Leading Pathogens Involved in Co-Infection and Super-Infection with COVID-19: Forensic Medicine Considerations after a Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Information Sources and Search Strategy
3. Results
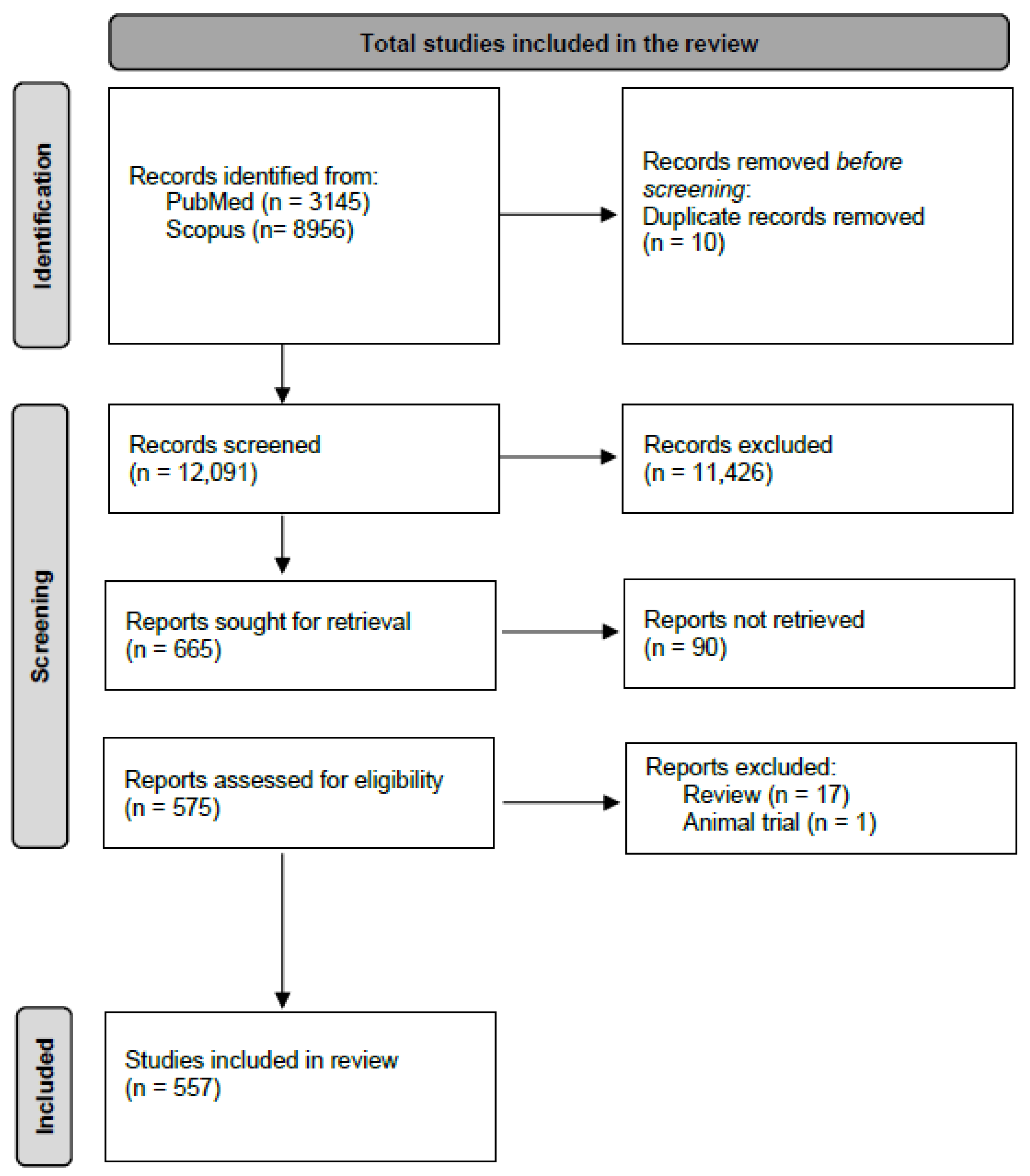
3.1. Study Selection
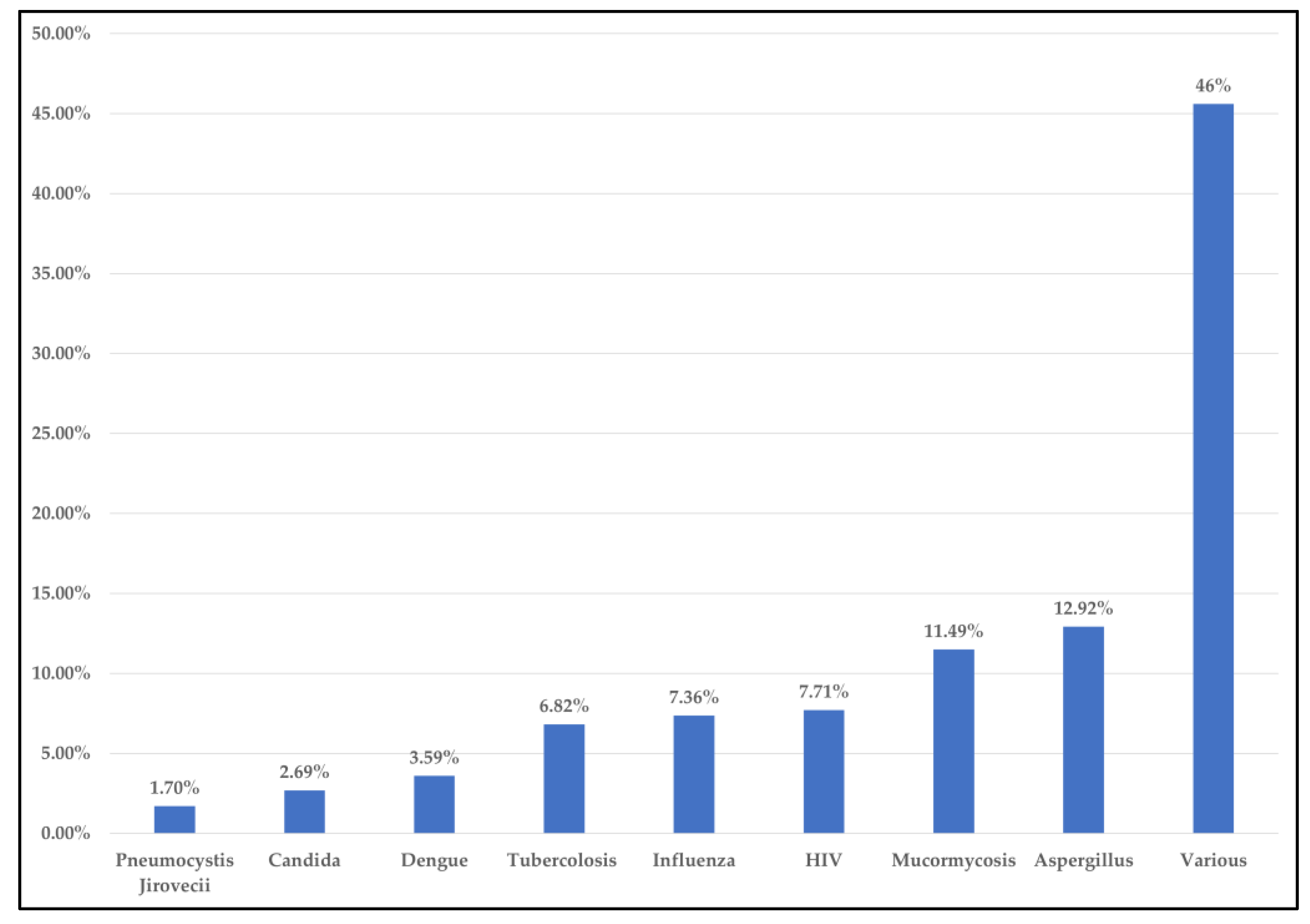
3.2. Results of Syntheses
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scendoni, R.; Marchesani, F.; Cannovo, N.; Fedeli, P.; Cingolani, M. Histopathology of COVID-19 pneumonia in two non-oncological, non-hospitalised cases as a reliable diagnostic benchmark. Diagn. Pathol. 2020, 15, 73. [Google Scholar] [CrossRef]
- Brandi, N.; Ciccarese, F.; Rimondi, M.R.; Balacchi, C.; Modolon, C.; Sportoletti, C.; Renzulli, M.; Coppola, F.; Golfieri, R. An Imaging Overview of COVID-19 ARDS in ICU Patients and Its Complications: A Pictorial Review. Diagnostics 2022, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, A.M.; Coppola, A.; Tortora, S.; Valconi, E.; Piacentino, F.; Fontana, F.; Stellato, E.; Cogliati, C.B.; Torzillo, D.; Giampalma, E.; et al. Gastrointestinal Bleeding in Patients with SARS-CoV-2 Infection Managed by Interventional Radiology. J. Clin. Med. 2021, 10, 4758. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; de la Court, J.R.; Buis, D.T.P.; Meijboom, L.J.; Schade, R.P.; Visser, C.E.; van Hest, R.; Kuijvenhoven, M.; Prins, J.M.; Nijman, S.F.M.; et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Murgia, F.; Fiamma, M.; Serra, S.; Olla, S.; Garau, M.C.; Cocco, E.; Lorefice, L.; Muntoni, S.; Paffi, P.; Porru, S.; et al. The impact of secondary infections in COVID-19 critically ill patients. J. Infect. 2022, 84, e116–e117. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, K.; Haque, M.; Nusrat, N.; Adnan, N.; Islam, S.; Lutfor, A.B.; Begum, D.; Rabbany, A.; Karim, E.; Malek, A.; et al. Management of Children Admitted to Hospitals across Bangladesh with Suspected or Confirmed COVID-19 and the Implications for the Future: A Nationwide Cross-Sectional Study. Antibiotics 2022, 11, 105. [Google Scholar] [CrossRef]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Dongen, M.V.; Founou, L.L. The COVID-19 pandemic: A threat to antimicrobial resistance containment. Future Sci. OA 2021, 7, FSO736. [Google Scholar] [CrossRef]
