Association between Mastitis Occurrence in Dairy Cows and Bedding Characteristics of Compost-Bedded Pack Barns
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd Selection and Study Protocols
2.2. Farm and Cow Characteristics
2.3. Milk and Bedding Sample Collection
2.4. Milk and Bedding Analysis
2.5. Data Analyses
3. Results
3.1. Farm and Cow Characteristics
3.2. Frequency of Mastitis-Causing Pathogens
3.3. Bedding Characteristics and Mastitis Indexes
4. Discussion
4.1. Frequency and Profile of Mastitis Pathogens
4.2. Bedding Characteristics and Mastitis Indexes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bewley, J.M.; Taraba, J.L.; Day, G.B.; Black, R.A. Compost Bedded Pack Barn Design: Features and Management Considerations; Cooperative Extension Publ. ID 206; University of Kentucky College of Agriculture: Lexington, KY, USA, 2012. [Google Scholar]
- Leso, L.; Barbari, M.; Lopes, M.A.; Damasceno, F.A.; Galama, P.; Taraba, J.L.; Kuipers, A. Invited review: Compost-bedded pack barns for dairy cows. J. Dairy Sci. 2020, 103, 1072–1099. [Google Scholar] [CrossRef] [PubMed]
- de Kogima, P.A.; Diesel, T.A.; Vieira, F.M.C.; Schogor, A.L.B.; Volpini, A.A.; Veloso, G.J.; Ferraz, P.F.P.; Zotti, M.L.A.N. The Welfare of Dairy Cows in Pasture, Free Stall, and Compost Barn Management Systems in a Brazilian Subtropical Region. Animals 2022, 12, 2215. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.G.B.S.; Ferraz, P.F.P.; Damasceno, F.A.; Zotti, M.L.A.N.; Barbari, M. Compost Barns: A Bibliometric Analysis. Animals 2022, 12, 2492. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Taraba, J.L.; Day, G.B.; Damasceno, F.A.; Newman, M.C.; Akers, K.A.; Wood, C.L.; McQuerry, K.J.; Bewley, J.M. The relationship between compost bedded pack performance, management, and bacterial counts. J. Dairy Sci. 2014, 97, 2669–2679. [Google Scholar] [CrossRef]
- Leso, L.; Uberti, M.; Morshed, W.; Barbari, M. A survey on Italian compost dairy barns. J. Agric. Eng. 2013, 44, 203–207. [Google Scholar] [CrossRef]
- Damasceno, F.A.; Day, G.B.; Taraba, J.L.; Oliveira, C.E.A.; Andrade, R.R.; Frigeri, K.D.M.; Vieira, F.M.C.; Barbari, M.; Bambi, G. Compost Dairy Barn Layout and Management Recommendations in Kentucky: A Descriptive Study. Animals 2022, 12, 3324. [Google Scholar] [CrossRef] [PubMed]
- Emanuelson, U.; Brügemann, K.; Klopčič, M.; Leso, L.; Ouweltjes, W.; Zentner, A.; Blanco-Penedo, I. Animal Health in Compost-Bedded Pack and Cubicle Dairy Barns in Six European Countries. Animals 2022, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.-M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-specific production losses in bovine mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef] [PubMed]
- Egyedy, A.F.; Ametaj, B.N. Mastitis: Impact of Dry Period, Pathogens, and Immune Responses on Etiopathogenesis of Disease and its Association with Periparturient Diseases. Dairy 2022, 3, 881–906. [Google Scholar] [CrossRef]
