Abstract
In Chagas disease, the mechanisms involved in cardiac damage are an active field of study. The factors underlying the evolution of lesions following infection by Trypanosoma cruzi and, in some cases, the persistence of its antigens and the host response, with the ensuing development of clinically observable cardiac damage, are analyzed in this review.
1. Introduction
Chagas disease is a complex zoonosis. Its natural history involves the interaction of transmitting arthropods with wild, peridomestic, and domestic mammals, and it has a great diversity of transmission forms. In a vertebrate host, the disease evolution is evinced by various clinical manifestations [1,2].
The disease is caused by Trypanosoma cruzi, a flagellated protozoan that is naturally transmitted by hematophagous Hemiptera insects (triatomines). The parasite was discovered in 1909 by Dr. Carlos Chagas in Minas Gerais, Brazil. Dr. Chagas also described a large part of the biological cycle, linking the parasite to the transmitting triatomine (Panstrongylus megistus). He was able to isolate the parasite and replicated the infection in experimental animals. Dr. Chagas also mentioned that rural housing conditions, which to date have not changed significantly in endemic countries, are important for the spread of the vector. As a clinical entity, Chagas disease is linked to poverty [1].
Chagas disease is endemic in 21 countries within continental Latin America. It is distributed from the south of the United States, in Central America, the Southern Cone, Andean countries, and Amazonian countries. In the Americas, 30,000 new cases are reported every year, 12,000 deaths on average, and 8600 newborns are infected at gestation. In 2019, a prevalence rate of 933.76 per 100,000 population was recorded. Currently, about 70 million people in the Americas live in areas at risk for the infection [3]. Humans become infected with T. cruzi by several mechanisms. The most important one is natural transmission, involving an infected triatomine bug. This transmission form is very common in rural areas, where housing traits and the ecotope favor a colonization of the domestic niche by insects. The second transmission form, restricted to urban areas, is related to the transfusion of blood or its components. This transmission form depends on the migration of rural population to cities, as more than 70% of the population in some cities had immigrated from high-prevalence areas of the disease [4].
The primary vectors for Chagas Disease are Triatoma infestans in Argentina, Bolivia, Brazil, Chile, Paraguay, Uruguay, and Peru; Rhodnius prolixus in Colombia, Venezuela, and Central America; T. dimidiata in Ecuador and Central America; and R. pallescens in Panama [3]. In the Americas, natural infection is associated with risk factors such as housing construction material and other characteristics that favor the colonization of human dwellings in rural areas. For this reason, it is considered as a neglected tropical disease [4]. PAHO/WHO, working with the endemic countries, have launched several Subregional Disease Prevention and Control Initiatives. These include the improvement of housing to halt vectorial transmission in 17 countries, and screening of blood donors in the 21 endemic countries, in addition to eliminating some vector species such as R. prolixus in El Salvador, Costa Rica, and Mexico, and T. infestans in Brazil and Uruguay [3,5].
Due to the increase in population mobility worldwide, Chagas disease is considered a major health problem, which has reached countries where vector transmission does not exist, due to the immigration of seropositive cases from endemic geographic areas. This poses a risk of transmission through transfusions, organ and tissue transplants, and even maternal-fetal transmission in the USA, Canada, and some European and Western Pacific countries. It has been estimated that Spain is the main non-endemic country in the number of transmissions, followed by the USA and Italy [6].
Chagas disease has two clinical phases: an acute phase and a chronic one. Most cases of acute Chagas disease are asymptomatic; only 5–10% of infected subjects develop symptoms, including persistent fever, asthenia, adynamia, headache, and hepatosple-nomegaly, all of which are nonspecific. Among these symptomatic individuals, the most frequent pathognomonic signs are the Romaña sign, characterized by unilateral bi-palpebral edema, which is observed in 50% of cases, and the indurated cutaneous chagoma, found in 25% of cases. Both signs are often accompanied by regional adeno-megaly. In the remaining 25% of patients, there is no sign of portal entry, but some of the nonspecific symptoms mentioned above can be found [7]. The most frequent symptom is fever, which is present in up to 95% of cases, usually without specific characteristics. All other signs and symptoms, including asthenia, adynamia, headache, and hepatosple-nomegaly, are nonspecific [3,5]. While T. cruzi can infect any nucleated cell, some strains exhibit a marked tropism for myocardial cells, smooth muscle cells of the digestive system, or nervous tissue, among other cell types. Cardiac manifestations include primarily organ enlargement (cardiomegaly) [7].
The chronic phase is divided into a chronic asymptomatic phase and a symptomatic phase; the former evolves without demonstrated pathology and can last 10–20 years; however, cases have been reported in minors in Mexico where this period lasts 2–7 years before the chronic form with cardiovascular symptomatology can be detected [8]; this phase is clinically asymptomatic and exhibits very low parasitemia, so that the methods of choice for diagnosis are serological, and confirmation requires two positive tests with different principles [9].
After this phase, patients progress to the chronic symptomatic phase, with a proven symptomatology, in which they develop mainly cardiac lesions and, to a lesser extent, digestive lesions, mainly in the esophagus and colon, and in a few cases in the peripheral nervous system. Cardiac lesions cause alterations in myocardial contractility and the electrical impulse, mainly in the bundle of His, the particular right bundle branch block with left anterior fascicular hemiblock, ventricular extrasystoles, and atrioventricular block [3,7]. The lesions in this cardiomyopathy involve several cardiac tissues, mainly the myocardium, and in severe cases, the endocardium pericardium; this can cause pleural effusion, which may evolve into sudden death, which is more frequent in cases with dilated heart disease and severe heart failure [10]. Carlos Chagas described digestive disorders linked to the disease in 1916, although Kidder and Fletcher had already done so in 1857, when they called it mal d’engasgo, i.e., “disease causing dysphagia” [11]. Esophageal involvement usually consists of a megaesophagus with slow esophageal transit disorders, along with pain and difficulty in swallowing. In cases of colon involvement, a megacolon and constipation are typical [12,13]. Chagas heart disease is clinically classified according to symptoms, and electrocardio-graphic, echocardiographic, and radiological abnormalities, especially changes in left ventricular function. Some risk factors predisposing one to a progression to the chronic phase include electrocardiographic abnormalities, male sex, systolic blood pressure less than 120 mmHg, altered systolic function, left ventricular dilatation, and complex arrhythmias; risk scores have been proposed to stratify the risk of death, although their clinical value is still under study [14]. In a consensus, several authors note that the main predictors of poor prognosis in chronic Chagas disease are a deterioration of left ventricular function, falling into classes III (fatigue, palpitations, dyspnea, or anginal pain) and VI (cardiomegaly and non-sustained ventricular tachycardia) of the New York Heart Association (NYHA) classification of heart failure [15]. Other risk scores have been proposed. Rassi uses a combination of clinical symptoms, cabinet test results, and demographic data [16]. On the other hand, de Sousa use a four-factor score that includes the QT dispersion interval on ECG, syncope, premature ventricular contractions, and left ventricular function [17].