- Kurra, N.; Woodard, P.I.; Gandrakota, N.; Gandhi, H.; Polisetty, S.R.; Ang, S.P.; Patel, K.P.; Chitimalla, V.; Ali Baig, M.M.; Samudrala, G. Opportunistic Infections in COVID-19: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e23687. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose Coronavirus Disease 2019-Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef] [PubMed]
- Brandi, N.; Ciccarese, F.; Balacchi, C.; Rimondi, M.R.; Modolon, C.; Sportoletti, C.; Capozzi, C.; Renzulli, M.; Paccapelo, A.; Castelli, A.; et al. Co-Infections and Superinfections in COVID-19 Critically Ill Patients Are Associated with CT Imaging Abnormalities and the Worst Outcomes. Diagnostics 2022, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef] [PubMed]
- Almamlouk, R.; Kashour, T.; Obeidat, S.; Bois, M.C.; Maleszewski, J.J.; Omrani, O.A.; Tleyjeh, R.; Berbari, E.; Chakhachiro, Z.; Zein-Sabatto, B.; et al. COVID-19-Associated cardiac pathology at the postmortem evaluation: A collaborative systematic review. Clin. Microbiol. Infect. 2021, 28, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Solarino, B.; Ferorelli, D.; Dell’Erba, A. Post-mortem routine practice in the era of the COVID-19 pandemic. J. Forensic Leg. Med. 2020, 74, 102010. [Google Scholar] [CrossRef]
- De Micco, F.; De Benedictis, A.; Fineschi, V.; Frati, P.; Ciccozzi, M.; Pecchia, L.; Alloni, R.; Petrosillo, N.; Filippi, S.; Ghilardi, G.; et al. From Syndemic Lesson after COVID-19 Pandemic to a “Systemic Clinical Risk Management” Proposal in the Perspective of the Ethics of Job Well Done. Int. J. Environ. Res. Public Health 2021, 19, 15. [Google Scholar] [CrossRef]
- Ferorelli, D.; Bolcato, M.; Aprile, A.; Dell’Erba, A. Editorial: Medico-legal aspects of clinical risk management and patient safety. Front. Public Health 2022, 10, 970258. [Google Scholar] [CrossRef]
- Tattoli, L.; Dell’Erba, A.; Ferorelli, D.; Gasbarro, A.; Solarino, B. Sepsis and Nosocomial Infections: The Role of Medico-Legal Experts in Italy. Antibiotics 2019, 8, 199. [Google Scholar] [CrossRef]
- De Micco, F.; Fineschi, V.; Banfi, G.; Frati, P.; Oliva, A.; Travaini, G.V.; Picozzi, M.; Curcio, G.; Pecchia, L.; Petitti, T.; et al. From COVID-19 Pandemic to Patient Safety: A New “Spring” for Telemedicine or a Boomerang Effect? Front. Med. 2022, 9, 901788. [Google Scholar] [CrossRef]