- Hogan, J.; Smith, K.L. Managing Environmental Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 217–224. [Google Scholar] [CrossRef]
- Ruegg, P.L. New Perspectives in Udder Health Management. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, J.; Piepers, S.; Supré, K.; De Vliegher, S. Pathogen-specific incidence rate of clinical mastitis in Flemish dairy herds, severity, and association with herd hygiene. J. Dairy Sci. 2014, 97, 6926–6934. [Google Scholar] [CrossRef] [PubMed]
- Kurban, D.; Roy, J.-P.; Kabera, F.; Fréchette, A.; Um, M.M.; Albaaj, A.; Rowe, S.; Godden, S.; Adkins, P.R.F.; Middleton, J.R.; et al. Diagnosing Intramammary Infection: Meta-Analysis and Mapping Review on Frequency and Udder Health Relevance of Microorganism Species Isolated from Bovine Milk Samples. Animals 2022, 12, 3288. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.K.; Sahibzada, S.; Annandale, H.C.; Robertson, I.D.; Waichigo, F.W.; Tufail, M.S.; Aleri, J.A. Bacterial pathogens associated with clinical and subclinical mastitis in a Mediterranean pasture-based dairy production system of Australia. Res. Vet. Sci. 2021, 141, 103–109. [Google Scholar] [CrossRef]
- Patel, K.; Godden, S.M.; Royster, E.; Crooker, B.A.; Timmerman, J.; Fox, L. Relationships among bedding materials, bedding bacteria counts, udder hygiene, milk quality, and udder health in US dairy herds. J. Dairy Sci. 2019, 102, 10213–10234. [Google Scholar] [CrossRef]
- Fávero, S.; Portilho, F.V.R.; Oliveira, A.C.R.; Langoni, H.; Pantoja, J.C.F. Factors associated with mastitis epidemiologic indexes, animal hygiene, and bulk milk bacterial concentrations in dairy herds housed on compost bedding. Livest. Sci. 2015, 181, 220–230. [Google Scholar] [CrossRef]
- Albino, R.L.; Taraba, J.L.; Marcondes, M.I.; Eckelkamp, E.A.; Bewley, J.M. Comparison of bacterial populations in bedding material, on teat ends, and in milk of cows housed in compost bedded pack barns. Anim. Prod. Sci. 2017, 58, 1686–1691. [Google Scholar] [CrossRef]
- Cole, K.J.; Hogan, J.S. Short communication: Environmental mastitis pathogen counts in freestalls bedded with composted and fresh recycled manure solids. J. Dairy Sci. 2016, 99, 1501–1505. [Google Scholar] [CrossRef]
- Godden, S.; Bey, R.; Lorch, K.; Farnsworth, R.; Rapnicki, P. Ability of Organic and Inorganic Bedding Materials to Promote Growth of Environmental Bacteria. J. Dairy Sci. 2008, 91, 151–159. [Google Scholar] [CrossRef]
- Sherwin, V.E.; Egan, S.A.; Green, M.J.; Leigh, J.A. Survival of Streptococcus uberis on bedding substrates. Vet. J. 2021, 276, 105731. [Google Scholar] [CrossRef] [PubMed]
- Wenz, J.R.; Garry, F.B.; Barrington, G.M. Comparison of disease severity scoring systems for dairy cattle with acute coliform mastitis. J. Am. Vet. Med. Assoc. 2006, 229, 259–262. [Google Scholar] [CrossRef] [PubMed]
- National Mastitis Council. Laboratory Handbook on Bovine Mastitis; National Mastitis Council: Madison, WI, USA, 2017; p. 148. [Google Scholar]