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease. Both drugs have been approved internationally. Antiparasitic treatment of these cardiomyopathies is accompanied by the administration of antiarrhythmics and pace-maker placement. In the most severe cases, heart transplantation can be required [5,7].
2. Pathogenic Mechanisms of Immune Response Evasion in Chagas Disease
Most of the mechanisms involved in evading the immune response occur in the acute phase, when trypomastigotes establish contact with immune cells of the vertebrate host. The parasite has evolved mechanisms to survive processes such as phagocytosis and the complement system, in addition to interfering with lymphocyte maturation. When metacyclic trypomastigotes contact host cells, either through skin lesions or mucous membranes, the immune response is activated. T. cruzi enters cells through two main mechanisms. The first one is lysosome-dependent, which favors Ca2+ mobilization [18]. In this stage, the parasite surface glycoprotein gp82 is crucial for cell adhesion and lysosomal fusion at the site of entry. Cruzipain has also been proven to be critical for calcium induction and lysosome recruitment. It is a cysteine-protease secreted by trypomastigotes [19,20]. The second mechanism involves invagination of the plasma membrane followed by lysosomal fusion. The acidification process of lysosomes containing the parasite is key to its differentiation into an amastigote, which is the replicative form. A disruption of cytoskeletal actin facilitates the mobilization of lysosomes towards the cell periphery, where they will fuse with the cytoplasmic membrane and contribute to the formation of a parasitic vacuole [21]. After several divisions in the parasitophorous vacuole, where sialic acid residues are added on the T. cruzi membrane by parasitic trans-sialidases, the phagolysosome is lysed. Thus, trypomastigotes are released into the cytoplasm and bloodstream to infect distant or adjacent tissues and cells [22].
T. cruzi can also be phagocytosed at the site of infection by tissue macrophages. Leishmania spp. also parasitizes macrophages and develops mechanisms similar to those of T. cruzi [23]. Some Leishmania species that parasitize cutaneous and peripheral blood macrophages and have been isolated in Latin America are L. mexicana, L. amazonensis, L. venezuelensis, and L. braziliensis; in the Mediterranean, we find L. infantum, and in Asia there are L. tropica and L. major [23]. It should be emphasized that these species, phylogenetically related to T. cruzi as Kinetoplastida, can inhibit the antiparasitic function of macrophages, and both genera use this strategy; in the case of Leishmania spp., they create a safe intracellular compartment and continue their life cycle in the mammal, and in the case of T. cruzi, they evade the phagolysosome and escape to the cytosol for replication [24,25]. To survive in this extremely oxidative environment inside the macrophages, the T.cruzi express antioxidant enzymes such as peroxidases, which protect it from reactive oxygen and nitrogen species within macrophages [26,27]. In this regard, an overexpression of TcCPX in T. cruzi has been shown to correlate with increased parasitemia and inflammatory infiltrates in the myocardium [28].
Notably, factors such as strain, the level of antioxidant enzyme expression, and the kinetics of association with the phagolysosome may in turn contribute to parasite evasion and persistence in the host. In contrast to other protozoa, which inhibit phagolysosome maturation, T. cruzi evade macrophage activity by the mechanisms mentioned above, and escape from the phagolysosome into the host cell cytoplasm, where they replicate [29]. Once outside the macrophages or in any infected tissue, T. cruzi can be recognized by their different PAMPs, which are mainly glycoinositolphospholipids (GIPLs) and lipopepti-doglycans (LPPG). These molecules have protective functions because they allow the parasite to survive in hydrolytic environments and promote adherence to mammalian cells for invasion. The complement system features a specialized pathway for mannose recognition in pathogenic organisms, and it is known that blood trypomastigotes activate this system; however, the parasite expresses a set of specific surface proteins for complement evasion [30,31]. T. cruzi trans-sialidases are crucial for host cell infection by transferring sialic acid from mammalian cells to their own glycocalyx [32]. Their presence is also known to reduce the recognition of anti-α-Gal antibodies in the bloodstream, in addition to evading the lytic effect of complement by promoting the conversion of C3 to inactive C3b (iC3b) [33].
T. cruzi calreticulin (TcCRT) is also involved in the evasion of the lectin pathway by binding to MBL or inhibiting the classical complement pathway by binding to C1q. These links inactivate the membrane attack complex (MAC) formation pathway [34]. Another mechanism by which the parasite controls the complement pathway is mediated by the CRP protein, which is anchored to the parasite membrane. This 160-kDa glycoprotein binds non-covalently to C3b and C4b to inhibit the assembly of C3 convertase, rendering it inactive to catalyze the cleavage of the complement system on the parasite surface. Another parasitic protein called T-DAF accelerates the decay of C3 and C5 convertase in the classical and alternative pathways of the complement system. The mucin-rich surface of T. cruzi can be recognized by Toll-like receptors, such as TLR-2, expressed on mac-rophages. This interaction induces the synthesis of proinflammatory cytokines such as IL-12 and TNF-α, and it favors the activation of the iNOS pathway in these phagocytes. In addition, cruzipain from T. cruzi has a proteolytic action on the NF-κB protein complex, inhibiting the transcription of proinflammatory cytokines such as IL-12 [35].
