West Nile, Sindbis and Usutu Viruses: Evidence of Circulation in Mosquitoes and Horses in Tunisia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
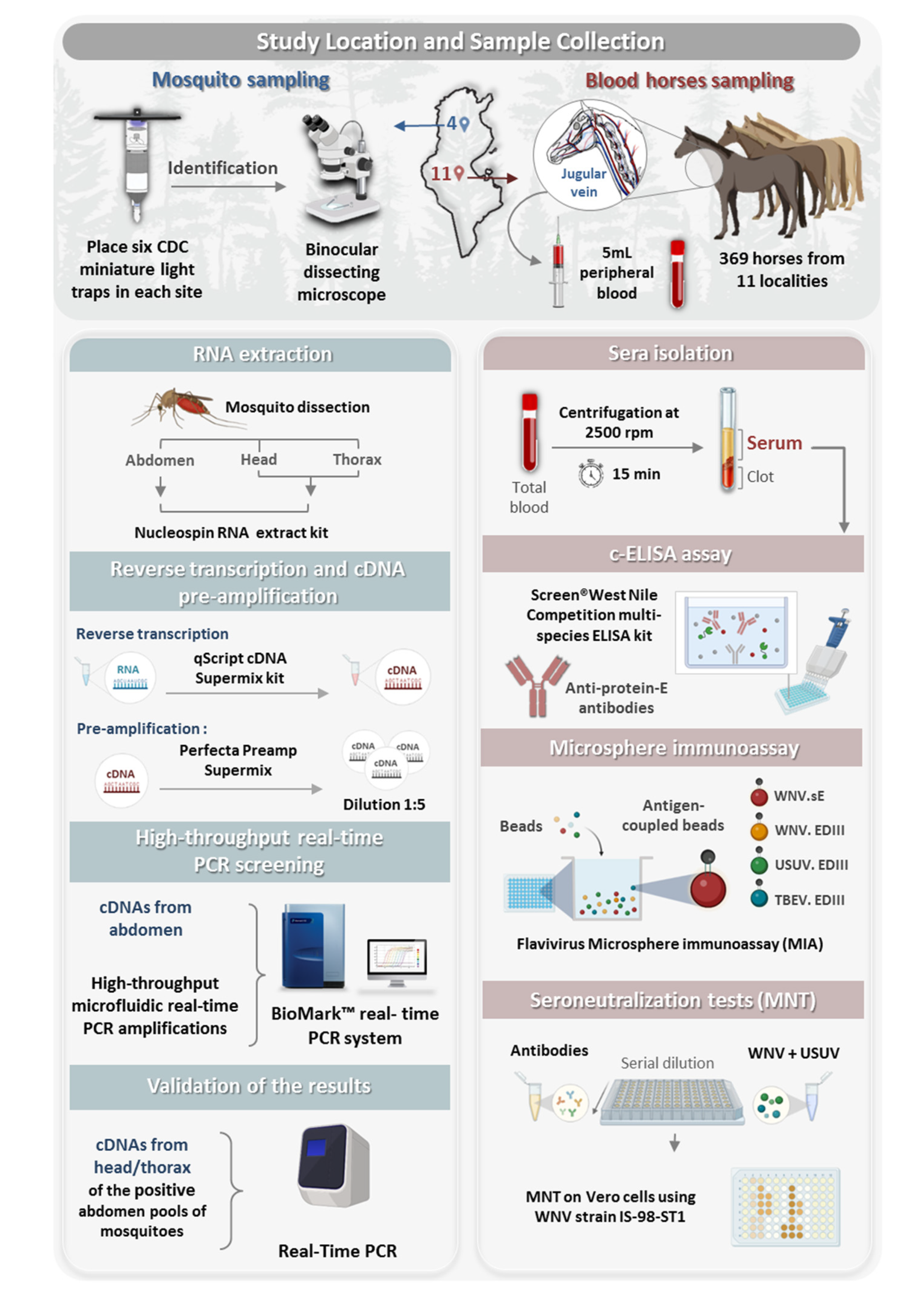
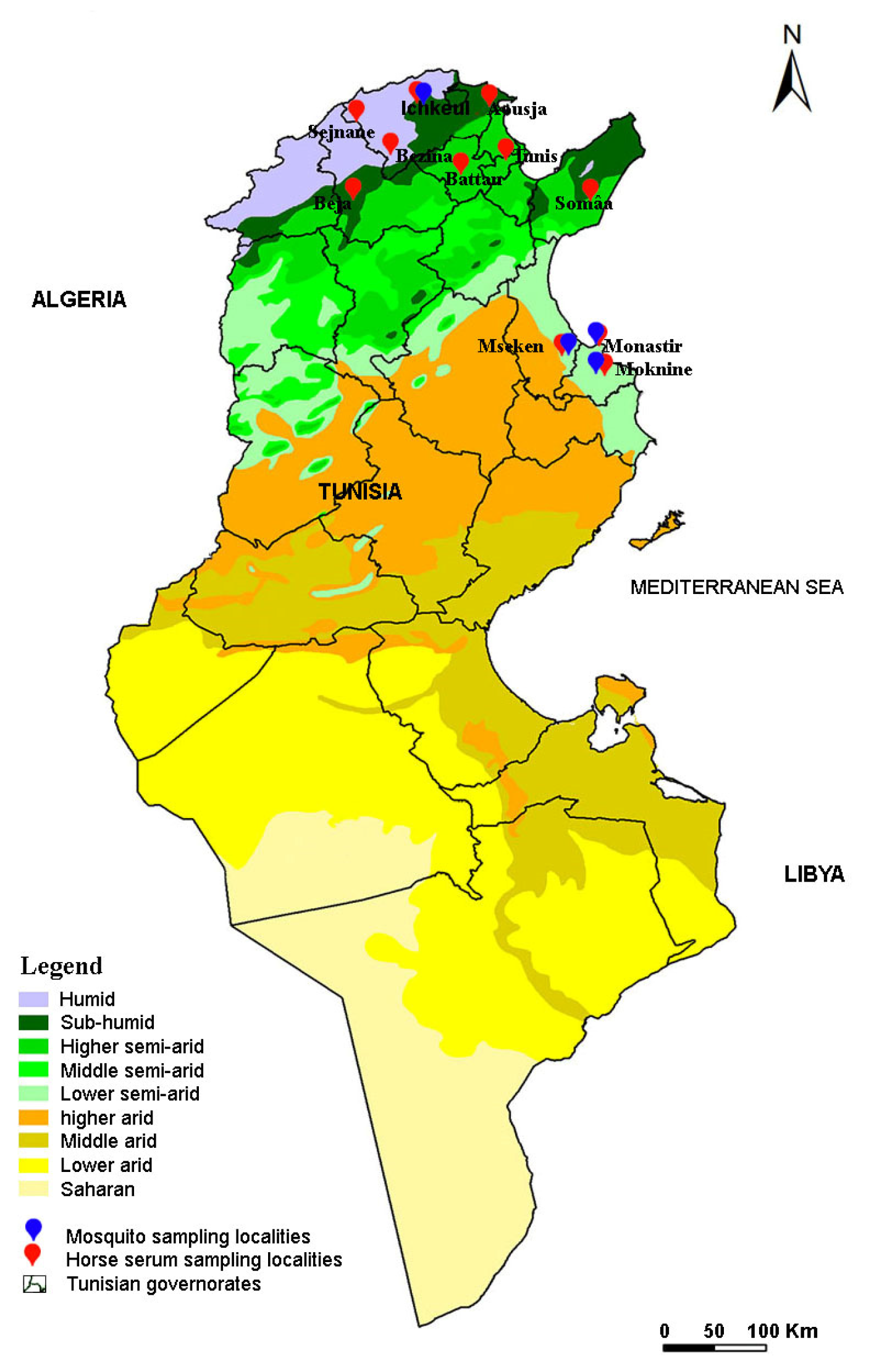
2.2. Study Location and Sample Collection
2.2.1. Mosquito Sampling
2.2.2. Sampling Horses for Sera
2.3. Sample Processing and RNA Extraction
2.4. Reverse Transcription and cDNA Pre-Amplification
2.5. High-Throughput Real-Time PCR Screening
2.6. Confirmation of a Disseminated Infection by Real-Time PCR of RBP
2.7. Serological Investigation of Flavivirus Infections in Horses by ELISA Assay
2.8. Microsphere Immunoassay
2.9. Virus Neutralization Tests
2.10. Ethical Statement
3. Results
3.1. High-Throughput Real-Time PCR Screening of Mosquito-Borne Viruses
3.2. WNV Disseminated Infections Confirmed in Culex perexiguus Sampled in Ichkeul
3.3. Seroprevalence of Flavivirus, WNV and USUV, Infections in Horses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swei, A.; Couper, L.I.; Coffey, L.L.; Kapan, D.; Bennett, S. Patterns, Drivers, and Challenges of Vector-Borne Disease Emergence. Vector Borne Zoonotic Dis. 2020, 20, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Failloux, A.-B.; Bouattour, A.; Faraj, C.; Gunay, F.; Haddad, N.; Harrat, Z.; Jancheska, E.; Kanani, K.; Kenawy, M.A.; Kota, M.; et al. Surveillance of Arthropod-Borne Viruses and Their Vectors in the Mediterranean and Black Sea Regions Within the MediLabSecure Network. Curr. Trop. Med. Rep. 2017, 4, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N.; Berenger, J.-M.; Laroche, M.; Ayhan, N.; Bitam, I.; Delaunay, P.; Parola, P. Neglected Vector-Borne Bacterial Diseases and Arboviruses in the Mediterranean Area. New Microbes New Infect. 2018, 26, S31–S36. [Google Scholar] [CrossRef]
- Eybpoosh, S.; Fazlalipour, M.; Baniasadi, V.; Pouriayevali, M.H.; Sadeghi, F.; Ahmadi Vasmehjani, A.; Karbalaie Niya, M.H.; Hewson, R.; Salehi-Vaziri, M. Epidemiology of West Nile Virus in the Eastern Mediterranean Region: A Systematic Review. PLoS Negl. Trop. Dis. 2019, 13, e0007081. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Gould, E.A. Taxonomy of the Virus Family Flaviviridae. Adv. Virus Res. 2003, 59, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef] [PubMed]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean Basin: 1950–2000. Ann. N. Y. Acad. Sci. 2001, 951, 117–126. [Google Scholar] [CrossRef]
- Johnson, N.; Fernández de Marco, M.; Giovannini, A.; Ippoliti, C.; Danzetta, M.L.; Svartz, G.; Erster, O.; Groschup, M.H.; Ziegler, U.; Mirazimi, A.; et al. Emerging Mosquito-Borne Threats and the Response from European and Eastern Mediterranean Countries. Int. J. Environ. Res. Public Health 2018, 15, 2775. [Google Scholar] [CrossRef]
- Papa, A. Emerging Arboviruses of Medical Importance in the Mediterranean Region. J. Clin. Virol. 2019, 115, 5–10. [Google Scholar] [CrossRef]
- Amraoui, F.; Krida, G.; Bouattour, A.; Rhim, A.; Daaboub, J.; Harrat, Z.; Boubidi, S.-C.; Tijane, M.; Sarih, M.; Failloux, A.-B. Culex Pipiens, an Experimental Efficient Vector of West Nile and Rift Valley Fever Viruses in the Maghreb Region. PLoS ONE 2012, 7, e36757. [Google Scholar] [CrossRef]