- Ricciardi, S.; Guarino, A.M.; Giaquinto, L.; Polishchuk, E.V.; Santoro, M.; Di Tullio, G.; Wilson, C.; Panariello, F.; Soares, V.C.; Dias, S.S.G.; et al. The role of NSP6 in the biogenesis of the SARS-CoV-2 replication organelle. Nature 2022, 606, 761–768. [Google Scholar] [CrossRef]
- Lundin, A.; Dijkman, R.; Bergström, T.; Kann, N.; Adamiak, B.; Hannoun, C.; Kindler, E.; Jónsdóttir, H.R.; Muth, D.; Kint, J.; et al. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the middle East respiratory syndrome virus. PLoS Pathog. 2014, 10, e1004166. [Google Scholar] [CrossRef] [PubMed]
- Rappe, J.C.F.; de Wilde, A.; Di, H.; Müller, C.; Stalder, H.; V’kovski, P.; Snijder, E.; Brinton, M.A.; Ziebuhr, J.; Ruggli, N.; et al. Antiviral activity of K22 against members of the order Nidovirales. Virus Res. 2018, 246, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Massey, B.W.; Jayathilake, K.; Meltzer, H.Y. Respiratory Microbial Co-Infection with SARS-CoV-2. Front. Microbiol. 2020, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- İslamoğlu, M.S.; Borku Uysal, B. Influenza and SARS-CoV-2 coinfection during the COVID-19 pandemic. Turk Hij. Deney. Biyol. Derg. 2021, 78, 373–378. [Google Scholar] [CrossRef]
- Schirmer, P.; Lucero-Obusan, C.; Sharma, A.; Sohoni, P.; Oda, G.; Holodniy, M. Respiratory co-infections with COVID-19 in the Veterans Health Administration, 2020. Diagn. Microbiol. Infect. Dis. 2021, 100, 115312. [Google Scholar] [CrossRef]
- Burrel, S.; Hausfater, P.; Dres, M.; Pourcher, V.; Luyt, C.E.; Teyssou, E.; Soulié, C.; Calvez, V.; Marcelin, A.G.; Boutolleau, D. Co-infection of SARS-CoV-2 with other respiratory viruses and performance of lower respiratory tract samples for the diagnosis of COVID-19. Int. J. Infect. Dis. 2021, 102, 10–13. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, L.; Cheng, X.; Han, H.; Li, C.; Li, D.; Liu, A.; Gao, G.; Zhou, F.; Liu, F.; et al. Clinical characteristics of COVID-19 patients with hepatitis B virus infection—A retrospective study. Liver Int. 2021, 41, 720–730. [Google Scholar] [CrossRef]
- Moin, S.; Farooqi, J.; Rattani, S.; Nasir, N.; Zaka, S.; Jabeen, K.C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med. Mycol. 2021, 59, 1238–1242. [Google Scholar] [CrossRef]
