Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Drugs
2.3. Cytotoxicity Assay
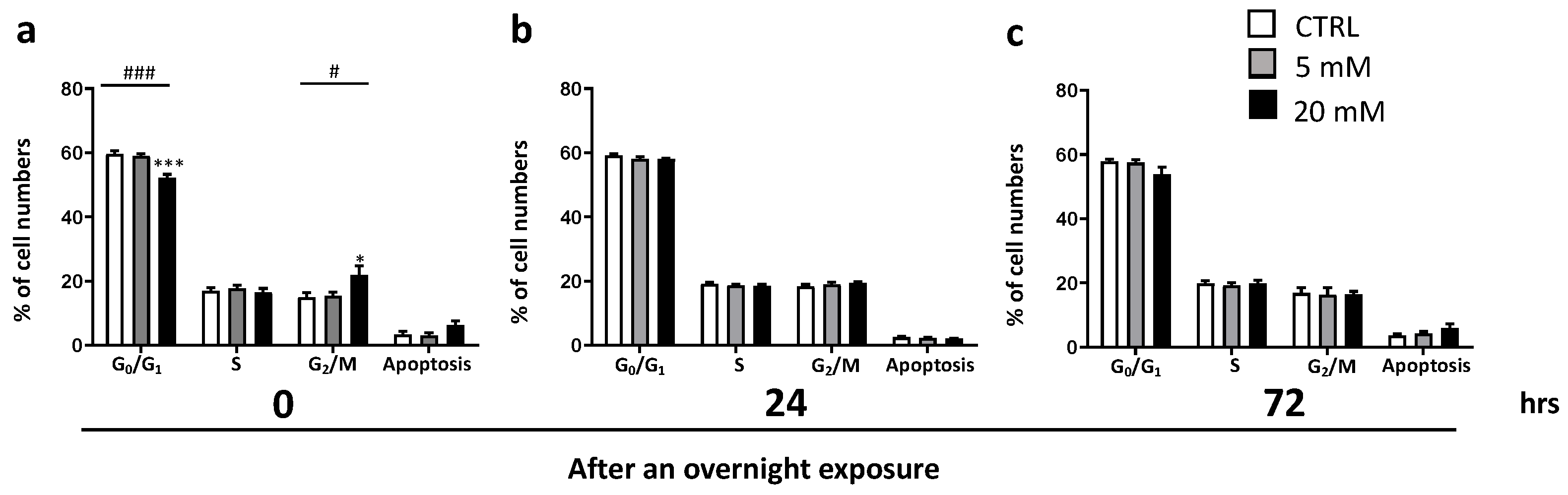
2.4. Cell Cycle Analysis
2.5. Plasma Membrane Cholesterol Content
2.6. Plasmids
2.7. Pseudotyped Lentiviral Particles Production
2.8. Single-Cycle Infectivity Assay
2.9. Curve Fit and Statistical Analysis
3. Results
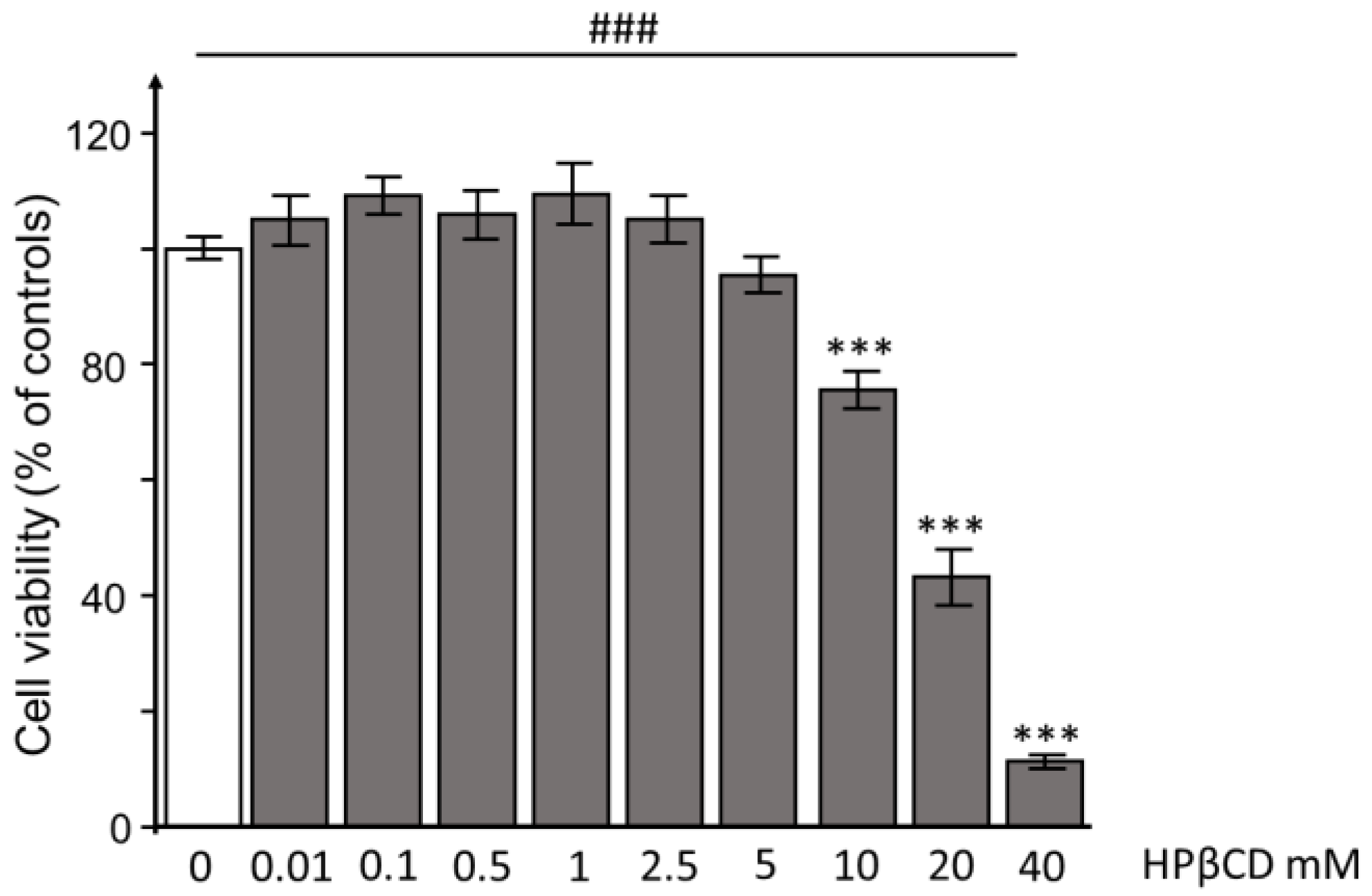
3.1. HPβCD Concentration-Dependent Effects on Cell Viability and Cell Cycle
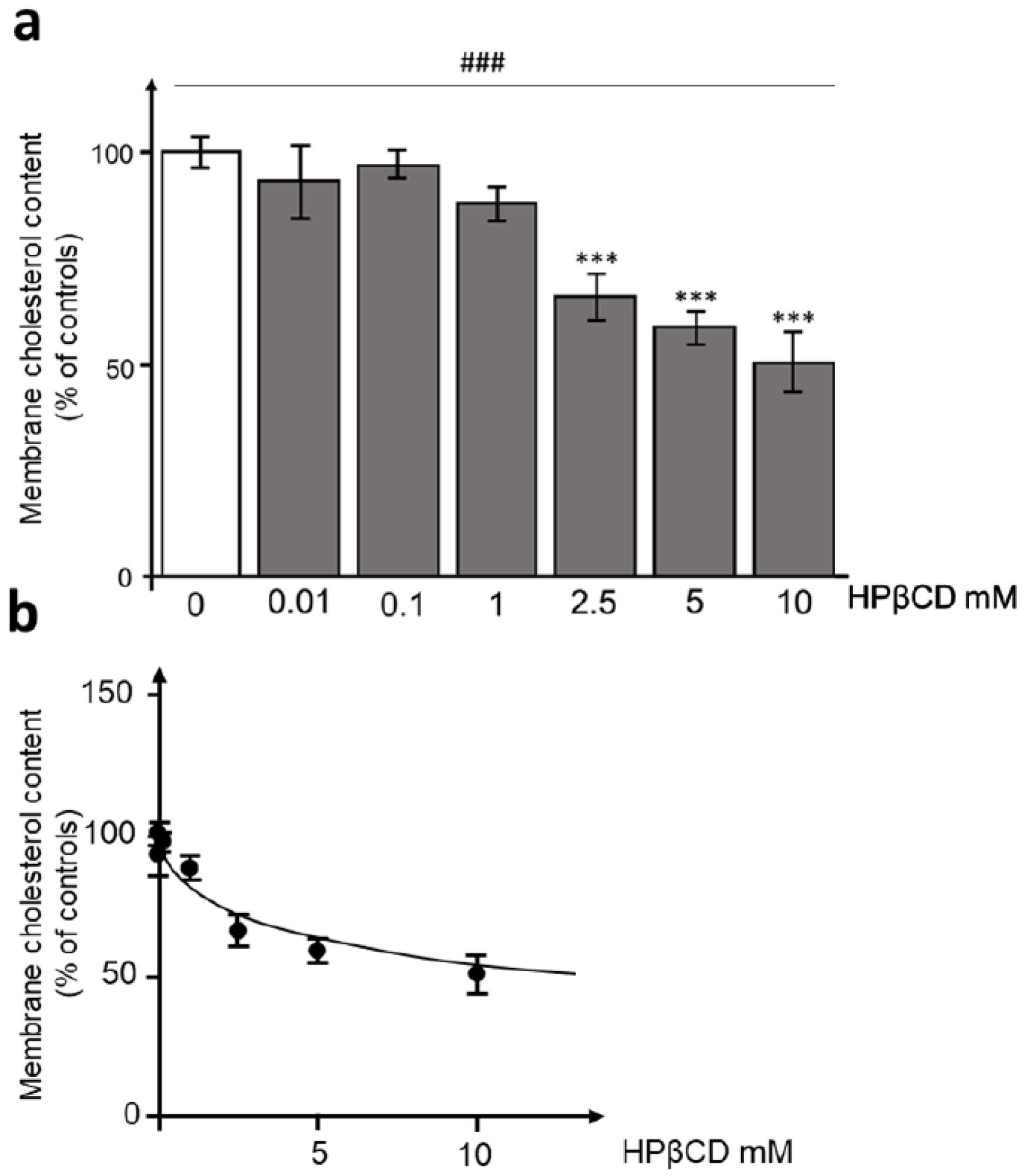
3.2. HPβCD Effects on Membrane Cholesterol Content
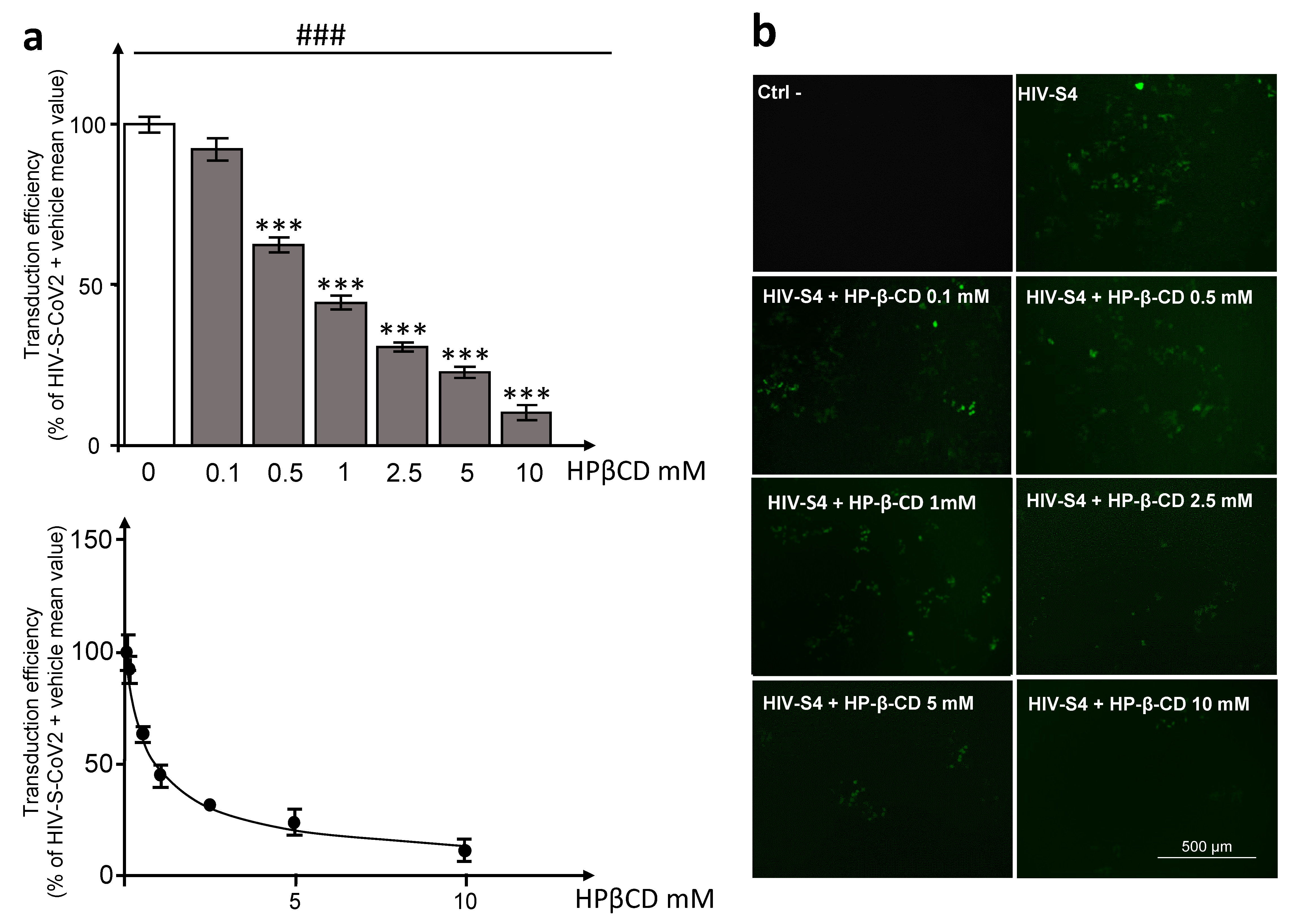
3.3. HPβCD Effects on Pseudotyped SARS-CoV-2 Particle Entry into HEK293T-ACE2hi Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Moran, M.M.; Cane, A.; Curcio, D.; Khan, F.; Malhotra, D.; Surinach, A.; Miles, A.; Swerdlow, D.; McLaughlin, J.M.; et al. Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. J. Med. Econ. 2021, 24, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef]
- Hagan, L.M.; McCormick, D.W.; Lee, C.; Sleweon, S.; Nicolae, L.; Dixon, T.; Banta, R.; Ogle, I.; Dusseau, C.; Salmonson, S.; et al. Outbreak of SARS-CoV-2 B.1.617.2 (delta) variant infections among incarcerated persons in a federal