3. Histopathological Mechanisms Related to Tissue Parasitism
The presence and replication by binary fission of intracellular amastigotes in the myocardiocyte and its ensuing lysis cause inflammation, the release of cellular components, and finally the destruction of cardiac tissue [36]. A direct correlation between the presence of the parasite and tissue inflammation has been reported [37], even when only the presence of T. cruzi antigens or DNA has been confirmed in chronic lesions [38]. The infection can affect skeletal, cardiac, and smooth muscle, as well as neuronal tissue. Parasitism in the myocardium causes destruction and fibrosis in the Schwann sheath and nerve fibers, which leads to neurogenic alterations, clinically expressed as arrhythmias, His bundle branch block, and even the presence of dyssynergic areas in the parietal function of the heart (Figure 1A). All of these are characteristic manifestations of the chronic phase of Chagas disease [39,40]. Another important mechanism described in myocardial injury is the occurrence of microvascular abnormalities caused by the increase in endothelin due to inflammation. On the other hand, blood trypomastigotes have been described to produce neuraminidase (Figure 1B). All these mechanisms increase platelet adhesiveness and thrombus formation, leading to myocardial ischemia and microinfarcts, which in turn cause tissue necrosis [41,42].
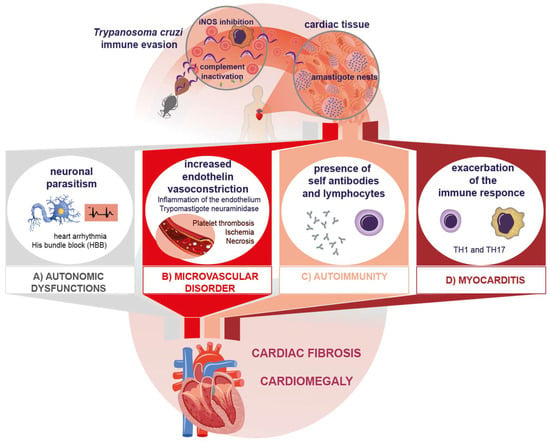
Figure 1.
Immunopathological mechanisms of Chagas disease. The four immunopathogenic mechanisms interacting to cause fibrosis and cardiomegaly in the chronic phase of Chagas disease.
As mentioned above, chronic myocardial inflammation and proinflammatory cytokines can affect myocardial microcirculation. Constant episodes of intense microcirculatory vasodilatation may occur, leading to decreased myocardial blood flow in the terminal distal portions of the coronary microcirculation, thus promoting myocardial ischemia of the apical infero/lateral and basal segments [43]. Rochitte et al. have observed this mechanism, determining by magnetic resonance imaging that the apical and inferolateral regions of the left ventricle show greater damage due to myocardial fibrosis [44]. This is consistent with experimental findings, where chagasic myocardial fibrosis is found in regions of terminal circulation, such as the apex (terminal circulation between the left anterior descending and right coronary artery) and the basal inferolateral segments (terminal circulation between the right coronary artery and the left circumflex coronary artery) [45]. As damage progresses, it leads to myocardial thinning, which results in hypokinesia of the cardiac wall, and eventually to apex aneurysms, a common finding in patients with severe Chagas disease [46].
4. Autoimmune Mechanisms in Chagas Disease
Several theories have been advanced on the development of lesions in heart disease. Among them, the presence of autoimmune phenomena and the persistence of the parasite stand out, without being mutually exclusive [47]. To date, there is no consensus regarding the autoimmune hypothesis in the pathogenesis of Chagas disease; however, several studies have demonstrated the participation of mechanisms that could contribute to tissue damage in the chronic phase [47,48]. Autoimmune damage processes can originate through several mechanisms: (1) In the acute phase, lysis of infected vertebrate cells leads to an exposure of cryptic antigens or with molecular mimicry between the parasite and host cell epitopes. (2) The release and presentation of self-antigens. The ensuing production of inflammatory cytokines may exceed the activation threshold, breaking self-tolerance and stimulating T and B lymphocytes [49]. (3) T cell activation without cytokine signaling (bystander activation). (4) Autoantibody-dependent cell-mediated cytotoxicity [50]. All four mechanisms may result in autoimmune damage.
The existence of molecular mimicry between proteins and other molecules that generate clones of autoreactive CD4+ and CD8+ T lymphocytes, which simultaneously recognize cardiac myosin and the B13 protein of the parasite, has been demonstrated [51]. The proteins with mimicry between host and T. cruzi antigens are shown in Table 1.

Table 1.
Main T. cruzi antigens that show molecular mimicry with vertebrate cell components.
Other processes inherent to the presentation of host antigens after myocardial lysis suggest the existence of cryptic antigens. This is the case in traumatic ophthalmopathies, which expose previously cryptic antigens. Since tolerance for these antigens has not been developed, autoreactive clones are produced. Similarly, some cryptic epitopes can show molecular mimicry with T. cruzi epitopes, which favors the generation of these clones [64]. Such a process has been reported in Chagas disease after host cell lysis (Figure 1C).
As shown in Table 2, there is evidence of the generation of autoreactive T cells in Chagas disease. Ribeiro Dos Santos et al. described myocarditis induced by CD4+ T cell lines from mice in the chronic phase that proliferate in response to any crude T. cruzi antigen or heart tissue extracts [65]. The existence of T lymphocyte populations that recognize cardiac myosin in inflammatory infiltrates during the chronic phase has been reported in T cells in asymptomatic patients that respond to B13 antigen stimulation, which causes increased interferon-γ production. Cardiac damage could be due to delayed hypersensitivity, initiated by antigens such as B13, which induce the release of inflammatory cytokines [66].

Table 2.
Evidence of autoimmune processes in Chagas disease.
The loss of tolerance of T lymphocytes could also be due to a defect in thymic reg-ulation of the formation of naïve T lymphocytes. Since T. cruzi cannot synthesize sialic acid, it expresses trans-sialidases that alter the maturation of thymocytes, causing the migration of undifferentiated cells to the circulation. In this regard, increased numbers of double positive TCD4+ and TCD8+ cells have been demonstrated in this infection [73].
5. Inflammatory and Cytotoxic Process in the Immunopathogenesis of Chagas Disease
The inflammatory process in Chagas disease starts in the acute phase, with the production of proinflammatory cytokines to recruit and induce the activation of monocytes to the site of infection. This process is important to control parasite replication through a Th1-type response, characterized by the production of interleukins (IL) such as IL-1, inter-feron gamma (IFN-γ), and the tumor necrosis factor alpha (TNF-α). The functions of IFN-γ predominate in this mechanism by inducing the production of nitric oxide (NO) in macrophages and initiating the control of blood parasitemia in this phase of the disease [74]. On the other hand, it has also been reported that IL-10 and IL-4, stimulated by antigens such as cruzipain, reduce the production of IFN-γ and NO in macrophages, inhibiting phagocytosis [75].