- Krida, G.; Rhim, A.; Daaboub, J.; Failloux, A.-B.; Bouattour, A. New Evidence for the Potential Role of Culex Pipiens Mosquitoes in the Transmission Cycle of West Nile Virus in Tunisia. Med. Vet. Entomol. 2015, 29, 124–128. [Google Scholar] [CrossRef]
- Benjelloun, A.; El Harrak, M.; Belkadi, B. West Nile Disease Epidemiology in North-West Africa: Bibliographical Review. Transbound. Emerg. Dis. 2016, 63, e153–e159. [Google Scholar] [CrossRef]
- Sule, W.F.; Oluwayelu, D.O.; Hernández-Triana, L.M.; Fooks, A.R.; Venter, M.; Johnson, N. Epidemiology and Ecology of West Nile Virus in Sub-Saharan Africa. Parasit Vectors 2018, 11, 414. [Google Scholar] [CrossRef]
- Nikolay, B. A Review of West Nile and Usutu Virus Co-Circulation in Europe: How Much Do Transmission Cycles Overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef]
- Triki, H.; Murri, S.; Le Guenno, B.; Bahri, O.; Hili, K.; Sidhom, M.; Dellagi, K. West Nile viral meningo-encephalitis in Tunisia. Med. Trop. (Mars) 2001, 61, 487–490. [Google Scholar]
- Fares, W.; Gdoura, M.; Dhrif, H.; Touzi, H.; Hogga, N.; Hannachi, N.; Mhalla, S.; Kacem, S.; Karray, H.; Bougatef, S.; et al. Genetic Characterization of West Nile Virus Strains during Neuroinvasives Infection Outbreak in Tunisia, 2018. Transbound. Emerg. Dis. 2021, 68, 2414–2421. [Google Scholar] [CrossRef]
- Gdoura, M.; Fares, W.; Bougatef, S.; Inoubli, A.; Touzi, H.; Hogga, N.; Ben Dhifallah, I.; Hannachi, N.; Argoubi, A.; Kacem, S.; et al. The Value of West Nile Virus RNA Detection by Real-Time RT-PCR in Urine Samples from Patients with Neuroinvasive Forms. Arch. Microbiol. 2022, 204, 238. [Google Scholar] [CrossRef] [PubMed]
- Monastiri, A.; Mechri, B.; Vázquez-González, A.; Ar Gouilh, M.; Chakroun, M.; Loussaief, C.; Mastouri, M.; Dimassi, N.; Boughzala, L.; Aouni, M.; et al. A Four-Year Survey (2011–2014) of West Nile Virus Infection in Humans, Mosquitoes and Birds, Including the 2012 Meningoencephalitis Outbreak in Tunisia. Emerg. Microbes Infect. 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.; Hassine, T.B.; Conte, A.; Amdouni, J.; De Massis, F.; Sghaier, S.; Hassen, S.B. West Nile Disease in Tunisia: An Overview of 60 Years. Vet. Ital. 2017, 53, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, A.; Lecollinet, S.; Hamza, F.; Nasri, I.; Neb, A.; Selmi, S. Exposure of Resident Sparrows to West Nile Virus Evidenced in South Tunisia. Epidemiol. Infect. 2015, 143, 3546–3549. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, F.; Dachraoui, K.; Cherni, S.; Bosworth, A.; Barhoumi, W.; Dowall, S.; Chelbi, I.; Derbali, M.; Zoghlami, Z.; Beier, J.C.; et al. West Nile Virus in Tunisia, 2014: First Isolation from Mosquitoes. Acta Trop. 2016, 159, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Bahri, O.; Dhifallah, I.; Ben Alaya-Bouafif, N.; Fekih, H.; Gargouri, J.; Triki, H. Sero-epidemiological study of West Nile virus circulation in human in Tunisia. Bull. Soc. Pathol. Exot. 2011, 104, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Amdouni, J.; Monaco, F.; Portanti, O.; Sghaier, S.; Conte, A.; Hassine, T.B.; Polci, A.; Valleriani, F.; Gennaro, A.D.; Zoueri, M.; et al. Detection of Enzootic Circulation of a New Strain of West Nile Virus Lineage 1 in Sentinel Chickens in the North of Tunisia. Acta Trop. 2020, 202, 105223. [Google Scholar] [CrossRef] [PubMed]
