) | 0.259 | 6.30 (3.28–28.85) | 3.75 (1.25–15.72) | 0.206 | 0.450; 0.500 | 0.028; −0.699 |
| IL-8 | 2287.00 (20.60–70573.00) | 243.00 (65.35–5667.00) | 0.876 | 480.40 (9.07–5972.00) | 32.21 (3.60–9340.00) | 0.420 | 0.450; 0.500 | 0.154; −0.487 |
| IL17 | 7.34 (1.30–84.92) | 3.81 (0.80–49.88) | 0.119 | 4.38 (0.80–12.73) | 2.06 (0.80–19.58) | 0.493 | 0.350; 0.600 | 0.024; −0.714 |
| IFN-γ | 9.49 (0.2–86.77) | 5.16 (0.20–17.35) | 0.391 | 4.55 (2.85–9.94) | 2.85 (0.20–12.11) | 0.169 | 0.400; 0.564 | 0.061; −0.61 |
| Chemokines | ||||||||
| Eotaxin | 4.78 (2.15–8.41) | 2.95 (1.49–6.90) | 0.036 | 5.14 (2.34–7.82) | 3.13 (1.34–8.49) | 0.196 | ||
| IP-10 | 585.70 (260.10–1490.00) | 720.90 (176.20–3037.00) | 0.713 | 252.40 (203.40–775.00) | 172.20 (46.67–3957.00) | 0.206 | 0.783; −0.200 | 0.918; 0.042 |
| MCP-1 | 120.40 (19.31–746.5) | 107.30 (22.59–4941.00) | 0.763 | 16.01 (6.58–24.42) | 9.00 (4.97–90.12) | 0.744 | 0.350; 0.600 | 0.395; −0.298 |
| MIP-1α | 32.91 (1.86–1557.00) | 6.25 (0.86–280.90) | 0.110 | 2.84 (0.86–8.49) | 2.17 (0.46–104.30) | 0.742 | 0.233; 0.700 | 0.004; −0.838 |
| MIP-1β | 261.80 (86.06–1637.00) | 131.90 (67.64–1589.00) | 0.042 | 143.90 (111.20–227.50) | 116.30 (42.36–253.20) | 0.206 | 0.683; 0.300 | 0.607; −0.187 |
| RANTES | 29,239.00 (1948.00–313,339.00) | 8427.00 (4175.00–232,903.00) | 0.266 | 19246.00 (1168.00–70380.00) | 7243.00 (1007.00–30303.00) | 0.206 | 0.233; 0.700 | 0.367; −0.321 |
| Growth Factors | ||||||||
| FGF | 20.59 (4.50–135.70) | 14.18 (5.50–64.73) | 0.056 | 19.10 (1.80–33.01) | 5.47 (1.91–47.62) | 0.526 | 0.133; 0.800 | 0.185; 0.455 |
| PDGF | 6158.00 (2095.00–9185.00) | 4728.00 (1672.00–8636.00) | 0.338 | 4458.00 (597.90–11803.00) | 1865.00 (508.00–7109.00) | 0.513 | 0.083; 0.900 | 0.427; −0.284 |
| VEGF | 298.80 (138.50–1384.00) | 133.90 (83.52–716.10) | 0.023 | 250.60 (11.26–257.10) | 58.04 (6.00–481.90) | 0.238 | 0.683; −0.300 | 0.386; −0.304 |
| G-CSF | 629.30 (43.91–6576.00) | 163.30 (30.04–3713.00) | 0.141 | 54.33 (12.90–171.90) | 54.98 (12.90–2115.00) | 1.000 | 0.350;0.600 | 0.037:−0.674 |
| GM-CSF | 0.20 (0.20–10.22) | 0.20 (0.20–4980.00) | 0.063 | 0.20 (0.20–1.78) | 0.20 (0.20–2.92) | 0.517 | 1.000; −0.111 | 1.000; −0.250 |
| IL-7 | 11.33 (1.540–17.49) | 7.36 (1.10–17.59) | 0.297 | 12.07 (1.54–31.98) | 1.54 (1.10–9.04) | 0.038 | 0.400; 0.552 | 0.305; −0.355 |
| IL-15 | 589.40 (291.3–941.6) | 428.60 (11.00–794.60) | 0.029 | 206.40 (11.00–762.30) | 398.8 (11.00–728.60) | 0.374 | 0.350; 0.600 | 0.367; −0.314 |
| Th Cytokines | ||||||||
| IL-2 | 4.94 (1.110–27.74) | 1.52 (0.19–17.13) | 0.013 | 1.11 (0.19–6.35) | 0.95 (0.67–11.07) | 0.566 | 0.067; 0.872 | 0.221; −0.423 |