As noted, while the Th1 response protects the host in the acute phase, its deregulation in the chronic phase leads to cardiac tissue damage by exacerbated, chronic inflammation (Figure 1D) [76]. Molecules that contribute to tissue damage (DAMPS), specifically of cytotoxic origin, are generated by inflammation [77]. In this regard, there is evidence that oxidative stress also induces myocardial lesions in these patients, contributing to lesion progression in Chagas disease [78].
As the condition progresses towards chronicity, further mechanisms of cytotoxic damage affect cardiac tissue. Initially, oxidative stress is triggered, and reactive oxygen species are released. These reactive oxygen species, mainly H2O2 and OH, can be pro-duced by cytotoxic immune responses that cause lysis in the tissues surrounding the infec-tion [79,80]. Another mechanism triggered by the production of reactive oxygen species is the increase in NO levels, which stimulates the production of proinflammatory cytokines and the activation of adhesion molecules for rolling, as well as an increased endothelial permeability [81]. Immune cells with cytotoxic activity, such as macrophages and neutrophils, cause oxidative stress. The inflammatory infiltrate generated in the response against pathogens can subsequently cause myocardial damage. Neutrophils are the first cell type involved in this process and contribute to tissue damage. It has been described that, after infarct resolution, the presence of neutrophils either decreases rapidly, or they may remain activated if inflammation is not resolved. Neutrophils are activated by IFN-γ, which activates inducible nitric oxide synthase (iNOS) and the generation of NO. This molecule peroxidizes lipids and proteins, degrades the membrane, diffuses to the mitochondria of cardiocytes and triggers apoptotic or necrotic cell death. This is detrimental to mammals because of its high potential for tissue damage, and it has been regarded as a factor for myocarditis progression [78,79,82].
Finally, CD8+ T cells, a population that regulates protection against T. cruzi infection from the acute to the chronic phase [83], either by secreting cytokines such as TNF-α and IFN-γ or by releasing cytotoxic granules containing granzymes and perforins, are also capable of damaging the myocardium by cytotoxicity. In the acute phase, the absence of these cells results in an exacerbated parasitemia in a murine model; however, the presence of CD8+ T cells, especially in the chronic phase, is associated with areas of myocardial damage [84]. The presence of a perforin producing CD8+ T phenotype has been proven near areas of cardiac damage [85].
6. Fibrotic Mechanisms in Chagas Disease
Fibrosis in cardiac tissue is a significant increase in the volume of collagen. It gradually produces scar tissue, which affects the overall cardiac function in the severe manifestations of the chronic phase of Chagas disease [86,87]. Fibroblasts are responsible for the production of structural proteins that make up the extracellular matrix. This process, either constructive or reconstructive, favors cardiac remodeling, which is a progressive mechanism in response to acute or chronic damage, regardless of its etiology. Remodeling leads to changes in the heart size, shape, and function, and it is clinically associated with a poor prognosis in patients with heart failure [37,88].
Fibroblast activation is induced by various stimuli or by tissue injury; these trigger the production of transforming growth factor beta (TGF-β), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FCF). Fibroblasts can also be activated by direct cell–cell communication and by contact with leukocytes through adhesion molecules such as the intercellular adhesion molecule-1 (ICAM-1) and the vascular cell adhesion molecule 1 (VCAM-1), or through reactive oxygen species or by complement [89]. This activation can lead to an excessive production and deposition of proteins in the extracellular matrix, a process known as fibrosis. In addition to being a source of collagenase, fibroblasts produce some cytokines (IL-1, IL-6) and enzymes such as matrix metalloproteinases (MMPs); this deposition of collagenase and fibers disrupts the connection between myocardial cells and compromises the structural and functional integrity of the heart. When these fibers are produced in excess, myocardial stiffness occurs. In turn, this condition shows disorders in parietal motility, with the consequent cavitary deformation (growth) and alteration in the diastolic and systolic functions of the heart [90].
Cardiac tissue fibrosis is accompanied by cellular inflammatory infiltrates characterized by the presence of macrophages, neutrophils, and eosinophils during the acute phase, and by lymphocytic infiltrates in the chronic phase. Acute or chronic fibrosis has been described in humans by immunohistochemistry in postmortem studies, pointing to the relevance of these proinflammatory cells and fibrotic accompaniment [91,92]. Cardiomyocytes have also been shown to actively participate in the inflammatory response by producing chemokines, cytokines, and NO. These factors can trigger leukocytes at-traction, and they have even been suggested to promote fibrosis by secreting fibro-blast-activating growth factors and cytokines [93]. It has been experimentally proven in primary cultures that soluble mediators synthesized by cardiomyocytes can regulate fibronectin synthesis [94].
Various works have studied in vitro the process of the extracellular matrix remodeling due to the presence of cytokines, mainly TGF-β, given that fibrosis is a characteristic process in Chagas chronicity. Other studies have reported that both TGF-β and IL-10 are major regulators of the Th1 response in the lapse preceding the onset of tissue damage, and decreasing the activation of phagocytic cells. On the other hand, TGF-β, IL-13, and IFN-γ are directly related to the severity of cardiac dysfunction due to degenerative fibrosis, which induces motility disorders and organ growth [94,95].
7. Conclusions
Altogether, the evasion of the immune response by T. cruzi and the proinflammatory immune response lead to autonomic heart dysfunction and microvascular disorders. They also activate autoimmune processes that result in the fibrosis that characterizes the chronic phase of the infection. These mechanisms, triggered by the presence of the parasite or its antigens, are responsible for the processes that underlie the immunopathogenesis of cardiac lesions in Chagas disease.
Author Contributions
Conceptualization and writing—original draft preparation M.C.D.A.-A., M.I.B.-T., N.R.-F. and P.M.S.-S., supervision E.Z.-G., visualization and editing image E.T.-G., editing text O.A.R.-D., M.C.-B. and Y.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially financed by UNAM, DGAPA/PAPIIT grant No. IN221121, and the Facultad de Medicina, UNAM, project numbers FM/DI/001/20, and FM/DI/078/20, FM/DI/22/17.