- Lafri, I.; Hachid, A.; Bitam, I. West Nile Virus in Algeria: A Comprehensive Overview. New Microbes New Infect. 2019, 27, 9–13. [Google Scholar] [CrossRef]
- Medrouh, B.; Lafri, I.; Beck, C.; Leulmi, H.; Akkou, M.; Abbad, L.; Lafri, M.; Bitam, I.; Lecollinet, S. First Serological Evidence of West Nile Virus Infection in Wild Birds in Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101415. [Google Scholar] [CrossRef]
- El Rhaffouli, H.; El Harrak, M.; Lotfi, C.; El Boukhrissi, F.; Bajjou, T.; Laraqui, A.; Hilali, F.; Kenfaoui, M.; Lahlou-Amine, I. Serologic Evidence of West Nile Virus Infection among Humans, Morocco. Emerg. Infect. Dis. 2012, 18, 880–881. [Google Scholar] [CrossRef]
- Assaid, N.; Mousson, L.; Moutailler, S.; Arich, S.; Akarid, K.; Monier, M.; Beck, C.; Lecollinet, S.; Failloux, A.-B.; Sarih, M. Evidence of Circulation of West Nile Virus in Culex Pipiens Mosquitoes and Horses in Morocco. Acta Trop. 2020, 205, 105414. [Google Scholar] [CrossRef]
- Assaid, N.; Arich, S.; Ezzikouri, S.; Benjelloun, S.; Dia, M.; Faye, O.; Akarid, K.; Beck, C.; Lecollinet, S.; Failloux, A.-B.; et al. Serological Evidence of West Nile Virus Infection in Human Populations and Domestic Birds in the Northwest of Morocco. Comp. Immunol. Microbiol. Infect. Dis. 2021, 76, 101646. [Google Scholar] [CrossRef]
- Benjelloun, A.; El Harrak, M.; Calistri, P.; Loutfi, C.; Kabbaj, H.; Conte, A.; Ippoliti, C.; Danzetta, M.L.; Belkadi, B. Seroprevalence of West Nile Virus in Horses in Different Moroccan Regions. Vet. Med. Sci. 2017, 3, 198–207. [Google Scholar] [CrossRef]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu Virus: A New Threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Ruiz, S.; Herrero, L.; Moreno, J.; Molero, F.; Magallanes, A.; Sánchez-Seco, M.P.; Figuerola, J.; Tenorio, A. West Nile and Usutu Viruses in Mosquitoes in Spain, 2008–2009. Am. J. Trop. Med. Hyg. 2011, 85, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Petrovic, T.; Savic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Klobucar, A.; Mrzljak, A.; Ilic, M.; Bogdanic, M.; et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnejad, F.; Otarod, V.; Fallah, M.H.; Lowenski, S.; Sedighi-Moghaddam, R.; Zavareh, A.; Durand, B.; Lecollinet, S.; Sabatier, P. Spread of West Nile Virus in Iran: A Cross-Sectional Serosurvey in Equines, 2008–2009. Epidemiol. Infect. 2011, 139, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.R.; Ziegler, U.; Linke, S.; Niedrig, M.; Modirrousta, H.; Hoffmann, B.; Groschup, M.H. West Nile Virus Monitoring in Migrating and Resident Water Birds in Iran: Are Common Coots the Main Reservoirs of the Virus in Wetlands? Vector-Borne Zoonotic Dis. 2011, 11, 1377–1381. [Google Scholar] [CrossRef]
- Ben Hassine, T.; De Massis, F.; Calistri, P.; Savini, G.; BelHaj Mohamed, B.; Ranen, A.; Di Gennaro, A.; Sghaier, S.; Hammami, S. First Detection of Co-Circulation of West Nile and Usutu Viruses in Equids in the South-West of Tunisia. Transbound Emerg. Dis. 2014, 61, 385–389. [Google Scholar] [CrossRef]
- Ayadi, T.; Hammouda, A.; Poux, A.; Boulinier, T.; Lecollinet, S.; Selmi, S. Evidence of Exposure of Laughing Doves (Spilopelia Senegalensis) to West Nile and Usutu Viruses in Southern Tunisian Oases. Epidemiol. Infect. 2017, 145, 2808–2816. [Google Scholar] [CrossRef]