prison—Texas, July–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Ito, K.; Anzai, A.; Kobayashi, T.; Piantham, C.; Rodríguez-Morales, A.J. Relative Reproduction Number of SARS-CoV-2 Omicron (B.1.1.529) Compared with Delta Variant in South Africa. J. Clin. Med. 2021, 11, 30. [Google Scholar] [CrossRef]
- Ito, K.; Piantham, C.; Nishiura, H. Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J. Med. Virol. 2022, 94, 2265–2268. [Google Scholar] [CrossRef]
- Yamasoba, D.; Uriu, K.; Plianchaisuk, A.; Kosugi, Y.; Pan, L.; Zahradnik, J.; The Genotype to Phenotype Japan (G2P-Japan) Consortium; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 Omicron XBB. 1.16 variant. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef]
- Di Cagno, M.P. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2017, 22, 1. [Google Scholar] [CrossRef]
- Irie, T.; Fukunaga, K.; Garwood, M.K.; Carpenter, T.O.; Pitha, J.; Pitha, J. Hydroxypropylcyclodextrins in parenteral use. II: Effects on transport and disposition of lipids in rabbit and humans. J. Pharm. Sci. 1992, 81, 524–528. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Pettifor, J.M.; Russel, R.M.; Pitha, J.; Mobarhan, S.; Ossip, M.S.; Wainer, S.; Anast, C.S. Severe Hypervitaminosis A in Siblings: Evidence of Variable Tolerance to Retinol Intake. J. Pediatr. 