Acknowledgments
We wish to thank to Mauro O. Vences-Blanco, Berenice González Rete, and Juan Francisco Rodríguez for their help in final copyediting.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chagas, C. Nova Tripanozomiaze Humana: Estudos Sobre a Morfolojia e o Ciclo Evolutivo Do Schizotrypanum Cruzi n. Gen., n. Sp., Ajente Etiolojico de Nova Entidade Morbida Do Homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- World Health Organization. Asamblea Mundial de la Salud. In Enfermedad de Chagas: Control y eliminación; World Health Organization: Geneva, Swithzerand, 2009; p. 4. [Google Scholar]
- Organización Panamericana de la Salud. Enfermedad de Chagas. Available online: https://bit.ly/3HrzTwF (accessed on 6 January 2023).
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Salud Manual De Procedimientos Para La Enfermedad De Chagas En México; Secretaría de Salud: Ciudad de México, México, 2019; pp. 32–33.
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Global Heart 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Schettino, P.M.; Bucio Torres, M.I.; Cabrera-Bravo, M.; De Alba-Alvarado, M.; Castillo-Saldaña, D.R.; Zenteno-Galindo, E.; Rojo-Medina, J.; Fernández-Santos, N.; Perera-Salazar, M.G. Enfermedad de Chagas en México. Rev. Fac. Med. UNAM 2016, 59, 6–16. [Google Scholar]
- Salazar-Schettino, P.M.; Perera, R.; Ruiz-Hernandez, A.L.; Bucio Torres, M.I.; Zamora-Gonzalez, C.; Cabrera-Bravo, M.; Harnden, A. Chagas Disease as a Cause of Symptomatic Chronic Myocardopathy in Mexican Children. Pediatr. Infect. Dis. J. 2009, 28, 1011–1013. [Google Scholar] [CrossRef]
- UNAM; OPS. WHO Manual para el Diagnostico de la Infección por Trypanosoma Cruzi; OPS/OMS: Mexico, Mexico, 2006; p. 45. [Google Scholar]
- Retyk, D.E.O. Consensus Statement on Primary and Secondary Prevention of Sudden Death. Argentine Society of Cardiology—Uruguayan Society of Cardiology (with the collaboration of the CONAREC). Rev. Argent. Cardiol. 2012, 80. [Google Scholar]
- Kidder, D.P.; Fletcher, D.P. Brazil and The Brazilians, Portrayed in Historical and Descriptive Sketches Kidder, 1st ed.; Childs & Peterson: Philadelphia, PA, USA, 1857; ISBN 13: 9781484118269. [Google Scholar]
- Salazar-Schettino, P.M.; Tay, J.; De Haro, I.; de Anzures, M.; Flores, A.G. Primer caso de megaesófago con serología positiva a Trypanosoma cruzi. Salud Pública México 1984, 26, 452–455. [Google Scholar]
- Tay, J.; Salazar-Schettino, P.M.; Ontiveros, A.; Jimenez, J. Estudio Epidemiológico de Enfermedad de Chagas en una Población de Oaxaca. Primer Caso de Megasigmoides en México. Parasitology 1985, 1, 17–24. [Google Scholar]
- Ribeiro, A.L.P.; Cavalvanti, P.S.; Lombardi, F.; Nunes, M.D.C.P.; Barros, M.V.L.; Rocha, M.O.D.C. Prognostic Value of Signal-Averaged Electrocardiogram in Chagas Disease. J. Cardiovasc. Electrophysiol. 2008, 19, 502–509. [Google Scholar] [CrossRef]
- Cardoso, C.S.; Sabino, E.C.; Oliveira, C.D.L.; de Oliveira, L.C.; Ferreira, A.M.; Cunha-Neto, E.; Bierrenbach, A.L.; Ferreira, J.E.; Haikal, D.S.; Reingold, A.L.; et al. Longitudinal Study of Patients with Chronic Chagas Cardiomyopathy in Brazil (SaMi-Trop Project): A Cohort Profile. BMJ Open 2016, 6, e011181. [Google Scholar] [CrossRef]
- Rassi, A.; Rassi, A.; Little, W.C.; Xavier, S.S.; Rassi, S.G.; Rassi, A.G.; Rassi, G.G.; Hasslocher-Moreno, A.; Sousa, A.S.; Scanavacca, M.I. Development and Validation of a Risk Score for Predicting Death in Chagas’ Heart Disease. N. Engl. J. Med. 2006, 355, 799–808. [Google Scholar] [CrossRef]
- Di Lorenzo Oliveira, C.; Nunes, M.C.P.; Colosimo, E.A.; de Lima, E.M.; Cardoso, C.S.; Ferreira, A.M.; de Oliveira, L.C.; Moreira, C.H.V.; Bierrenbach, A.L.; Haikal, D.S.; et al. Risk Score for Predicting 2-Year Mortality in Patients with Chagas Cardiomyopathy From Endemic Areas: SaMi-Trop Cohort Study. JAHA 2020, 9, e014176. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Schettino, P.M.; Majumder, S.; Kierszenbaum, F. Regulatory Effect of the Level of Free Ca2+ of the Host Cell on the Capacity of Trypanosoma Cruzi to Invade and Multiply Intracellularly. J. Parasitol. 1995, 81, 597–602. [Google Scholar] [CrossRef]
- Giordanengo, L.; Guiñazú, N.; Stempin, C.; Fretes, R.; Cerbán, F.; Gea, S. Cruzipain, a MajorTrypanosoma Cruziantigen, Conditions the Host Immune Response in Favor of Parasite. Eur. J. Immunol. 2002, 32, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Woolsey, A.M.; Sunwoo, L.; Petersen, C.A.; Brachmann, S.M.; Cantley, L.C.; Burleigh, B.A. Novel PI 3-Kinase-Dependent Mechanisms of Trypanosome Invasion and Vacuole Maturation. J. Cell Sci. 2003, 116, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Salassa, B.N.; Cueto, J.A.; Gambarte Tudela, J.; Romano, P.S. Endocytic Rabs Are Recruited to the Trypanosoma Cruzi Parasitophorous Vacuole and Contribute to the Process of Infection in Non-Professional Phagocytic Cells. Front. Cell. Infect. Microbiol. 2020, 10, 536985. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.R.L.; Hecht, M.M.; Guimaro, M.C.; Sousa, A.O.; Nitz, N. Pathogenesis of Chagas’ Disease: Parasite Persistence and Autoimmunity. Clin. Microbiol. Rev. 2011, 24, 592–630. [Google Scholar] [CrossRef]
- Mauël, J. Intracellular Survival of Protozoan Parasites with Special Reference to Leishmania Spp., Toxoplasma Gondil and Trypanosoma Cruzi. In Advances in Parasitology; Elsevier: Amsterdam, Netherlands, 1996; Volume 38, pp. 1–51. ISBN 978-0-12-031738-7. [Google Scholar]
- Ministerio del Poder Popular para la Salud., PAHO, y World Health Organization. Programa de Control de Leishmaniasis, Normas, Pautas y Procedimientos para el Diagnóstico y Control, 1st ed.; PAHO/WHO: Caracas, Venezuela, 2019; p. 58. Available online: https://iris.paho.org/bitstream/handle/10665.2/51880/9789806678095_spa.pdf?sequence=1&isAllowed=y (accessed on 10 January 2023).