- Benbetka, S.; Hachid, A.; Benallal, K.; Benbetka, C.; Khaldi, A.; Bitam, I.; Harrat, Z. First Field Evidence Infection of Culex Perexiguus by West Nile Virus in Sahara Oasis of Algeria. J. Vector Borne Dis. 2018, 55, 305. [Google Scholar] [CrossRef]
- Eiden, M.; Gil, P.; Ziegler, U.; Rakotoarivony, I.; Marie, A.; Frances, B.; L’Ambert, G.; Simonin, Y.; Foulongne, V.; Groschup, M.H.; et al. Emergence of Two Usutu Virus Lineages in Culex Pipiens Mosquitoes in the Camargue, France, 2015. Infect. Genet. Evol. 2018, 61, 151–154. [Google Scholar] [CrossRef]
- Tamba, M.; Bonilauri, P.; Bellini, R.; Calzolari, M.; Albieri, A.; Sambri, V.; Dottori, M.; Angelini, P. Detection of Usutu Virus Within a West Nile Virus Surveillance Program in Northern Italy. Vector-Borne Zoonotic Dis. 2011, 11, 551–557. [Google Scholar] [CrossRef]
- Calzolari, M.; Gaibani, P.; Bellini, R.; Defilippo, F.; Pierro, A.; Albieri, A.; Maioli, G.; Luppi, A.; Rossini, G.; Balzani, A.; et al. Mosquito, Bird and Human Surveillance of West Nile and Usutu Viruses in Emilia-Romagna Region (Italy) in 2010. PLoS ONE 2012, 7, e38058. [Google Scholar] [CrossRef]
- Calzolari, M.; Chiapponi, C.; Bonilauri, P.; Lelli, D.; Baioni, L.; Barbieri, I.; Lavazza, A.; Pongolini, S.; Dottori, M.; Moreno, A. Co-Circulation of Two Usutu Virus Strains in Northern Italy between 2009 and 2014. Infect. Genet. Evol. 2017, 51, 255–262. [Google Scholar] [CrossRef]
- Vazquez, A.; Jimenez-Clavero, M.; Franco, L.; Donoso-Mantke, O.; Sambri, V.; Niedrig, M.; Zeller, H.; Tenorio, A. Usutu Virus: Potential Risk of Human Disease in Europe. Eurosurveillance 2011, 16, 19935. [Google Scholar] [CrossRef]
- Jansen, S.; Heitmann, A.; Lühken, R.; Leggewie, M.; Helms, M.; Badusche, M.; Rossini, G.; Schmidt-Chanasit, J.; Tannich, E. Culex Torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe. Viruses 2019, 11, 492. [Google Scholar] [CrossRef]
- Camp, J.V.; Kolodziejek, J.; Nowotny, N. Targeted Surveillance Reveals Native and Invasive Mosquito Species Infected with Usutu Virus. Parasit Vectors 2019, 12, 46. [Google Scholar] [CrossRef]
- Mancini, G.; Montarsi, F.; Calzolari, M.; Capelli, G.; Dottori, M.; Ravagnan, S.; Lelli, D.; Chiari, M.; Santilli, A.; Quaglia, M.; et al. Mosquito Species Involved in the Circulation of West Nile and Usutu Viruses in Italy. Vet. Ital. 2017, 53, 97–110. [Google Scholar] [CrossRef]
- Fortuna, C.; Remoli, M.E.; Severini, F.; Di Luca, M.; Toma, L.; Fois, F.; Bucci, P.; Boccolini, D.; Romi, R.; Ciufolini, M.G. Evaluation of Vector Competence for West Nile Virus in Italian Stegomyia Albopicta (=Aedes Albopictus) Mosquitoes. Med. Vet. Entomol. 2015, 29, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Petrić, D.; Hrnjakovic, I.; Radovanov, J.; Cvjetkovic, D.; Patic, V.; Milosevic, V.; Kovacevic, G.; Zgomba, M.; Ignjatovic Cupina, A.; Konjevic, A.; et al. West Nile Virus Surveillance in Humans and Mosquitoes and Detection of Cell Fusing Agent Virus in Vojvodina Province (Serbia). Healthmed 2012, 6, 462–468. [Google Scholar]
- Tick-Borne Encephalitis—Annual Epidemiological Report for 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/tick-borne-encephalitis-annual-epidemiological-report-2017 (accessed on 10 January 2023).