1987, 111, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Favari, E.; Ronda, N.; Granata, A.; Bellosta, S.; Arnaboldi, L.; Corsini, A.; Gatti, R.; Bernini, F. Free cholesterol alters macrophage morphology and mobility by an ABCA1 dependent mechanism. Atherosclerosis 2011, 215, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Abels, E.R.; Redzic, J.S.; Margulis, J.; Finkbeiner, S.; Breakefield, X.O. Potential transfer of polyglutamine and CAG repeat RNA in extracellular vesicles in Huntington’s disease: Background and evaluation in cell culture. Cell. Mol. Neurobiol. 2016, 36, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Yokoo, M.; Kubota, Y.; Motoyama, K.; Higashi, T.; Taniyoshi, M.; Tokumaru, H.; Tabe, Y.; Nishiyama, R.; Mochinaga, S.; Sato, A.; et al. 2-Hydroxypropyl-β-Cyclodextrin Acts as a Novel Anticancer Agent. PLoS ONE 2015, 10, e0141946. [Google Scholar] [CrossRef]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Martínez-Mier, G.; Quistián-Galván, J.; Muñoz-Pérez, A.; Bernal-Dolores, V.; del Ángel, R.M.; et al. Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry. Front. Immunol. 2021, 12, 796855. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Fan, M.; Zhang, J.; Peng, Y.; Huang, F.; Wang, N.; He, L.; Zhang, L.; Holmdahl, R.; et al. Dependence of SARS-CoV-2 infection on cholesterol-rich lipid raft and endosomal acidification. Comput. Struct Biotechnol. J. 2021, 19, 1933–1943. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.E. Construction and applications of SARS-CoV-2 pseudoviruses: A mini review. Int. J. Biol. Sci. 2021, 17, 1574–1580. [Google Scholar] [CrossRef]
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The Ins and Outs of Lipid Rafts: Functions in Intracellular Cholesterol Homeostasis, Microparticles, and Cell membranes. J. Lipid Res. 2020, 61, 676–686. [Google Scholar] [CrossRef]
- Van IJzendoorn, S.C.D.; Agnetti, J.; Gassama-Diagne, A. Mechanisms Behind the Polarized Distribution of Lipids in Epithelial Cells. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183145. [Google Scholar] [CrossRef]
- López, C.A.; De Vries, A.H.; Marrink, S.J. Molecular Mechanism of Cyclodextrin Mediated Cholesterol Extraction. PLOS Comput. Biology 2011, 7, e1002020. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Hudák, A.; Letoha, A.; Szilák, L.; Letoha, T. Contribution of Syndecans to the Cellular Entry of SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 5336. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Kondo, Y.; Larabee, J.L.; Gao, L.; Shi, H.; Shao, B.; Hoover, C.M.; McDaniel, J.M.; Ho, Y.; Silasi-Mansat, R.; Archer-Hartmann, S.A.; et al. L-SIGN is a receptor on liver sinusoidal endothelial cells for SARS-CoV-2 virus. JCI Insight 2021, 6, e148999. [Google Scholar] [CrossRef]
- Wei, C.; Wan, L.; Yan, Q.; Wang, X.; Zhang, J.; Yang, X.; Zhang, Y.; Fan, C.; Li, D.; Deng, Y.; et al. HDL Scavenger Receptor B Type 1 Facilitates SARS-CoV-2 Entry. Nat. Metab. 2020, 2, 1391–1400. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal. Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Sarosh, V. Emerging Variants of SARS-CoV-2 and novel therapeutics against Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Qu, P.; Evans, J.P.; Zheng, Y.M.; Carlin, C.; Saif, L.J.; Oltz, E.M.; Xu, K.; Gumina, R.J.; Liu, S.L. Evasion of neutralizing antibody responses by the SARS-CoV-2 BA.2.75 variant. Cell Host Microbe 2022, 30, 1518–1526. [Google Scholar] [CrossRef]
- EMA. Background Review for Cyclodextrins Used as Excipients; 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/12/WC500177936.pdf (accessed on 18 January 2016).