- Desjardins, M.; Descoteaux, A. Inhibition of Phagolysosomal Biogenesis by the Leishmania Lipophosphoglycan. J. Exp. Med. 1997, 185, 2061–2068. [Google Scholar] [CrossRef]
- Maia, C.; Rolão, N.; Nunes, M.; Gonçalves, L.; Campino, L. Infectivity of Five Different Types of Macrophages by Leishmania Infantum. Acta Trop. 2007, 103, 150–155. [Google Scholar] [CrossRef]
- Piacenza, L.; Peluffo, G.; Alvarez, M.N.; Martínez, A.; Radi, R. Trypanosoma Cruzi Antioxidant Enzymes as Virulence Factors in Chagas Disease. Antioxid. Redox Signal. 2013, 19, 723–734. [Google Scholar] [CrossRef]
- Alvarez, M.N.; Peluffo, G.; Piacenza, L.; Radi, R. Intraphagosomal Peroxynitrite as a Macrophage-Derived Cytotoxin against Internalized Trypanosoma Cruzi. J. Biol. Chem. 2011, 286, 6627–6640. [Google Scholar] [CrossRef] [PubMed]
- De Diego, J.; Punzón, C.; Duarte, M.; Fresno, M. Alteration of Macrophage Function by a Trypanosoma Cruzi Membrane Mucin. J. Immunol. 1997, 159, 4983–4989. [Google Scholar] [CrossRef] [PubMed]
- De Lima Rivero, A.R.; Farías Tamoy, M.N.; Tortolero Leal, E.; Navarro Aguilera, M.C.; Contreras Alvarez, V.T. [Partial purification and use of Trypanosoma cruzi glycosidic fractions for Chagas disease diagnosis]. Acta Cient. Venez. 2001, 52, 235–247. [Google Scholar] [PubMed]
- Cummings, R.D.; Hokke, C.H.; Haslam, S.M. Parasitic Infections. In Essentials of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Nardy, A.F.F.R.; Freire-de-Lima, C.G.; Pérez, A.R.; Morrot, A. Role of Trypanosoma Cruzi Trans-Sialidase on the Escape from Host Immune Surveillance. Front. Microbiol. 2016, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Cestari, I.; Ramirez, M.I. Inefficient Complement System Clearance of Trypanosoma Cruzi Metacyclic Trypomastigotes Enables Resistant Strains to Invade Eukaryotic Cells. PLoS ONE 2010, 5, e9721. [Google Scholar] [CrossRef]
- Ferreira, V.; Valck, C.; Sánchez, G.; Gingras, A.; Tzima, S.; Molina, M.C.; Sim, R.; Schwaeble, W.; Ferreira, A. The Classical Activation Pathway of the Human Complement System Is Specifically Inhibited by Calreticulin from Trypanosoma Cruzi. J. Immunol. 2004, 172, 3042–3050. [Google Scholar] [CrossRef]
- Doyle, P.S.; Zhou, Y.M.; Hsieh, I.; Greenbaum, D.C.; McKerrow, J.H.; Engel, J.C. The Trypanosoma Cruzi Protease Cruzain Mediates Immune Evasion. PLoS Pathog. 2011, 7, e1002139. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Gazzinelli, R.T.; Silva, J.S. Chemokines, Inflammation and Trypanosoma Cruzi Infection. Trends Parasitol. 2002, 18, 262–265. [Google Scholar] [CrossRef]
- De Alba-Alvarado, M.; Bucio-Torres, M.I.; Zenteno, E.; Sampedro-Carrillo, E.; Hernández-Lopez, M.; Reynoso-Ducoing, O.; Torres-Gutiérrez, E.; Guevara-Gomez, Y.; Guerrero-Alquicira, R.; Cabrera-Bravo, M.; et al. Response to Infection by Trypanosoma Cruzi in a Murine Model. Front. Vet. Sci. 2020, 7, 568745. [Google Scholar] [CrossRef]
- Jones, E.M.; Colley, D.G.; Tostes, S.; Lopes, E.R.; Vnencak-Jones, C.L.; McCurley, T.L. A Trypanosoma Cruzi DNA Sequence Amplified from Inflammatory Lesions in Human Chagasic Cardiomyopathy. Trans. Assoc. Am. Physicians 1992, 105, 182–189. [Google Scholar]
- Köberle, F. Chagas’ Disease and Chagas’ Syndromes: The Pathology of American Trypanosomiasis. In Advances in Parasitology; Elsevier: Amsterdam, Netherlands, 1968; Volume 6, pp. 63–116. ISBN 978-0-12-031706-6. [Google Scholar]
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simões, M.V. Pathogenesis of Chronic Chagas Heart Disease. Circulation 2007, 115, 1109–1123. [Google Scholar] [CrossRef]
- Herrera, R.N.; Díaz, E.; Pérez-Aguilar, R.; Bianchi, J.; Berman, S.; Luciardi, H.L. Prothrombotic state in early stages of chronic Chagas’ disease. Its association with thrombotic risk factors. Arch. Cardiol. Méx. 2005, 75, 38–48. [Google Scholar]
- Teixeira, A.R.; Nascimento, R.J.; Sturm, N.R. Evolution and Pathology in Chagas Disease: A Review. Mem. Inst. Oswaldo Cruz 2006, 101, 463–491. [Google Scholar] [CrossRef]
- Higuchi, M. Pathophysiology of the Heart in Chagas’ Disease: Current Status and New Developments. Cardiovasc. Res. 2003, 60, 96–107. [Google Scholar] [CrossRef]