- Fares, W.; Dachraoui, K.; Cherni, S.; Barhoumi, W.; Slimane, T.B.; Younsi, H.; Zhioua, E. Tick-Borne Encephalitis Virus in Ixodes Ricinus (Acari: Ixodidae) Ticks, Tunisia. Ticks Tick Borne Dis. 2021, 12, 101606. [Google Scholar] [CrossRef] [PubMed]
- Steffen, R. Epidemiology of Tick-Borne Encephalitis (TBE) in International Travellers to Western/Central Europe and Conclusions on Vaccination Recommendations. J. Travel Med. 2016, 23, taw018. [Google Scholar] [CrossRef]
- Kaiser, R. Tick-Borne Encephalitis. Infect. Dis. Clin. N. Am. 2008, 22, 561–575. [Google Scholar] [CrossRef]
- Ling, J.; Smura, T.; Lundström, J.O.; Pettersson, J.H.-O.; Sironen, T.; Vapalahti, O.; Lundkvist, Å.; Hesson, J.C. Introduction and Dispersal of Sindbis Virus from Central Africa to Europe. J. Virol. 2019, 93, e00620-19. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, S.; Human, S.; Williams, J.; van Wilpe, E.; Pretorius, M.; Swanepoel, R.; Venter, M. Sindbis and Middelburg Old World Alphaviruses Associated with Neurologic Disease in Horses, South Africa. Emerg. Infect. Dis. 2015, 21, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Sigei, F.; Nindo, F.; Mukunzi, S.; Ng’ang’a, Z.; Sang, R. Evolutionary Analyses of Sindbis Virus Strains Isolated from Mosquitoes in Kenya. Arch. Virol. 2018, 163, 2465–2469. [Google Scholar] [CrossRef]
- Korhonen, E.M.; Suvanto, M.T.; Uusitalo, R.; Faolotto, G.; Smura, T.; Sane, J.; Vapalahti, O.; Huhtamo, E. Sindbis Virus Strains of Divergent Origin Isolated from Humans and Mosquitoes During a Recent Outbreak in Finland. Vector Borne Zoonotic Dis. 2020, 20, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, N.; Hachid, A.; Thirion, L.; Benallal, K.E.; Pezzi, L.; Khardine, F.A.; Benbetka, C.; Benbetka, S.; Harrat, Z.; Charrel, R. Detection and Isolation of Sindbis Virus from Field Collected Mosquitoes in Timimoun, Algeria. Viruses 2022, 14, 894. [Google Scholar] [CrossRef] [PubMed]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis Virus as a Human Pathogen-Epidemiology, Clinical Picture and Pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef]
- Engler, O.; Savini, G.; Papa, A.; Figuerola, J.; Groschup, M.H.; Kampen, H.; Medlock, J.; Vaux, A.; Wilson, A.J.; Werner, D.; et al. European Surveillance for West Nile Virus in Mosquito Populations. Int. J. Environ. Res. Public Health 2013, 10, 4869–4895. [Google Scholar] [CrossRef]
- Mixão, V.; Bravo Barriga, D.; Parreira, R.; Novo, M.T.; Sousa, C.A.; Frontera, E.; Venter, M.; Braack, L.; Almeida, A.P.G. Comparative Morphological and Molecular Analysis Confirms the Presence of the West Nile Virus Mosquito Vector, Culex Univittatus, in the Iberian Peninsula. Parasit Vectors 2016, 9, 601. [Google Scholar] [CrossRef]
- Orshan, L.; Bin, H.; Schnur, H.; Kaufman, A.; Valinsky, A.; Shulman, L.; Weiss, L.; Mendelson, E.; Pener, H. Mosquito Vectors of West Nile Fever in Israel. J. Med. Entomol. 2008, 45, 939–947. [Google Scholar] [CrossRef]
- la Puente, J.M.-D.; Ferraguti, M.; Ruiz, S.; Roiz, D.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M.; Soriguer, R.; Figuerola, J. Mosquito Community Influences West Nile Virus Seroprevalence in Wild Birds: Implications for the Risk of Spillover into Human Populations. Sci. Rep. 2018, 8, 2599. [Google Scholar] [CrossRef] [PubMed]
- Osório, H.C.; Zé-Zé, L.; Alves, M.J. Host-Feeding Patterns of Culex Pipiens and Other Potential Mosquito Vectors (Diptera: Culicidae) of West Nile Virus (Flaviviridae) Collected in Portugal. J. Med. Entomol. 2012, 49, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Hesson, J.C.; Lundström, J.O.; Tok, A.; Östman, Ö.; Lundkvist, Å. Temporal Variation in Sindbis Virus Antibody Prevalence in Bird Hosts in an Endemic Area in Sweden. PLoS ONE 2016, 11, e0162005. [Google Scholar] [CrossRef] [PubMed]
- Brunhes, J.; Hassaïne, K.; Rhaiem, A.; Hervy, J.-P. Les Culicides de l’Afrique méditerranéenne: Espèces présentes et répartition (Diptera, Nematocera). Bull. De La Société Entomol. De Fr. 2000, 105, 195–204. [Google Scholar] [CrossRef]
- Moutailler, S.; Yousfi, L.; Mousson, L.; Devillers, E.; Vazeille, M.; Vega-Rúa, A.; Perrin, Y.; Jourdain, F.; Chandre, F.; Cannet, A.; et al. A New High-Throughput Tool to Screen Mosquito-Borne Viruses in Zika Virus Endemic/Epidemic Areas. Viruses 2019, 11, 904. [Google Scholar] [CrossRef]
- Nielsen, E.M.; Andersen, M.T. Detection and Characterization of Verocytotoxin-Producing Escherichia Coli by Automated 5′ Nuclease PCR Assay. J. Clin. Microbiol. 2003, 41, 2884–2893. [Google Scholar] [CrossRef]