- Laszkowska, M.; Kim, J.; Faye, A.S.; Joelson, A.M.; Ingram, M.; Truong, H.; Silver, E.R.; May, B.; Greendyke, W.G.; Zucker, J.; et al. Prevalence of Clostridioides difficile and Other Gastrointestinal Pathogens in Patients with COVID-19. Dig. Dis. Sci. 2021, 66, 4398–4405. [Google Scholar] [CrossRef]
- Scott, H.; Zahra, A.; Fernandes, R.; Fries, B.C.; Thode, H.C., Jr.; Singer, A.J. Bacterial infections and death among patients with COVID-19 versus non COVID-19 patients with pneumonia. Am. J. Emerg. Med. 2021, 51, 1–5. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.B.; Mahé, C.; Chaves, S.S. Uncertain effects of the pandemic on respiratory viruses. Science 2021, 372, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, R.; Al Busaidi, N.; Al Umairi, R.; Al Busaidy, M.; Al Naabi, H.H.; Khamis, F. COVID-19 and Pulmonary Mycobacterium Tuberculosis Coinfection. Oman Med. J. 2021, 36, e298. [Google Scholar] [CrossRef] [PubMed]
- Scendoni, R.; Gattari, D.; Cingolani, M. COVID-19 Pulmonary Pathology, Ventilator-Induced Lung Injury (VILI), or Sepsis-Induced Acute Respiratory Distress Syndrome (ARDS)? Healthcare Considerations Arising from an Autopsy Case and Miny-Review. Clin. Pathol. 2022, 15, 2632010X221083223. [Google Scholar] [CrossRef]
- Sreenath, K.; Batra, P.; Vinayaraj, E.V.; Bhatia, R.; SaiKiran, K.; Singh, V.; Singh, S.; Verma, N.; Singh, U.B.; Mohan, A.; et al. Coinfections with Other Respiratory Pathogens among Patients with COVID-19. Microbiol. Spectr. 2021, 9, e0016321. [Google Scholar] [CrossRef]
- Cataño-Correa, J.C.; Cardona-Arias, J.A.; Porras Mancilla, J.P.; García, M.T. Bacterial superinfection in adults with COVID-19 hospitalized in two clinics in Medellín-Colombia, 2020. PLoS ONE 2021, 16, e0254671. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Esposito, M.; Salerno, M.; Scoto, E.; Di Nunno, N.; Sessa, F. The Impact of the COVID-19 Pandemic on the Practice of Forensic Medicine: An Overview. Healthcare 2022, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Malhotra, A.; Visonà, S.D.; Finley, S.J.; Osculati, A.M.M.; Javan, G.T. The roles of medical examiners in the COVID-19 era: A comparison between the United States and Italy. Forensic Sci. Med. Pathol. 2021, 17, 262–270. [Google Scholar] [CrossRef]