- Rochitte, C.E.; Oliveira, P.F.; Andrade, J.M.; Ianni, B.M.; Parga, J.R.; Ávila, L.F.; Kalil-Filho, R.; Mady, C.; Meneghetti, J.C.; Lima, J.A.C.; et al. Myocardial Delayed Enhancement by Magnetic Resonance Imaging in Patients with Chagas’ Disease. J. Am. Coll. Cardiol. 2005, 46, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.d.L.; Fukasawa, S.; De Brito, T.; Parzianello, L.C.; Bellotti, G.; Ramires, J.A.F. Different Microcirculatory and Interstitial Matrix Patterns in Idiopathic Dilated Cardiomyopathy and Chagas’ Disease: A Three Dimensional Confocal Microscopy Study. Heart 1999, 82, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Rochitte, C.E.; Nacif, M.S.; de Oliveira Júnior, A.C.; Siqueira-Batista, R.; Marchiori, E.; Uellendahl, M.; de Lourdes Higuchi, M. Cardiac Magnetic Resonance in Chagas’ Disease. Artif. Organs 2007, 31, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, F.R.S.; Guedes, P.M.M.; Gazzinelli, R.T.; Silva, J.S. The Role of Parasite Persistence in Pathogenesis of Chagas Heart Disease: Parasite Persistence in Chagas Heart Disease. Parasite Immunol. 2009, 31, 673–685. [Google Scholar] [CrossRef]
- Kierszenbaum, F. Views on the Autoimmunity Hypothesis for Chagas Disease Pathogenesis. FEMS Immunol. Med. Microbiol. 2003, 37, 1–11. [Google Scholar] [CrossRef]
- Cunha-Neto, E.; Teixeira, P.C.; Nogueira, L.G.; Kalil, J. Autoimmunity. In Advances in Parasitology; Elsevier: Amsterdam, Netherlands, 2011; Volume 76, pp. 129–152. ISBN 978-0-12-385895-5. [Google Scholar]
- De Bona, E.; Lidani, K.C.F.; Bavia, L.; Omidian, Z.; Gremski, L.H.; Sandri, T.L.; Messias Reason, I.J. de Autoimmunity in Chronic Chagas Disease: A Road of Multiple Pathways to Cardiomyopathy? Front. Immunol. 2018, 9, 1842. [Google Scholar] [CrossRef]
- Cunha-Neto, E.; Coelho, V.; Guilherme, L.; Fiorelli, A.; Stolf, N.; Kalil, J. Autoimmunity in Chagas’ Disease. Identification of Cardiac Myosin-B13 Trypanosoma Cruzi Protein Crossreactive T Cell Clones in Heart Lesions of a Chronic Chagas’ Cardiomyopathy Patient. J. Clin. Invest. 1996, 98, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Sterin-Borda, L.; Cantore, M.; Pascual, J.; Borda, E.; Cossio, P.; Arana, R.; Passeron, S. Chagasic IgG Binds and Interacts with Cardiac Beta Adrenoceptor-Coupled Adenylate Cyclase System. Int. J. Immunopharmacol. 1986, 8, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, I.; Levin, M.J.; Wallukat, G.; Elies, R.; Lebesgue, D.; Chiale, P.; Elizari, M.; Rosenbaum, M.; Hoebeke, J. Molecular Mimicry between the Immunodominant Ribosomal Protein P0 of Trypanosoma Cruzi and a Functional Epitope on the Human Beta 1-Adrenergic Receptor. J. Exp. Med. 1995, 182, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bergami, P.L.; Meckert, P.C.; Kaplan, D.; Levitus, G.; Elias, F.; Quintana, F.; Van Regenmortel, M.; Laguens, R.; Levin, M.J. Immunization with Recombinant Trypanosoma Cruzi Ribosomal P2β Protein Induces Changes in the Electrocardiogram of Immunized Mice. FEMS Immunol. Med. Microbiol. 1997, 18, 75–85. [Google Scholar] [CrossRef]
- Oya Masuda, M.; Levin, M.; Farias De Oliveira, S.; Dos Santos Costa, P.C.; Lopez Bergami, P.; Dos Santos Almeida, N.A.; Pedrosa, R.C.; Ferrari, I.; Hoebeke, J.; Campos De Carvalho, A.C. Functionally Active Cardiac Antibodies in Chronic Chagas’ Disease Are Specifically Blocked by Trypanosoma Cruzi Antigens. FASEB J. 1998, 12, 1551–1558. [Google Scholar] [CrossRef]
- Mahler, E.; Sepulveda, P.; Jeannequin, O.; Liegeard, P.; Gounon, P.; Wallukat, G.; Eftekhari, P.; Levin, M.J.; Hoebeke, J.; Hontebeyrie, M. A Monoclonal Antibody against the Immunodominant Epitope of the Ribosomal P2β Protein OfTrypanosoma Cruzi Interacts with the Human β 1-Adrenergic Receptor. Eur. J. Immunol. 2001, 31, 2210–2216. [Google Scholar] [CrossRef]
- Zwirner, N.W.; Malchiodi, E.L.; Chiaramonte, M.G.; Fossati, C.A. A Lytic Monoclonal Antibody to Trypanosoma Cruzi Bloodstream Trypomastigotes Which Recognizes an Epitope Expressed in Tissues Affected in Chagas’ Disease. Infect. Immun. 1994, 62, 2483–2489. [Google Scholar] [CrossRef]
- Motrán, C.C.; Cerbán, F.M.; Rivarola, W.; Iosa, D.; de Cima, E.V. Trypanosoma Cruzi: Immune Response and Functional Heart Damage Induced in Mice by the Main Linear B-Cell Epitope of Parasite Ribosomal P Proteins. Exp. Parasitol. 1998, 88, 223–230. [Google Scholar] [CrossRef]
- Al-Sabbagh, A.; Garcia, C.A.A.C.; Diaz-Bardales, B.M.; Zaccarias, C.; Sakurada, J.K.; Santos, L.M.B. Evidence for Cross-Reactivity between Antigen Derived FromTrypanosoma Cruziand Myelin Basic Protein in Experimental Chagas Disease. Exp. Parasitol. 1998, 89, 304–311. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Bijovsky, A.T.; Carvalho, T.U.; Souza, W.; Alves, M.M.; Colli, W. A Monoclonal Antibody to Trypanosoma Cruzi Trypomastigotes Recognizes a Myosin Tail Epitope. Parasitol. Res. 2001, 87, 1043–1049. [Google Scholar] [CrossRef]