- Michelet, L.; Delannoy, S.; Devillers, E.; Umhang, G.; Aspan, A.; Juremalm, M.; Chirico, J.; van der Wal, F.J.; Sprong, H.; Boye Pihl, T.P.; et al. High-Throughput Screening of Tick-Borne Pathogens in Europe. Front. Cell. Infect. Microbiol. 2014, 4, 103. [Google Scholar] [CrossRef]
- Linke, S.; Ellerbrok, H.; Niedrig, M.; Nitsche, A.; Pauli, G. Detection of West Nile Virus Lineages 1 and 2 by Real-Time PCR. J. Virol. Methods 2007, 146, 355–358. [Google Scholar] [CrossRef]
- Beck, C.; Desprès, P.; Paulous, S.; Vanhomwegen, J.; Lowenski, S.; Nowotny, N.; Durand, B.; Garnier, A.; Blaise-Boisseau, S.; Guitton, E.; et al. A High-Performance Multiplex Immunoassay for Serodiagnosis of Flavivirus-Associated Neurological Diseases in Horses. Biomed. Res. Int. 2015, 2015, 678084. [Google Scholar] [CrossRef]
- Vanhomwegen, J.; Beck, C.; Desprès, P.; Figuerola, A.; García, R.; Lecollinet, S.; López-Roig, M.; Manuguerra, J.-C.; Serra-Cobo, J. Circulation of Zoonotic Arboviruses in Equine Populations of Mallorca Island (Spain). Vector Borne Zoonotic Dis. 2017, 17, 340–346. [Google Scholar] [CrossRef]
- Kampen, H.; Tews, B.A.; Werner, D. First Evidence of West Nile Virus Overwintering in Mosquitoes in Germany. Viruses 2021, 13, 2463. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Chinikar, S.; Moosa-Kazemi, S.H.; Sedaghat, M.M.; Kayedi, M.H.; Lühken, R.; Schmidt-Chanasit, J. West Nile Virus Lineage-2 in Culex Specimens from Iran. Trop. Med. Int. Health 2017, 22, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Moosa-Kazemi, S.H.; Sedaghat, M.M.; Wong, G.; Chinikar, S.; Hajivand, Z.; Mokhayeri, H.; Nowotny, N.; Kayedi, M.H. Autochthonous Transmission of West Nile Virus by a New Vector in Iran, Vector-Host Interaction Modeling and Virulence Gene Determinants. Viruses 2020, 12, 1449. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, A.; Ferguson, H.H.; Méndez-Sánchez, J.D.; Danis-Lozano, R.; Casas-Martínez, M.; Bond, J.G.; García-Zebadúa, J.C.; Orozco-Bonilla, A.; Juárez-Ordaz, J.A.; Farfan-Ale, J.A.; et al. West Nile Virus Activity in Mosquitoes and Domestic Animals in Chiapas, México. Vector Borne Zoonotic Dis. 2009, 9, 555–560. [Google Scholar] [CrossRef]
- Nabli, B.; Chippaux-Hyppolite, C.; Chippaux, A.; Tamalet, J. Enquête Sérologique En Tunisie Sur Les Arbovirus. Bull. World Health Organ. 1970, 42, 297–303. [Google Scholar]
- Chastel, C.; Bach-Hamba, D.; Launay, H.; Le Lay, G.; Hellal, H.; Beaucournu, J.C. Arbovirus infections in Tunisia: New serological survey of small wild mammals. Bull. Soc. Pathol. Exot. Fil. 1983, 76, 21–33. [Google Scholar]
- Ben Hassine, T.; Conte, A.; Calistri, P.; Candeloro, L.; Ippoliti, C.; De Massis, F.; Danzetta, M.L.; Bejaoui, M.; Hammami, S. Identification of Suitable Areas for West Nile Virus Circulation in Tunisia. Transbound. Emerg. Dis. 2017, 64, 449–458. [Google Scholar] [CrossRef]
- Bargaoui, R.; Lecollinet, S.; Lancelot, R. Mapping the Serological Prevalence Rate of West Nile Fever in Equids, Tunisia. Transbound. Emerg. Dis. 2015, 62, 55–66. [Google Scholar] [CrossRef]
- Ayadi, T.; Hammouda, A.; Beck, C.; Boulinier, T.; Lecollinet, S.; Selmi, S. Flaviviruses in Migratory Passerines during Spring Stopover in a Desert Oasis. Zoonoses Public Health 2019, 66, 495–503. [Google Scholar] [CrossRef]
- Beasley, D.W.C.; Holbrook, M.R.; Travassos Da Rosa, A.P.A.; Coffey, L.; Carrara, A.-S.; Phillippi-Falkenstein, K.; Bohm, R.P.; Ratterree, M.S.; Lillibridge, K.M.; Ludwig, G.V.; et al. Use of a Recombinant Envelope Protein Subunit Antigen for Specific Serological Diagnosis of West Nile Virus Infection. J. Clin. Microbiol. 2004, 42, 2759–2765. [Google Scholar] [CrossRef]
- Zohaib, A.; Saqib, M.; Beck, C.; Hussain, M.H.; Lowenski, S.; Lecollinet, S.; Sial, A.; Asi, M.N.; Mansoor, M.K.; Saqalein, M.; et al. High Prevalence of West Nile Virus in Equines from the Two Provinces of Pakistan. Epidemiol. Infect. 2015, 143, 1931–1935. [Google Scholar] [CrossRef] [PubMed]
- Napp, S.; Llorente, F.; Beck, C.; Jose-Cunilleras, E.; Soler, M.; Pailler-García, L.; Amaral, R.; Aguilera-Sepúlveda, P.; Pifarré, M.; Molina-López, R.; et al. Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019. Viruses 2021, 13, 2404. [Google Scholar] [CrossRef] [PubMed]
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D.; et al. Epidemic West Nile Encephalitis, New York, 1999: Results of a Household-Based Seroepidemiological Survey. Lancet 2001, 358, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Pierro, A.; Gaibani, P.; Spadafora, C.; Ruggeri, D.; Randi, V.; Parenti, S.; Finarelli, A.C.; Rossini, G.; Landini, M.P.; Sambri, V. Detection of Specific Antibodies against West Nile and Usutu Viruses in Healthy Blood Donors in Northern Italy, 2010–2011. Clin. Microbiol. Infect. 2013, 19, E451–E453. [Google Scholar] [CrossRef]