- Meredith, M.E.; Salameh, T.S.; Banks, W.A. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J. 2015, 17, 780–787. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta 2007, 1768, 1311–1324. [Google Scholar] [CrossRef]
- Ohvo, H.; Slotte, J.P. Cyclodextrin-mediated removal of sterols from monolayers: Effects of sterol structure and phospholipids on desorption rate. Biochemistry 1996, 35, 8018–8024. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential effects of alpha-, beta- and gammacyclodextrins on human erythrocytes. Eur. J. Biochem. 1989, 186, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Papakyriakopoulou, P.; Manta, K.; Kostantini, C.; Kikionis, S.; Banella, S.; Ioannou, E.; Christodoulou, E.; Rekkas, D.M.; Dallas, P.; Vertzoni, M.; et al. Nasal powders of quercetin-β-cyclodextrin derivatives complexes with mannitol/lecithin microparticles for Nose-to-Brain delivery: In vitro and ex vivo evaluation. Int. J. Pharm. 2021, 607, 121016. [Google Scholar] [CrossRef] [PubMed]
- Badana, A.; Chintala, M.; Varikuti, G.; Pudi, N.; Kumari, S.; Kappala, V.R.; Malla, R.R. Lipid Raft Integrity Is Required for Survival of Triple Negative Breast Cancer Cells. J. Breast Cancer 2016, 19, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Lichtenhan, J.T.; Hirose, K.; Buchman, C.A.; Duncan, R.K.; Salt, A.N. Direct administration of 2-Hydroxypropyl-beta-cyclodextrin into guinea pig cochleae: Effects on physiological and histological measurements. PLoS ONE 2017, 1, e0175236. [Google Scholar] [CrossRef]
- Bezerra, B.B.; Silva, G.P.D.D.; Coelho, S.V.A.; Correa, I.A.; Souza, M.R.M.; Macedo, K.V.G.; Matos, B.M.; Tanuri, A.; Matassoli, F.L.; Costa, L.J.D.; et al. Hydroxypropyl-beta-cyclodextrin (HP-BCD) inhibits SARS-CoV-2 replication and virus-induced inflammatory cytokines. Antivir. Res. 2022, 205, 105373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alboni, S.; Secco, V.; Papotti, B.; Vilella, A.; Adorni, M.P.; Zimetti, F.; Schaeffer, L.; Tascedda, F.; Zoli, M.; Leblanc, P.; et al. Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells. Pathogens 2023, 12, 647. https://doi.org/10.3390/pathogens12050647
Alboni S, Secco V, Papotti B, Vilella A, Adorni MP, Zimetti F, Schaeffer L, Tascedda F, Zoli M, Leblanc P, et al. Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells. Pathogens. 2023; 12(5):647. https://doi.org/10.3390/pathogens12050647
Chicago/Turabian StyleAlboni, Silvia, Valentina Secco, Bianca Papotti, Antonietta Vilella, Maria Pia Adorni, Francesca Zimetti, Laurent Schaeffer, Fabio Tascedda, Michele Zoli, Pascal Leblanc, and et al. 2023. "Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells" Pathogens 12, no. 5: 647. https://doi.org/10.3390/pathogens12050647
APA StyleAlboni, S., Secco, V., Papotti, B., Vilella, A., Adorni, M. P., Zimetti, F., Schaeffer, L., Tascedda, F., Zoli, M., Leblanc, P., & Villa, E. (2023). Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells. Pathogens, 12(5), 647. https://doi.org/10.3390/pathogens12050647








