Colonization of Extramammary Sites with Mastitis-Associated S. aureus Strains in Dairy Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd and Animal Selection
2.2. Sampling
2.3. Identification of Staphylococcus aureus
2.4. Data Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergonier, D.; de Crémoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef] [PubMed]
- Menzies, P.I.; Ramanoon, S.Z. Mastitis of Sheep and Goats. Vet. Clin. North Am. Food Anim. Pract. 2001, 17, 333–358. [Google Scholar] [CrossRef] [PubMed]
- Sommerhäuser, J.; Kloppert, B.; Wolter, W.; Zschöck, M.; Sobiraj, A.; Failing, K. The epidemiology of Staphylococcus aureus infections from subclinical mastitis in dairy cows during a control programme. Vet. Microbiol. 2003, 96, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Lyman, R.; Moury, K.; Ray, D.; Watson, D.; Correa, M. Molecular epidemiology of Staphylococcus aureus mastitis in dairy heifers. J. Dairy Sci. 2012, 95, 4921–4930. [Google Scholar] [CrossRef]
- Capurro, A.; Aspán, A.; Unnerstad, H.E.; Waller, K.P.; Artursson, K. Identification of potential sources of Staphylococcus aureus in herds with mastitis problems. J. Dairy Sci. 2010, 93, 180–191. [Google Scholar] [CrossRef]
- Matos, J.; White, D.; Harmon, R.; Langlois, B. Isolation of Staphylococcus aureus from Sites Other than the Lactating Mammary Gland. J. Dairy Sci. 1991, 74, 1544–1549. [Google Scholar] [CrossRef]
- Mørk, T.; Kvitle, B.; Jørgensen, H. Reservoirs of Staphylococcus aureus in meat sheep and dairy cattle. Vet. Microbiol. 2012, 155, 81–87. [Google Scholar] [CrossRef]
- Roberson, J.; Fox, L.; Hancock, D.; Gay, J.; Besser, T. Ecology of Staphylococcus aureus Isolated from Various Sites on Dairy Farms. J. Dairy Sci. 1994, 77, 3354–3364. [Google Scholar] [CrossRef]
- Van den Borne, B.H.P.; Nielen, M.; van Schaik, G.; Melchior, M.B.; Lam, T.J.G.M.; Zadoks, R.N. Host adaptation of bovine Staphylococcus aureus seems associated with bacteriological cure after lactational antimicrobial treatment. J. Dairy Sci. 2010, 93, 2550–2558. [Google Scholar] [CrossRef]
- Exel, C.E.; Gerritsen, K.; Spaninks, M.; Duim, B.; Koop, G.; Benedictus, L. Association of Staphylococcus aureus genotypes with milk or colonization of extramammary sites in Dutch dairy cattle indicates strain variation in reservoirs for intramammary infections. Res. Vet. Sci. 2023, 154, 138–144. [Google Scholar] [CrossRef]
- Leuenberger, A.; Sartori, C.; Boss, R.; Resch, G.; Oechslin, F.; Steiner, A.; Moreillon, P.; Graber, H. Genotypes of Staphylococcus aureus: On-farm epidemiology and the consequences for prevention of intramammary infections. J. Dairy Sci. 2019, 102, 3295–3309. [Google Scholar] [CrossRef]
- Valle, J.; Piriz, S.; de la Fuente, R.; Vadillo, S. Staphylococci Isolated from Healthy Goats. J. Vet. Med. Ser. B 1991, 38, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Espinosa-Gongora, C.; Stamphøj, I.; Larsen, A.R.; Guardabassi, L. Carriage frequency, diversity and methicillin resistance of Staphylococcus aureus in Danish small ruminants. Vet. Microbiol. 2013, 163, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mørk, T.; Kvitle, B.; Mathisen, T.; Jørgensen, H. Bacteriological and molecular investigations of Staphylococcus aureus in dairy goats. Vet. Microbiol. 