- Hadjichristodoulou, C.; Pournaras, S.; Mavrouli, M.; Marka, A.; Tserkezou, P.; Baka, A.; Billinis, C.; Katsioulis, A.; Psaroulaki, A.; Papa, A.; et al. West Nile Virus Seroprevalence in the Greek Population in 2013: A Nationwide Cross-Sectional Survey. PLoS ONE 2015, 10, e0143803. [Google Scholar] [CrossRef]
- Tezcan, S.; Kızıldamar, S.; Ulger, M.; Aslan, G.; Tiftik, N.; Ozkul, A.; Emekdaş, G.; Niedrig, M.; Ergünay, K. Flavivirus seroepidemiology in blood donors in Mersin province, Turkey. Mikrobiyol. Bul. 2014, 48, 606–617. [Google Scholar] [CrossRef]
- Zakhia, R.; Dupuis, A.P.; Khodr, F.; Fadel, M.; Kramer, L.D.; Haddad, N. Evidence of West Nile Virus Circulation in Lebanon. Viruses 2021, 13, 994. [Google Scholar] [CrossRef]
- Batieha, A.; Saliba, E.K.; Graham, R.; Mohareb, E.; Hijazi, Y.; Wijeyaratne, P. Seroprevalence of West Nile, Rift Valley, and Sandfly Arboviruses in Hashimiah, Jordan. Emerg. Infect. Dis. 2000, 6, 358–362. [Google Scholar] [CrossRef]
- Meshkat, Z.; Chinikar, S.; Shakeri, M.; Manavifar, L.; Moradi, M.; Mirshahabi, H.; Jalali, T.; Khakifirouz, S.; Shahhosseini, N. Prevalence of West Nile Virus in Mashhad, Iran: A Population-Based Study. Asian Pac. J. Trop. Med. 2015, 8, 203–205. [Google Scholar] [CrossRef]
- Khbou, M.K.; Romdhane, R.; Foughali, A.A.; Sassi, L.; Suin, V.; Rekik, M.; Benzarti, M. Presence of Antibodies against Tick-Borne Encephalitis Virus in Sheep in Tunisia, North Africa. BMC Vet. Res. 2020, 16, 1–8. [Google Scholar] [CrossRef]
- Beck, C.; Leparc-Goffart, I.; Desoutter, D.; Debergé, E.; Bichet, H.; Lowenski, S.; Dumarest, M.; Gonzalez, G.; Migné, C.; Vanhomwegen, J.; et al. Serological Evidence of Infection with Dengue and Zika Viruses in Horses on French Pacific Islands. PLoS Negl. Trop. Dis. 2019, 13, e0007162. [Google Scholar] [CrossRef] [PubMed]
- Bouattour, A.; Khrouf, F.; Rhim, A.; M’ghirbi, Y. First Detection of the Asian Tiger Mosquito, Aedes (Stegomyia) Albopictus (Diptera: Culicidae), in Tunisia. J. Med. Entomol. 2019, 56, 1112–1115. [Google Scholar] [CrossRef]
- Bohers, C.; Mousson, L.; Madec, Y.; Vazeille, M.; Rhim, A.; M’ghirbi, Y.; Bouattour, A.; Failloux, A.-B. The Recently Introduced Aedes Albopictus in Tunisia Has the Potential to Transmit Chikungunya, Dengue and Zika Viruses. PLoS Negl. Trop. Dis. 2020, 14, e0008475. [Google Scholar] [CrossRef] [PubMed]
- Durand, B.; Haskouri, H.; Lowenski, S.; Vachiery, N.; Beck, C.; Lecollinet, S. Seroprevalence of West Nile and Usutu Viruses in Military Working Horses and Dogs, Morocco, 2012: Dog as an Alternative WNV Sentinel Species? Epidemiol. Infect. 2016, 144, 1857–1864. [Google Scholar] [CrossRef]
- Lafri, I.; Prat, C.M.; Bitam, I.; Gravier, P.; Besbaci, M.; Zeroual, F.; Ben-Mahdi, M.H.; Davoust, B.; Leparc-Goffart, I. Seroprevalence of West Nile Virus Antibodies in Equids in the North-East of Algeria and Detection of Virus Circulation in 2014. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 8–12. [Google Scholar] [CrossRef]
- Constant, O.; Bollore, K.; Clé, M.; Barthelemy, J.; Foulongne, V.; Chenet, B.; Gomis, D.; Virolle, L.; Gutierrez, S.; Desmetz, C.; et al. Evidence of Exposure to USUV and WNV in Zoo Animals in France. Pathogens 2020, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Iapaolo, F.; Polci, A.; Marcacci, M.; Di Gennaro, A.; Teodori, L.; Curini, V.; Di Lollo, V.; Secondini, B.; Scialabba, S.; et al. West Nile Virus Lineage 2 Overwintering in Italy. Trop. Med. Infect. Dis. 2022, 7, 160. [Google Scholar] [CrossRef]
- Beji, M.; Rhim, A.; Roiz, D.; Bouattour, A. Ecophysiological Characterization and Molecular Differentiation of Culex Pipiens Forms (Diptera: Culicidae) in Tunisia. Parasites Vectors 2017, 10, 327. [Google Scholar] [CrossRef]
- Muñoz, J.; Ruiz, S.; Soriguer, R.; Alcaide, M.; Viana, D.S.; Roiz, D.; Vázquez, A.; Figuerola, J. Feeding Patterns of Potential West Nile Virus Vectors in South-West Spain. PLoS ONE 2012, 7, e39549. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J.; Juricová, Z. West Nile Fever in Czechland. Emerg. Infect. Dis. 1999, 5, 594–595. [Google Scholar] [CrossRef]
- Zeller, H.G.; Schuffenecker, I. West Nile Virus: An Overview of Its Spread in Europe and the Mediterranean Basin in Contrast to Its Spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Randolph, S.E. Climate Change and Vector-Borne Diseases. Adv. Parasitol. 2006, 62, 345–381. [Google Scholar] [CrossRef] [PubMed]