- Barberg, A.E.; Endres, M.I.; Janni, K.A. Compost Dairy Barns in Minnesota: A Descriptive Study. Appl. Eng. Agric. 2007, 23, 231–238. [Google Scholar] [CrossRef]
- Barcelos, M.M.; Martins, L.; Grenfell, R.C.; Juliano, L.; Anderson, K.L.; dos Santos, M.V.; Gonçalves, J.L. Comparison of standard and on-plate extraction protocols for identification of mastitis-causing bacteria by MALDI-TOF MS. Braz. J. Microbiol. 2019, 50, 849–857. [Google Scholar] [CrossRef]
- Conesa, A.; Dieser, S.; Barberis, C.; Bonetto, C.; Lasagno, M.; Vay, C.; Odierno, L.; Porporatto, C.; Raspanti, C. Differentiation of non-aureus staphylococci species isolated from bovine mastitis by PCR-RFLP of groEL and gap genes in comparison to MALDI-TOF mass spectrometry. Microb. Pathog. 2020, 149, 104489. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Manual de Métodos Analíticos Oficiais Para Fertilizantes e Corretivos; Secretaria de Defesa Agropecuária; MAPA: Ribeira, Brazil, 2017; p. 240. [Google Scholar]
- Zdanowicz, M.; Shelford, J.A.; Tucker, C.B.; Weary, D.M.; von Keyserlingk, M.A.G. Bacterial Populations on Teat Ends of Dairy Cows Housed in Free Stalls and Bedded with Either Sand or Sawdust. J. Dairy Sci. 2004, 87, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.R.; Leslie, K.E. Evaluation of changes in somatic cell counts as indicators of new intramammary infections. Prev. Vet. Med. 1991, 10, 225–237. [Google Scholar] [CrossRef]
- Fréchette, A.; Fecteau, G.; Côté, C.; Dufour, S. Clinical Mastitis Incidence in Dairy Cows Housed on Recycled Manure Solids Bedding: A Canadian Cohort Study. Front. Vet. Sci. 2021, 8, 742868. [Google Scholar] [CrossRef]
- Tomazi, T.; Ferreira, G.C.; Orsi, A.M.; Gonçalves, J.L.; Ospina, P.A.; Nydam, D.V.; Moroni, P.; dos Santos, M.V. Association of herd-level risk factors and incidence rate of clinical mastitis in 20 Brazilian dairy herds. Prev. Vet. Med. 2018, 161, 9–18. [Google Scholar] [CrossRef]
- Oliveira, L.; Ruegg, P.L. Treatments of clinical mastitis occurring in cows on 51 large dairy herds in Wisconsin. J. Dairy Sci. 2014, 97, 5426–5436. [Google Scholar] [CrossRef]
- Klaas, I.C.; Zadoks, R.N. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef]
- Gelgie, A.E.; Korsa, M.G.; Kerro Dego, O. Mycoplasma bovis mastitis. Curr. Res. Microb. Sci. 2022, 3, 100123. [Google Scholar] [CrossRef] [PubMed]
- Schukken, Y.H.; Smit, J.A.H.; Grommers, F.J.; Vandegeer, D.; Brand, A. Effect of Freezing on Bacteriologic Culturing of Mastitis Milk Samples. J. Dairy Sci. 1989, 72, 1900–1906. [Google Scholar] [CrossRef]
- Ruegg, P.L. Making Antibiotic Treatment Decisions for Clinical Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Suojala, L.; Kaartinen, L.; Pyörälä, S. Treatment for bovine Escherichia coli mastitis—An evidence-based approach. J. Vet. Pharmacol. Ther. 2013, 36, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Hogan, J.S. Environmental Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 1993, 9, 489–498. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 12, 10381–10397. [Google Scholar] [CrossRef]