2010, 141, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.; Saei, H.D.; Ahmadi, M. Nasal Carriage of Staphylococcus aureus: Frequency and Antibiotic Resistance in Healthy Ruminants. Jundishapur J. Microbiol. 2015, 8, e22413. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, M.; Li, H.; Yang, H.; Li, X.; Song, X.; Wang, Z. Prevalence and molecular characterization of Staphylococcus aureus isolated from goats in Chongqing, China. BMC Vet. Res. 2017, 13, 352. [Google Scholar] [CrossRef]
- Francois, P.; Pittet, D.; Bento, M.; Pepey, B.; Vaudaux, P.; Lew, D.; Schrenzel, J. Rapid Detection of Methicillin-Resistant Staphylococcus aureus Directly from Sterile or Nonsterile Clinical Samples by a New Molecular Assay. J. Clin. Microbiol. 2003, 41, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Hallin, M.; Friedrich, A.W.; Struelens, M.J. spa Typing for Epidemiological Surveillance of Staphylococcus aureus. In Molecular Epidemiology of Microorganisms; Humana Press: Totowa, NJ, USA, 2009; Volume 551, pp. 189–202. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 27.0; IBM Corp: New York, NY, USA, 2020. [Google Scholar]
- Madsen, A.M.; Kurdi, I.; Feld, L.; Tendal, K. Airborne MRSA and Total Staphylococcus aureus as Associated With Particles of Different Sizes on Pig Farms. Ann. Work. Expo. Health 2018, 62, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Feld, L.; Bay, H.; Angen, Ø.; Larsen, A.R.; Madsen, A.M. Survival of LA-MRSA in Dust from Swine Farms. Ann. Work. Expo. Health 2018, 62, 147–156. [Google Scholar] [CrossRef]
- Schulz, J.; Friese, A.; Klees, S.; Tenhagen, B.A.; Fetsch, A.; Rösler, U.; Hartung, J. Longitudinal Study of the Contamination of Air and of Soil Surfaces in the Vicinity of Pig Barns by Livestock-Associated Methicillin-Resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 5666–5671. [Google Scholar] [CrossRef]
- Bos, M.E.H.; Verstappen, K.M.; Cleef, B.A.G.L.V.; Dohmen, W.; Dorado-García, A.; Graveland, H.; Duim, B.; A Wagenaar, J.; Kluytmans, J.A.J.W.; Heederik, D.J.J. Transmission through air as a possible route of exposure for MRSA. J. Expo. Sci. Environ. Epidemiol. 2014, 26, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, J.G.; Aebi, S.; Oppliger, A.; Hilty, M. The Indoor-Air Microbiota of Pig Farms Drives the Composition of the Pig Farmers’ Nasal Microbiota in a Season-Dependent and Farm-Specific Manner. Appl. Environ. Microbiol. 2019, 85, e03038-18. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, J.G.; Ramette, A.; Aebi, S.; Oppliger, A.; Hilty, M. Influence of Pig Farming on the Human Nasal Microbiota: Key Role of Airborne Microbial Communities. Appl. Environ. Microbiol. 2018, 84, e02470-17. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.; Haslinger-Löffler, B.; Westh, H.; Boye, K.; Peters, G.; Neumann, C.; Kahl, B.C. Non- spa -Typeable Clinical Staphylococcus aureus Strains Are Naturally Occurring Protein A Mutants. J. Clin. Microbiol. 2009, 47, 3624–3629. [Google Scholar] [CrossRef]