| Localities (nb of Tested Pools, nb of Tested Mosquito Specimens) a | Mosquito Species (nb of Tested Pools, nb of Mosquito Specimens) | Arboviruses Infection by High-Throughput Microfluidic Real-Time PCR (nb of Infected Pools Composed of 10 Abdomens + or 10 Whole Mosquitoes) | Arboviruses Infection Confirmation by Real-Time PCR (nb of RBP Confirmed/nb of RBP Tested) b |
|---|---|---|---|
| Ichkeul (159, 1590) | Ochlerotatus caspius (12, 120) | Negative | n/a |
| Ochlerotatus detritus (6, 60) | Negative | n/a | |
| Culex perexiguus (116, 1160) | SINV (1 + 2) | Negative (10/10) | |
| WNV (1 + 9) | WNV (2/10) | ||
| WNV and SINV (1 + 2) | WNV and SINV (1/10) | ||
| WNV and USUV (0 + 1) | n/a c | ||
| Culex pipiens (25, 250) | Negative | n/a | |
| Monastir (47, 470) | Oc. caspius (16, 160) | Negative | n/a |
| Culex perexiguus (23, 230) | n/a | ||
| Culex pipiens (8, 80) | n/a | ||
| Moknine (8, 80) | Oc. caspius (3, 30) | Negative | n/a |
| Culex perexiguus (4, 40) | n/a | ||
| Culex pipiens (1, 10) | n/a | ||
| Mseken (34, 340) | Oc. caspius (24, 240) | Negative | n/a |
| Oc. detritus (6, 60) | n/a | ||
| Culex perexiguus (3, 30) | n/a | ||
| Culex pipiens (1, 10) | n/a |
| Localities (nb of Tested Sera by cELISA) | cELISA, WNV Results | MIA Analysis of Positive cELISA Horse Sera | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Nb of cELISA Positive Sera/nb of Sera Analysed in MIA | Negative | Undetermined Flavivirus | WNV | USUV | TBEV | WNV + TBEV | USUV + TBEV | WNV + USUV | |
| Aousja (15) | 10 | 5/4 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| Battan (36) | 26 | 10/9 | 3 | 0 | 3 | 3 | 0 | 0 | 0 | 0 |
| Beja (11) | 1 | 10/10 | 1 | 1 | 7 | 1 | 0 | 0 | 0 | 0 |
| Bezina (2) | 0 | 2/2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Ichkeul (38) | 5 | 33/17 | 0 | 0 | 14 | 2 | 0 | 0 | 1 | 0 |
| Moknine (32) | 15 | 17/17 | 1 | 2 | 11 | 1 | 0 | 0 | 0 | 2 |
| Monastir (32) | 18 | 14/14 | 2 | 0 | 10 | 1 | 0 | 0 | 0 | 1 |
| Mseken (22) | 5 | 17/17 | 0 | 1 | 15 | 0 | 0 | 0 | 0 | 1 |
| Sejnène (4) | 0 | 4/4 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Somâa (18) | 12 | 6/2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Tunis (159) | 131 | 28/8 | 2 | 2 | 4 | 0 | 0 | 0 | 0 | 0 |
| Total (369) | 223 | 146/104 | 9 | 7 | 74 | 8 | 0 | 1 | 1 | 4 |
| Localities (nb cELISA Positive Sera/nb of Sera Analysed by MNT) | Negative | Undetermined flavivirus | WNV (Range of Titers) | USUV (Range of Titers) | TBEV | WNV and/or USUV |
|---|---|---|---|---|---|---|
| Aousja (5/4) a | 1 | 0 | 3 (40–80) | 0 | 0 | 0 |
| Battan (10/9) | 3 | 0 | 3 (80–160) | 3 (10–20) | 0 | 0 |
| Beja (10/10) b | 1 | 0 | 7 (20–≥320) | 0 | 0 | 2 |
| Bezina (2/2) c | 0 | 0 | 1 (160) | 0 | 0 | 1 |
| Ichkeul (33/17) | 0 | 0 | 14 (20–160) | 3 (40–320) | 0 | 0 |
| Moknine (17/17) d | 1 | 0 | 11 (40–≥320) | 2 (20–80) | 0 | 3 |
| Monastir (14/14) e | 2 | 0 | 11 (20–≥320) | 0 | 0 | 1 |
| Mseken (17/17) f | 1 | 0 | 12 (20–≥320) | 0 | 0 | 4 |
| Sejnène (4/4) | 0 | 0 | 3 (80–≥320) | 0 | 0 | 1 |
| Somâa (6/2) | 0 | 0 | 2 (80–160) | 0 | 0 | 0 |
| Tunis (28/8) g | 2 | 0 | 4 (80–≥320) | 0 | 0 | 0 |
| Total (146/104) | 11 | 0 | 71 | 8 | 0 | 12 |
| Assay | Flavivirus Species Detected | nb of Positive Samples/nb of Tested Samples (%) |
|---|---|---|
| cELISA | Flavivirus | 146/369 (39.5) |
| MIA | WNV | 74/104 (71.2) |
| MNT | WNV | 71/104 (68.3) |
| MIA | USUV | 8/104 (7.7) |
| MNT | USUV | 8/104 (7.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’ghirbi, Y.; Mousson, L.; Moutailler, S.; Lecollinet, S.; Amaral, R.; Beck, C.; Aounallah, H.; Amara, M.; Chabchoub, A.; Rhim, A.; et al. West Nile, Sindbis and Usutu Viruses: Evidence of Circulation in Mosquitoes and Horses in Tunisia. Pathogens 2023, 12, 360. https://doi.org/10.3390/pathogens12030360
M’ghirbi Y, Mousson L, Moutailler S, Lecollinet S, Amaral R, Beck C, Aounallah H, Amara M, Chabchoub A, Rhim A, et al. West Nile, Sindbis and Usutu Viruses: Evidence of Circulation in Mosquitoes and Horses in Tunisia. Pathogens. 2023; 12(3):360. https://doi.org/10.3390/pathogens12030360
Chicago/Turabian StyleM’ghirbi, Youmna, Laurence Mousson, Sara Moutailler, Sylvie Lecollinet, Rayane Amaral, Cécile Beck, Hajer Aounallah, Meriem Amara, Ahmed Chabchoub, Adel Rhim, and et al. 2023. "West Nile, Sindbis and Usutu Viruses: Evidence of Circulation in Mosquitoes and Horses in Tunisia" Pathogens 12, no. 3: 360. https://doi.org/10.3390/pathogens12030360
APA StyleM’ghirbi, Y., Mousson, L., Moutailler, S., Lecollinet, S., Amaral, R., Beck, C., Aounallah, H., Amara, M., Chabchoub, A., Rhim, A., Failloux, A.-B., & Bouattour, A. (2023). West Nile, Sindbis and Usutu Viruses: Evidence of Circulation in Mosquitoes and Horses in Tunisia. Pathogens, 12(3), 360. https://doi.org/10.3390/pathogens12030360