- Keefe, G. Update on Control of Staphylococcus aureus and Streptococcus agalactiae for Management of Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 203–216. [Google Scholar] [CrossRef]
- Mesquita, A.A.; Rocha Christian, M.B.M.; Bruhn, F.R.P.; Custódio, D.A.C.; Braz, M.S.; Pinto, S.M.; Silva, D.B.; Costa, G.M. Staphylococcus aureus and Streptococcus agalactiae: Prevalence, resistance to antimicrobials, and their relationship with the milk quality of dairy cattle herds in Minas Gerais state, Brazil. Pesqui. Vet. Bras. 2019, 39, 308–316. [Google Scholar] [CrossRef]
- Eckelkamp, E.A.; Taraba, J.L.; Akers, K.A.; Harmon, R.J.; Bewley, J.M. Sand bedded freestall and compost bedded pack effects on cow hygiene, locomotion, and mastitis indicators. Livest. Sci. 2016, 190, 48–57. [Google Scholar] [CrossRef]
- Janni, K.A.; Endres, M.I.; Reneau, J.K.; Schoper, W.W. Compost Dairy Barn Layout and Management Recommendations. Appl. Eng. Agric. 2007, 23, 97–102. [Google Scholar] [CrossRef]
- Northeast Resource Agriculture and Engineering Service (NRAES-54). On-Farm Composting Handbook; NRAES-54: Ithaca, NY, USA, 1992; p. 204. [Google Scholar]
- Biasato, I.; D’Angelo, A.; Bertone, I.; Odore, R.; Bellino, C. Compost bedded-pack barn as an alternative housing system for dairy cattle in Italy: Effects on animal health and welfare and milk and milk product quality. Ital. J. Anim. Sci. 2019, 18, 1142–1153. [Google Scholar] [CrossRef]
- Schalm, O.W.; Woods, G.M. Characteristics of coliform mastitis and treatment with dihydrostreptomycin. J. Am. Vet. Med. Assoc. 1952, 120, 385–388. [Google Scholar] [PubMed]

| Variable | N 1 | Mean | SD 2 | Min 3 | Max 4 |
|---|---|---|---|---|---|
| Lactating cows (n) | 42 | 111.7 | 52.3 | 45.0 | 208.0 |
| BMSCC 5 (1000 scc/mL) | 30 | 391.4 | 203.5 | 133.0 | 816.0 |
| Milk production (L/cow/day) | 42 | 29.0 | 4.0 | 21.4 | 35.7 |
| Stocking density (m2/cow) | 42 | 11.8 | 1.5 | 8.3 | 16.0 |
| Bedding tilling (times/day) | 42 | 2.4 | 0.5 | 2.0 | 3.0 |
| Herd * | Lactating Cows 1 | Cow Milk Yield (L/Day) 2 | Stocking Density (m2/Cow) | Bedding Tilling (Times/Day) | Bedding Samples (n) 3 | CM 4 Samples (n) | SCC 5 Samples (n) |
|---|---|---|---|---|---|---|---|
| A | 175 | 24.0 | 11.1 | 2 | 6 | 114 | 1040 |
| B | 56 | 23.7 | 13.0 | 3 | 6 | 0 | 337 |
| C | 195 | 32.1 | 13.0 | 3 | 6 | 0 | 1163 |
| D | 71 | 27.4 | 11.8 | 2 | 6 | 24 | 427 |
| E | 132 | 33.3 | 12.8 | 3 | 6 | 68 | 771 |
| F | 78 | 29.9 | 11.0 | 2 | 6 | 38 | 463 |
| G | 76 | 32.7 | 9.8 | 2 | 6 | 28 | 424 |
| Total | - | - | - | - | 42 | 272 | 4625 |
| Pathogens | n | % |
|---|---|---|
| No growth | 117 | 43.01 |
| Gram-positive | ||
| Strep. dysgalactiae | 22 | 8.09 |
| Strep. uberis | 19 | 6.99 |
| Non-aureus staphylococci | ||
| Staph. chromogenes | 17 | 6.25 |
| Staph. haemolyticus | 3 | 1.10 |
| Staph. hyicus | 1 | 0.37 |
| Staph. pasteuri | 1 | 0.37 |
| Staph. simulans | 1 | 0.37 |
| Staph. xylosus | 1 | 0.37 |
| Staph. aureus | 9 | 3.30 |
| Corynebacterium spp. | 6 | 2.20 |