- Maiese, A.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Autopsy findings in COVID-19-related deaths: A literature review. Forensic Sci. Med. Pathol. 2021, 17, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Bertozzi, G.; Cipolloni, L.; Baldari, B.; Cantatore, S.; D’Errico, S.; Di Mizio, G.; Asmundo, A.; Castorina, S.; Salerno, M.; et al. Clinical-Forensic Autopsy Findings to Defeat COVID-19 Disease: A Literature Review. J. Clin. Med. 2020, 9, 2026. [Google Scholar] [CrossRef]
- Jakubec, P.; Fišerová, K.; Genzor, S.; Kolář, M. Pulmonary Complications after COVID-19. Life 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Tsai, P.H.; Chou, Y.C.; Lu, K.C.; Chang, F.Y.; Wu, C.C. Biomarkers Linked with Dynamic Changes of Renal Function in Asymptomatic and Mildly Symptomatic COVID-19 Patients. J. Pers. Med. 2021, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Bozzato, A.M.; Di Bella, S.; Occhipinti, A.A.; Martingano, P.; Cavallaro, M.F.M.; Luzzati, R.; Monica, F.; Cova, M.A.; Crocè, L.S. Spontaneous Rectal Perforation in a Patient with SARS-CoV-2 Infection. J. Pers. Med. 2020, 10, 157. [Google Scholar] [CrossRef]
- Tudoran, M.; Tudoran, C.; Lazureanu, V.E.; Marinescu, A.R.; Pop, G.N.; Pescariu, A.S.; Enache, A.; Cut, T.G. Alterations of Left Ventricular Function Persisting during Post-Acute COVID-19 in Subjects without Previously Diagnosed Cardiovascular Pathology. J. Pers. Med. 2021, 11, 225. [Google Scholar] [CrossRef]
- Domènech-Montoliu, S.; Puig-Barberà, J.; Pac-Sa, M.R.; Vidal-Utrillas, P.; Latorre-Poveda, M.; Del Rio-González, A.; Ferrando-Rubert, S.; Ferrer-Abad, G.; Sánchez-Urbano, M.; Aparisi-Esteve, L.; et al. Complications Post-COVID-19 and Risk Factors among Patients after Six Months of a SARS-CoV-2 Infection: A Population-Based Prospective Cohort Study. Epidemiologia 2022, 3, 49–67. [Google Scholar] [CrossRef]
- Fineschi, V.; Aprile, A.; Aquila, I.; Arcangeli, M.; Asmundo, A.; Bacci, M.; Cingolani, M.; Cipolloni, L.; D’Errico, S.; De Casamassimi, I.; et al. Management of the corpse with suspect, probable or confirmed COVID-19 respiratory infection—Italian interim recommendations for personnel potentially exposed to material from corpses, including body fluids, in morgue structures and during autopsy practice. Pathologica 2020, 112, 64–77. [Google Scholar]
- Cadena, J.; Thompson, G.R., 3rd; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, J.M.; Miceli, M.H. Mucormycosis. Infect. Dis. Clin. N. Am. 2021, 35, 435–452. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 3 March 2023).