- A Votintseva, A.; Fung, R.; Miller, R.R.; Knox, K.; Godwin, H.; Wyllie, D.H.; Bowden, R.; Crook, D.W.; Walker, A.S. Prevalence of Staphylococcus aureus protein A (spa) mutants in the community and hospitals in Oxfordshire. BMC Microbiol. 2014, 14, 63. [Google Scholar] [CrossRef]
- Hoekstra, J.; Rutten, V.P.; Hout, M.V.D.; Spaninks, M.; Benedictus, L.; Koop, G. Differences between Staphylococcus aureus lineages isolated from ovine and caprine mastitis but not between isolates from clinical or subclinical mastitis. J. Dairy Sci. 2019, 102, 5430–5437. [Google Scholar] [CrossRef]
- Polveiro, R.C.; Granja, M.M.C.; Roldão, T.C.B.; Lopes, I.D.S.; Vidigal, P.M.P.; Lima, M.C.; Moreira, M.A.S. Multilocus sequence analysis reveals genetic diversity in Staphylococcus aureus isolate of goat with mastitis persistent after treatment with enrofloxacin. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Porrero, M.C.; Hasman, H.; Vela, A.I.; Fernández-Garayzábal, J.F.; Domínguez, L.; Aarestrup, F.M. Clonal diversity of Staphylococcus aureus originating from the small ruminants goats and sheep. Vet. Microbiol. 2012, 156, 157–161. [Google Scholar] [CrossRef]
- Romanò, A.; Gazzola, A.; Bianchini, V.; Cortimiglia, C.; Maisano, A.M.; Cremonesi, P.; Graber, H.U.; Vezzoli, F.; Luini, M. Staphylococcus aureus From Goats Are Genetically Heterogeneous and Distinct to Bovine Ones. Front. Vet. Sci. 2020, 7, 628. [Google Scholar] [CrossRef]
- Merz, A.; Stephan, R.; Johler, S. Staphylococcus aureus Isolates from Goat and Sheep Milk Seem to Be Closely Related and Differ from Isolates Detected from Bovine Milk. Front. Microbiol. 2016, 7, 319. [Google Scholar] [CrossRef]
- Bar-Gal, G.K.; Blum, S.; Hadas, L.; Ehricht, R.; Monecke, S.; Leitner, G. Host-specificity of Staphylococcus aureus causing intramammary infections in dairy animals assessed by genotyping and virulence genes. Vet. Microbiol. 2015, 176, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Pichette-Jolette, S.; Millette, G.; Demontier, E.; Bran-Barrera, D.; Cyrenne, M.; Ster, C.; Haine, D.; Keefe, G.; Malouin, F.; Roy, J. Partial prediction of the duration and the clinical status of Staphylococcus aureus bovine intramammary infections based on the phenotypic and genotypic analysis of isolates. Vet. Microbiol. 2018, 228, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ikawaty, R.; Brouwer, E.; Jansen, M.; van Duijkeren, E.; Mevius, D.; Verhoef, J.; Fluit, A. Characterization of Dutch Staphylococcus aureus from bovine mastitis using a Multiple Locus Variable Number Tandem Repeat Analysis. Vet. Microbiol. 2009, 136, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Antók, F.I.; Mayrhofer, R.; Marbach, H.; Masengesho, J.C.; Keinprecht, H.; Nyirimbuga, V.; Fischer, O.; Lepuschitz, S.; Ruppitsch, W.; Ehling-Schulz, M.; et al. Characterization of Antibiotic and Biocide Resistance Genes and Virulence Factors of Staphylococcus Species Associated with Bovine Mastitis in Rwanda. Antibiotics 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.; Vargas, K.; Reyes-Vélez, J.; Jiménez, N.; Blanchard, A.; Olivera-Angel, M. High genetic diversity and zoonotic potential of Staphylococcus aureus strains recovered from bovine intramammary infections in Colombians dairy herds. Comp. Immunol. Microbiol. Infect. Dis. 2023, 93, 101940. [Google Scholar] [CrossRef]