| Strep. gallolyticus | 4 | 1.47 |
| Enterococcus faecalis | 2 | 0.73 |
| Strep. agalactiae | 2 | 0.73 |
| Strep. canis | 2 | 0.73 |
| Corynebacterium bovis | 1 | 0.37 |
| Strep. alactolyticus | 1 | 0.37 |
| Enterococcus faecium | 1 | 0.37 |
| Lysinibacillus boronitolerans | 1 | 0.37 |
| Strep. pluranimalium | 1 | 0.37 |
| Gram-negative | ||
| Escherichia coli | 34 | 12.50 |
| Klebsiella pneumoniae | 14 | 5.15 |
| Serratia marcescens | 2 | 0.73 |
| Enterobacter cloacae | 1 | 0.37 |
| Paenibacillus cookii | 1 | 0.37 |
| Pseudomonas aeruginosa | 1 | 0.37 |
| Stenotrophomonas maltophilia | 1 | 0.37 |
| Others | ||
| Candida tropicalis | 2 | 0.73 |
| Candida kefyr | 1 | 0.37 |
| Candida rugosa | 1 | 0.37 |
| Prototheca spp. | 1 | 0.37 |
| Contaminated 1 | 1 | 0.37 |
| Total | 272 | 100.00 |
| Pathogens | n | % |
|---|---|---|
| No growth | 629 | 40.24 |
| Gram-positive | ||
| Non-aureus staphylococci | ||
| Staph. chromogenes | 389 | 24.89 |
| Staph. simulans | 39 | 2.50 |
| Staph. hyicus | 20 | 1.28 |
| Staph. haemolyticus | 12 | 0.77 |
| Staph. xylosus | 5 | 0.32 |
| Staph. saprophyticus | 3 | 0.19 |
| Staph. capitis | 2 | 0.13 |
| Staph. auricularis | 1 | 0.06 |
| Staph. epidermidis | 1 | 0.06 |
| Staph. hominis | 1 | 0.06 |
| Staph. sciuri | 1 | 0.06 |
| Staph. warnieri | 1 | 0.06 |
| Staph. spp. 1 | 5 | 0.32 |
| Strep. agalactiae | 84 | 5.38 |
| Staph. aureus | 64 | 4.09 |
| Corynebacterium bovis | 45 | 2.88 |
| Strep. uberis | 44 | 2.82 |
| Strep. dysgalactiae | 31 | 1.98 |
| Corynebacterium spp. | 21 | 1.34 |
| Lactococcus garvieae | 10 | 0.64 |
| Lactococcus lactis | 9 | 0.58 |
| Other Gram-positive 2 | 39 | 2.50 |
| Gram-negative | ||
| Escherichia coli | 11 | 0.70 |
| Klebsiella pneumoniae | 8 | 0.51 |
| Serratia marcescens | 6 | 0.38 |
| Pseudomonas aeruginosa | 3 | 0.19 |
| Klebsiella variicola | 2 | 0.13 |
| Pseudomonas spp. | 1 | 0.06 |
| Serratia aureylitica | 1 | 0.06 |
| Other Gram-negative 3 | 21 | 1.34 |
| Others | ||
| Mixed culture 4 | 39 | 2.50 |
| Prototheca spp. | 7 | 0.45 |
| Candida rugosa | 3 | 0.19 |
| Candida kefyr | 2 | 0.13 |
| Candida krusei | 1 | 0.06 |
| Candida parapsilosis | 1 | 0.06 |
| Contaminated 5 | 1 | 0.06 |
| Total | 1563 | 100.00 |
| Variable | N 1 | Mean | SD 2 | CV 3 | Min 4 | Max 5 |
|---|---|---|---|---|---|---|
| Bedding physical-chemical characteristics 6 | ||||||
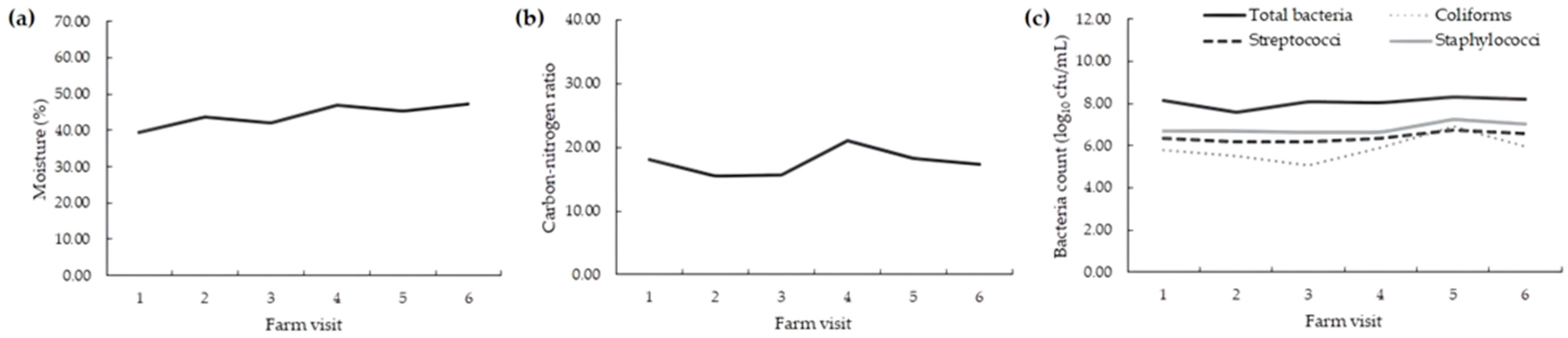
| Moisture (%) | 42 | 44.2 | 8.3 | 18.8 | 30.4 | 61.9 |
| pH | 42 | 8.3 | 0.5 | 6.1 | 7.4 | 9.5 |
| Organic matter (%) | 42 | 55.7 | 11.6 | 20.9 | 33.8 | 77.1 |
| Carbon-nitrogen ratio | 42 | 17.6 | 7.8 | 43.9 | 9.0 | 48.0 |
| Temperature (°C) | ||||||
| Surface | 42 | 35.9 | 4.9 | 13.7 | 26.4 | 45.6 |
| Deep layer | 42 | 41.4 | 6.8 | 16.4 | 27.7 | 55.0 |
| Bedding bacteria counting 7 | ||||||
| Total bacteria (log10 cfu/g) | 42 | 8.1 | 0.5 | 6.1 | 6.6 | 8.8 |
| Coliforms (log10 cfu/g) | 42 | 5.9 | 0.9 | 15.7 | 3.3 | 7.6 |
| Streptococci (log10 cfu/g) | 42 | 6.4 | 0.6 | 8.7 | 5.3 | 7.4 |
| Staphylococci (log10 cfu/g) | 42 | 6.8 | 0.6 | 8.4 | 5.0 | 7.9 |