| IL-4 | 0.19 (0.10–7.35) | 1.33 (0.10–11.31) | 0.125 | 0.38 (0.10–0.89) | 0.10 (0.10–3.75) | 0.077 | 0.250; 0.657 | 0.600; −0.363 |
| IL-5 | 39.71 (10.00–333.50) | 10.00 (10.00–269.70) | 0.024 | 196.00 (10.00–535.40) | 10.00 (10.00–2.79) | 0.054 | 0.950; 0.100 | 1.000; −0.214 |
| IL-9 | 384.30 (186.8–518.50) | 286.9 (90.74–380.70) | 0.006 | 279.30 (242.30–400.70) | 239.60 (101.10–350.90) | 0.164 | 0.100; 0.894 | 0.448; −0.272 |
| IL-12 | 4.85 (0.30–14.02) | 1.94 (0.30–4.14) | 0.081 | 4.85 (2.70–7.57) | 0.90 (0.30–5.54) | 0.016 | 0.125; 0.585 | 0.452; 0.341 |
| IL-13 | 0.15 (0.15–1.09) | 0.15 (0.15–6.15) | 1.000 | 0.15 (0.15–0.33) | 0.15 (0.15–0.15) | 0.333 | 0.800; 0.344 | 0.435; −0.273 |
| Anti-Inflammatory Cytokines | ||||||||
| IL-10 | 3.67 (0.40–7.18) | 0.40 (0.40–3.67) | 0.018 | 1.62 (0.40–4.31) | 0.40 (0.40–3.04) | 0.136 | 0.600; 0.289 | 0.300; 0.430 |
| IL-1ra | 100.30 (20.00–849.10) | 20.00 (18.19–583.7) | 0.015 | 100.30 (0.20–317.90) | 20.00 (20.00–418.20) | 0.502 | 0.200; 0.803 | 0.067; 0.872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelini, P.; d’Angelo, P.; Zavaglio, F.; Soleymaninejadian, E.; Mariani, L.; Perotti, F.; Dominoni, M.; Tonello, S.; Sainaghi, P.; Minisini, R.; et al. Inflammatory and Immune Responses during SARS-CoV-2 Infection in Vaccinated and Non-Vaccinated Pregnant Women and Their Newborns. Pathogens 2023, 12, 664. https://doi.org/10.3390/pathogens12050664
Zelini P, d’Angelo P, Zavaglio F, Soleymaninejadian E, Mariani L, Perotti F, Dominoni M, Tonello S, Sainaghi P, Minisini R, et al. Inflammatory and Immune Responses during SARS-CoV-2 Infection in Vaccinated and Non-Vaccinated Pregnant Women and Their Newborns. Pathogens. 2023; 12(5):664. https://doi.org/10.3390/pathogens12050664
Chicago/Turabian StyleZelini, Paola, Piera d’Angelo, Federica Zavaglio, Ehsan Soleymaninejadian, Liliana Mariani, Francesca Perotti, Mattia Dominoni, Stelvio Tonello, Pierpaolo Sainaghi, Rosalba Minisini, and et al. 2023. "Inflammatory and Immune Responses during SARS-CoV-2 Infection in Vaccinated and Non-Vaccinated Pregnant Women and Their Newborns" Pathogens 12, no. 5: 664. https://doi.org/10.3390/pathogens12050664
APA StyleZelini, P., d’Angelo, P., Zavaglio, F., Soleymaninejadian, E., Mariani, L., Perotti, F., Dominoni, M., Tonello, S., Sainaghi, P., Minisini, R., Apostolo, D., Lilleri, D., Spinillo, A., & Baldanti, F. (2023). Inflammatory and Immune Responses during SARS-CoV-2 Infection in Vaccinated and Non-Vaccinated Pregnant Women and Their Newborns. Pathogens, 12(5), 664. https://doi.org/10.3390/pathogens12050664