- Monecke, S.; Kuhnert, P.; Hotzel, H.; Slickers, P.; Ehricht, R. Microarray based study on virulence-associated genes and resistance determinants of Staphylococcus aureus isolates from cattle. Vet. Microbiol. 2007, 125, 128–140. [Google Scholar] [CrossRef]
- Feltrin, F.; Alba, P.; Kraushaar, B.; Ianzano, A.; Argudín, M.A.; Di Matteo, P.; Porrero, M.C.; Aarestrup, F.M.; Butaye, P.; Franco, A.; et al. A Livestock-Associated, Multidrug-Resistant, Methicillin-Resistant Staphylococcus aureus Clonal Complex 97 Lineage Spreading in Dairy Cattle and Pigs in Italy. Appl. Environ. Microbiol. 2016, 82, 816–821. [Google Scholar] [CrossRef]
- Annamanedi, M.; Sheela, P.; Sundareshan, S.; Isloor, S.; Gupta, P.; Jasmeen, P.; Gargi, M.; Mallick, S.; Hegde, N.R. Molecular fingerprinting of bovine mastitis-associated Staphylococcus aureus isolates from India. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Luini, M.; Cremonesi, P.; Magro, G.; Bianchini, V.; Minozzi, G.; Castiglioni, B.; Piccinini, R. Methicillin-resistant Staphylococcus aureus (MRSA) is associated with low within-herd prevalence of intra-mammary infections in dairy cows: Genotyping of isolates. Vet. Microbiol. 2015, 178, 270–274. [Google Scholar] [CrossRef]
- Luzzago, C.; Locatelli, C.; Franco, A.; Scaccabarozzi, L.; Gualdi, V.; Viganò, R.; Sironi, G.; Besozzi, M.; Castiglioni, B.; Lanfranchi, P.; et al. Clonal diversity, virulence-associated genes and antimicrobial resistance profile of Staphylococcus aureus isolates from nasal cavities and soft tissue infections in wild ruminants in Italian Alps. Vet. Microbiol. 2014, 170, 157–161. [Google Scholar] [CrossRef]
- Achek, R.; El-Adawy, H.; Hotzel, H.; Tomaso, H.; Ehricht, R.; Hamdi, T.M.; Azzi, O.; Monecke, S. Short communication: Diversity of staphylococci isolated from sheep mastitis in northern Algeria. J. Dairy Sci. 2020, 103, 890–897. [Google Scholar] [CrossRef]
- Agabou, A.; Ouchenane, Z.; Essebe, C.N.; Khemissi, S.; Chehboub, M.T.E.; Chehboub, I.B.; Sotto, A.; Dunyach-Remy, C.; Lavigne, J.-P. Emergence of Nasal Carriage of ST80 and ST152 PVL+ Staphylococcus aureus Isolates from Livestock in Algeria. Toxins 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Gavier-Widén, D.; Hotzel, H.; Peters, M.; Guenther, S.; Lazaris, A.; Loncaric, I.; Müller, E.; Reissig, A.; Ruppelt-Lorz, A.; et al. Diversity of Staphylococcus aureus Isolates in European Wildlife. PLoS ONE 2016, 11, e0168433. [Google Scholar] [CrossRef] [PubMed]
- Shittu, A.O.; Taiwo, F.F.; Froböse, N.J.; Schwartbeck, B.; Niemann, S.; Mellmann, A.; Schaumburg, F. Genomic analysis of Staphylococcus aureus from the West African Dwarf (WAD) goat in Nigeria. Antimicrob. Resist. Infect. Control. 2021, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Exel, C.E.; Halasa, T.; Koop, G.; Steeneveld, W.; Lam, T.J.; Benedictus, L.; Gussmann, M. A stochastic modelling approach to determine the effect of diverse Staphylococcus aureus strains on the economic and epidemiological outcomes of mastitis intervention strategies in dairy cattle. Prev. Vet. Med. 2021, 199, 105566. [Google Scholar] [CrossRef]