- Filbrun, A.B.; Richardson, J.C.; Khanal, P.C.; Tzeng, Y.L.; Dickson, R.M. Rapid, label-free antibiotic susceptibility determined directly from positive blood culture. Cytom. Part A J. Int. Soc. Anal. Cytol. 2022, 101, 564–576. [Google Scholar] [CrossRef]
- Alfonso-Sanchez, J.L.; Agurto-Ramirez, A.; Chong-Valbuena, M.A.; De-Jesús-María, I.; Julián-Paches, P.; López-Cerrillo, L.; Piedrahita-Valdés, H.; Giménez-Azagra, M.; Martín-Moreno, J.M. The Influence of Infection and Colonization on Outcomes in Inpatients with COVID-19: Are We Forgetting Something? Front. Public Health 2021, 9, 747791. [Google Scholar] [CrossRef]
- Rockett, R.J.; Draper, J.; Gall, M.; Sim, E.M.; Arnott, A.; Agius, J.E.; Johnson-Mackinnon, J.; Fong, W.; Martinez, E.; Drew, A.P.; et al. Co-infection with SARS-CoV-2 Omicron and Delta variants revealed by genomic surveillance. Nat. Commun. 2022, 13, 2745. [Google Scholar] [CrossRef] [PubMed]
- Vatteroni, M.L.; Capria, A.L.; Spezia, P.G.; Frateschi, S.; Pistello, M. Co-infection with SARS-CoV-2 omicron BA.1 and BA.2 subvariants in a non-vaccinated woman. Lancet Microbe 2022, 3, e478. [Google Scholar] [CrossRef]
- Scendoni, R.; Ferrante, L.; Stramazzotti, D.; Tagliabracci, A. Analysis of immunohistochemical expression of inducible nitric oxide synthase for the evaluation of agonal time in forensic medicine. Int. J. Leg. Med. 2016, 130, 1639–1646. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Ervin, J.N.; Kahn, J.M.; Cohen, T.R.; Weingart, L.R. Teamwork in the intensive care unit. Am. Psychol. 2018, 73, 468–477. [Google Scholar] [CrossRef]
- Oishi, K.; Horiuchi, S.; Minkoff, J.M.; ten Oever, B.R. The Host Response to Influenza A Virus Interferes with SARS-CoV-2 Replication during Coinfection. J. Virol. 2022, 96, e0076522. [Google Scholar] [CrossRef]

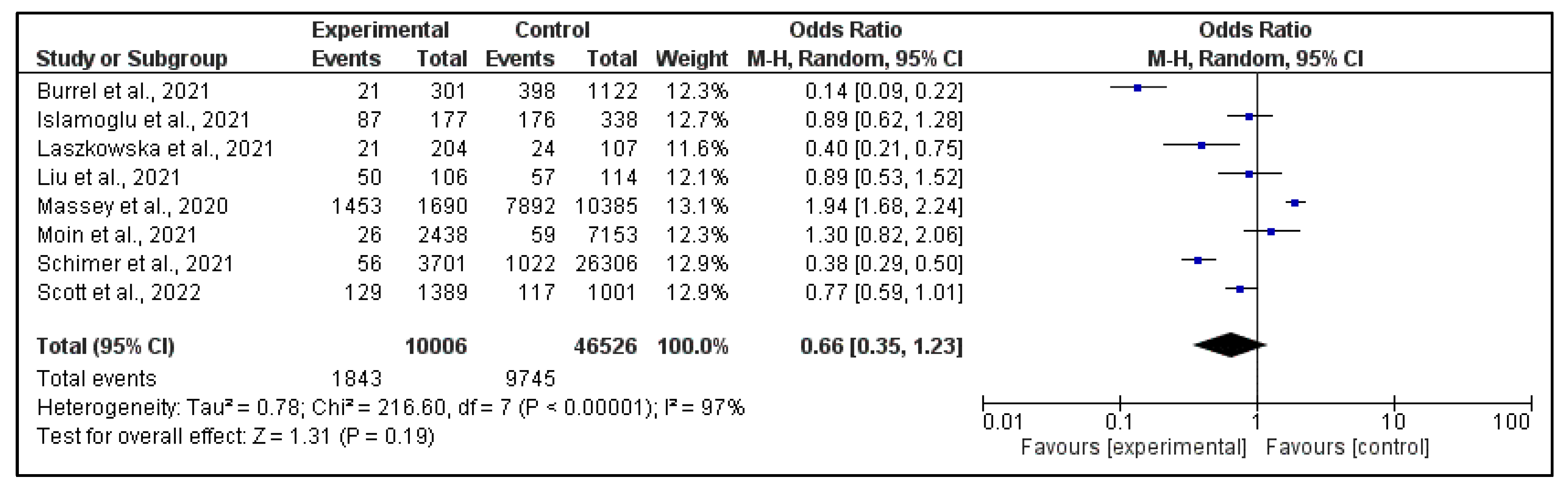
| Author (Year) | IC | NIC | ICON | NICON |
|---|---|---|---|---|
| Massey BW et al. (2020) [24] | 1453 in 1690 | 237 in 1690 | 7892 in 10,385 | 2493 in 10,385 |