| Mastitis indexes | ||||||
| Incidence of CM (all pathogens) 8 | 26 | 8.9 | 3.6 | 40.5 | 4.3 | 19.5 |
| Incidence of environmental CM 9 | 26 | 3.4 | 2.9 | 84.3 | 0.0 | 10.7 |
| Incidence of SCM 10 | 35 | 21.2 | 9.6 | 45.2 | 3.1 | 44.4 |
| Prevalence of SCM | 42 | 38.7 | 7.8 | 20.1 | 23.0 | 56.0 |
| Outcome (Bold Letters) and Explanatory Variables | Estimate | SE 1 | p-Value |
|---|---|---|---|
| Incidence of CM (all pathogens) 2 | |||
| Bedding moisture (%) | 0.49 | 0.14 | 0.004 |
| Counting of staphylococci (log10 cfu/g) | 4.46 | 1.10 | 0.001 |
| Incidence of environmental CM 3 | |||
| Bedding moisture (%) | 0.27 | 0.09 | 0.005 |
| Organic matter (%) | −0.12 | 0.07 | 0.105 |
| Counting of streptococci (log10 cfu/g) | 1.40 | 0.85 | 0.115 |
| Counting of staphylococci (log10 cfu/g) | 1.86 | 1.34 | 0.178 |
| Incidence of SCM 4 | |||
| Organic matter (%) | −0.23 | 0.16 | 0.171 |
| Carbon-nitrogen ratio | −0.56 | 0.42 | 0.190 |
| Counting of total bacteria (log10 cfu/g) | 8.85 | 5.67 | 0.130 |
| Prevalence of SCM | |||
| Counting of coliforms (log10 cfu/g) | 2.77 | 1.20 | 0.026 |
| Variable | Estimate | SE 1 | p-Value |
|---|---|---|---|
| Incidence of CM (all pathogens) 2 | |||
| Intercept | −14.23 | 6.99 | |
| Bedding moisture (%) | 0.49 | 0.14 | 0.004 |
| Incidence of environmental CM 3 | |||
| Intercept | −9.39 | 4.20 | |
| Bedding moisture (%) | 0.27 | 0.09 | 0.005 |
| Incidence of SCM 4 | |||
| Intercept | −49.32 | 41.49 | |
| Carbon-nitrogen ratio | −0.92 | 0.42 | 0.037 |
| Counting of total bacteria (log10 cfu/g) | 10.53 | 5.21 | 0.055 |
| Prevalence of SCM | |||
| Intercept | 22.98 | 7.23 | |
| Counting of coliforms (log10 cfu/g) | 2.77 | 1.20 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freu, G.; Garcia, B.L.N.; Tomazi, T.; Di Leo, G.S.; Gheller, L.S.; Bronzo, V.; Moroni, P.; Dos Santos, M.V. Association between Mastitis Occurrence in Dairy Cows and Bedding Characteristics of Compost-Bedded Pack Barns. Pathogens 2023, 12, 583. https://doi.org/10.3390/pathogens12040583
Freu G, Garcia BLN, Tomazi T, Di Leo GS, Gheller LS, Bronzo V, Moroni P, Dos Santos MV. Association between Mastitis Occurrence in Dairy Cows and Bedding Characteristics of Compost-Bedded Pack Barns. Pathogens. 2023; 12(4):583. https://doi.org/10.3390/pathogens12040583
Chicago/Turabian StyleFreu, Gustavo, Breno Luis Nery Garcia, Tiago Tomazi, Gabriela Siqueira Di Leo, Larissa Schneider Gheller, Valerio Bronzo, Paolo Moroni, and Marcos Veiga Dos Santos. 2023. "Association between Mastitis Occurrence in Dairy Cows and Bedding Characteristics of Compost-Bedded Pack Barns" Pathogens 12, no. 4: 583. https://doi.org/10.3390/pathogens12040583
APA StyleFreu, G., Garcia, B. L. N., Tomazi, T., Di Leo, G. S., Gheller, L. S., Bronzo, V., Moroni, P., & Dos Santos, M. V. (2023). Association between Mastitis Occurrence in Dairy Cows and Bedding Characteristics of Compost-Bedded Pack Barns. Pathogens, 12(4), 583. https://doi.org/10.3390/pathogens12040583