- Fournier, C.; Kuhnert, P.; Frey, J.; Miserez, R.; Kirchhofer, M.; Kaufmann, T.; Steiner, A.; Graber, H. Bovine Staphylococcus aureus: Association of virulence genes, genotypes and clinical outcome. Res. Vet. Sci. 2008, 85, 439–448. [Google Scholar] [CrossRef]
- Haveri, M.; Taponen, S.; Vuopio-Varkila, J.; Salmenlinna, S.; Pyörälä, S. Bacterial Genotype Affects the Manifestation and Persistence of Bovine Staphylococcus aureus Intramammary Infection. J. Clin. Microbiol. 2005, 43, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Jácome, I.S.; Sousa, F.G.; De Leon, C.M.; A Spricigo, D.; Saraiva, M.M.; Givisiez, P.E.; A Gebreyes, W.; Vieira, R.F.; Oliveira, C.J. Pre-parturition staphylococcal mastitis in primiparous replacement goats: Persistence over lactation and sources of infection. Vet. Res. 2014, 45, 115. [Google Scholar] [CrossRef][Green Version]
- Benedictus, L.; Ravesloot, L.; Poppe, K.; Daemen, I.; Boerhout, E.; van Strijp, J.; Broere, F.; Rutten, V.; Koets, A.; Eisenberg, S. Immunization of young heifers with staphylococcal immune evasion proteins before natural exposure to Staphylococcus aureus induces a humoral immune response in serum and milk. BMC Vet. Res. 2019, 15, 15. [Google Scholar] [CrossRef]
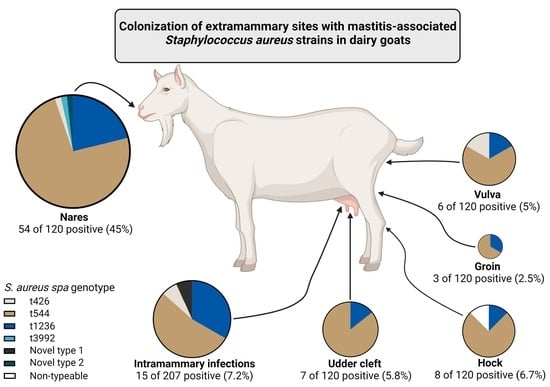
| Milk | Nares | Vulva | Hock | Groin | Udder Cleft | Extramammary Colonization 1 | |
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus positive culture | 15 | 54 | 6 | 8 | 3 | 7 | 62 |
| Total | 207 | 120 | 120 | 120 | 120 | 120 | 120 |
| Observed prevalence (95% CI 2) | 7.2% 3 (4.4–11.6) | 45% (36.4–53.9) | 5% (2.3–10.5) | 6.7% (3.4–12.6) | 2.5% (0.9–7.1) | 5.8% (2.9–11.6) | 51.7% (42.8–60.4) |
| Spa Type | Location | ||||||
|---|---|---|---|---|---|---|---|
| Milk (%) | Extramammary Site (%) | Nares | Vulva | Hock | Groin | Udder Cleft | |
| t426 | 1 (6.7%) | 2 (3.2%) | 1 | 1 | |||
| t544 | 8 (53.3%) | 51 (82.3%) | 42 | 4 | 6 | 2 | 6 |
| t1236 | 5 (33.3%) | 14 (22.6%) | 12 | 1 | 1 | 1 | 1 |
| t3992 | 1 (1.6%) | 1 | |||||
| Non-typeable 1 | 1 (1.6%) | 1 | |||||
| Novel type 1 | 1 (6.7%) | ||||||
| Novel type 2 | 1 (1.6%) | 1 | |||||
| Total | 15 | 62 | 57 | 6 | 8 | 3 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Exel, C.E.; Geus, Y.d.; Spaninks, M.; Koop, G.; Benedictus, L. Colonization of Extramammary Sites with Mastitis-Associated S. aureus Strains in Dairy Goats. Pathogens 2023, 12, 515. https://doi.org/10.3390/pathogens12040515
Exel CE, Geus Yd, Spaninks M, Koop G, Benedictus L. Colonization of Extramammary Sites with Mastitis-Associated S. aureus Strains in Dairy Goats. Pathogens. 2023; 12(4):515. https://doi.org/10.3390/pathogens12040515
Chicago/Turabian StyleExel, Catharina Elizabeth, Yvette de Geus, Mirlin Spaninks, Gerrit Koop, and Lindert Benedictus. 2023. "Colonization of Extramammary Sites with Mastitis-Associated S. aureus Strains in Dairy Goats" Pathogens 12, no. 4: 515. https://doi.org/10.3390/pathogens12040515
APA StyleExel, C. E., Geus, Y. d., Spaninks, M., Koop, G., & Benedictus, L. (2023). Colonization of Extramammary Sites with Mastitis-Associated S. aureus Strains in Dairy Goats. Pathogens, 12(4), 515. https://doi.org/10.3390/pathogens12040515