| İslamoğlu MS et al. (2021) [25] | 87 in 177 | 90 in 177 | 176 in 338 | 162 in 338 |
| Schirmer P et al. (2021) [26] | 56 in 3701 | 3645 in 3701 | 1022 in 26,306 | 25,284 in 26,306 |
| Burrel S et al. (2021) [27] | 21 in 301 | 280 in 301 | 398 in 1122 | 724 in 1122 |
| Liu R et al. (2021) [28] | 50 in 106 | 56 in 106 | 57 in 114 | 57 in 114 |
| Moin S et al. (2021) [29] | 26 in 2438 | 2412 in 2438 | 59 in 7153 | 7094 in 7153 |
| Laszkowska M et al. (2021) [30] | 21 in 204 | 183 in 204 | 24 in 107 | 83 in 107 |
| Scott H et al. (2022) [31] | 129 in 1389 | 1216 in 1389 | 117 in 1001 | 884 in 1001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scendoni, R.; Bury, E.; Lima Arrais Ribeiro, I.; Cingolani, M.; Cameriere, R.; De Benedictis, A.; De Micco, F. Leading Pathogens Involved in Co-Infection and Super-Infection with COVID-19: Forensic Medicine Considerations after a Systematic Review and Meta-Analysis. Pathogens 2023, 12, 646. https://doi.org/10.3390/pathogens12050646
Scendoni R, Bury E, Lima Arrais Ribeiro I, Cingolani M, Cameriere R, De Benedictis A, De Micco F. Leading Pathogens Involved in Co-Infection and Super-Infection with COVID-19: Forensic Medicine Considerations after a Systematic Review and Meta-Analysis. Pathogens. 2023; 12(5):646. https://doi.org/10.3390/pathogens12050646
Chicago/Turabian StyleScendoni, Roberto, Emanuele Bury, Isabella Lima Arrais Ribeiro, Mariano Cingolani, Roberto Cameriere, Anna De Benedictis, and Francesco De Micco. 2023. "Leading Pathogens Involved in Co-Infection and Super-Infection with COVID-19: Forensic Medicine Considerations after a Systematic Review and Meta-Analysis" Pathogens 12, no. 5: 646. https://doi.org/10.3390/pathogens12050646
APA StyleScendoni, R., Bury, E., Lima Arrais Ribeiro, I., Cingolani, M., Cameriere, R., De Benedictis, A., & De Micco, F. (2023). Leading Pathogens Involved in Co-Infection and Super-Infection with COVID-19: Forensic Medicine Considerations after a Systematic Review and Meta-Analysis. Pathogens, 12(5), 646. https://doi.org/10.3390/pathogens12050646