- Gironès, N.; Rodríguez, C.I.; Basso, B.; Bellon, J.M.; Resino, S.; Muñoz-Fernández, M.A.; Gea, S.; Moretti, E.; Fresno, M. Antibodies to an Epitope from the Cha Human Autoantigen Are Markers of Chagas’ Disease. Clin. Diagn. Lab. Immunol. 2001, 8, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Barcellos, L.; Gimenez, L.; Cabarcas, R.; Garcia, S.; Pedrosa, R.; Nascimento, J.; Kurtenbach, E.; Masuda, M.; Camposdecarvalho, A. Human Chagasic IgGs Bind to Cardiac Muscarinic Receptors and Impair L-Type Ca Currents. Cardiovasc. Res. 2003, 58, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.H.; López, N.C.; Ramírez, G.A.; Valck, C.E.; Molina, M.C.; Aguilar, L.; Rodríguez, M.; Maldonado, I.; Martínez, R.; González, C.; et al. Trypanosoma Cruzi Calreticulin: A Possible Role in Chagas’ Disease Autoimmunity. Mol. Immunol. 2009, 46, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Gironès, N.; Rodríguez, C.I.; Carrasco-Marín, E.; Hernáez, R.F.; de Rego, J.L.; Fresno, M. Dominant T- and B-Cell Epitopes in an Autoantigen Linked to Chagas’ Disease. J. Clin. Invest. 2001, 107, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-dos-Santos, R.; Pirmez, C.; Savino, W. Role of Autoreactive Immunological Mechanisms in Chagasic Carditis. Res. Immunol. 1991, 142, 134–137. [Google Scholar] [CrossRef]
- Hontebeyrie-Joskowicz, M.; Said, G.; Milon, G.; Marchal, G.; Eisen, H. L3T4+ T Cells Able to Mediate Parasite-Specific Delayed-Type Hypersensitivity Play a Role in the Pathology of Experimental Chagas’ Disease. Eur. J. Immunol. 1987, 17, 1027–1033. [Google Scholar] [CrossRef]
- Laguens, R.P.; Meckert, P.C.; Chambo, G.; Gelpi, R.J. Chronic Chagas Disease in the Mouse. II. Transfer of the Heart Disease by Means of Immunocompetent Cells. Medicina 1981, 41, 40–43. [Google Scholar]
- dos Santos, R.R.; Rossi, M.A.; Laus, J.L.; Silva, J.S.; Savino, W.; Mengel, J. Anti-CD4 Abrogates Rejection and Reestablishes Long-Term Tolerance to Syngeneic Newborn Hearts Grafted in Mice Chronically Infected with Trypanosoma Cruzi. J. Exp. Med. 1992, 175, 29–39. [Google Scholar] [CrossRef]
- Silva-Barbosa, S.D.; Cotta-de-Almeida, V.; Riederer, I.; De Meis, J.; Dardenne, M.; Bonomo, A.; Savino, W. Involvement of Laminin and Its Receptor in Abrogation of Heart Graft Rejection by Autoreactive T Cells from Trypanosoma Cruzi-Infected Mice. J. Immunol. 1997, 159, 997–1003. [Google Scholar] [CrossRef]
- Leon, J.S.; Godsel, L.M.; Wang, K.; Engman, D.M. Cardiac Myosin Autoimmunity in Acute Chagas’ Heart Disease. Infect. Immun. 2001, 69, 5643–5649. [Google Scholar] [CrossRef]
- Leon, J.S.; Wang, K.; Engman, D.M. Myosin Autoimmunity Is Not Essential for Cardiac Inflammation in Acute Chagas’ Disease. J. Immunol. 2003, 171, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Pontes-De-Carvalho, L.; Santana, C.C.; Soares, M.B.P.; Oliveira, G.G.S.; Cunha-Neto, E.; Ribeiro-Dos-Santos, R. Experimental Chronic Chagas’ Disease Myocarditis Is an Autoimmune Disease Preventable by Induction of Immunological Tolerance to Myocardial Antigens. J. Autoimmun. 2002, 18, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Morrot, A. The Development of Unconventional Extrathymic Activated CD4 + CD8 + T Cells in Chagas Disease. ISRN Infect. Dis. 2013, 2013, 801975. [Google Scholar] [CrossRef]
- Camargo, M.M.; Andrade, A.C.; Almeida, I.C.; Travassos, L.R.; Gazzinelli, R.T. Glycoconjugates Isolated from Trypanosoma Cruzi but Not from Leishmania Species Membranes Trigger Nitric Oxide Synthesis as Well as Microbicidal Activity in IFN-Gamma-Primed Macrophages. J. Immunol. 1997, 159, 6131–6139. [Google Scholar] [CrossRef]
- Verdot, L.; Lalmanach, G.; Vercruysse, V.; Hartmann, S.; Lucius, R.; Hoebeke, J.; Gauthier, F.; Vray, B. Cystatins Up-Regulate Nitric Oxide Release from Interferon-γ- Activated Mouse Peritoneal Macrophages. J. Biol. Chem. 1996, 271, 28077–28081. [Google Scholar] [CrossRef]
- Cardoni, R.L.; Antúnez, M.I.; Abrami, A.A. TH1 response in the experimental infection with Trypanosoma cruzi. Medicina 1999, 59, 84–90. [Google Scholar]
- Guiñazú, N.; Pellegrini, A.; Carrera-Silva, E.A.; Aoki, M.P.; Cabanillas, A.M.; Gìronés, N.; Fresno, M.; Cano, R.; Gea, S. Immunisation with a Major Trypanosoma Cruzi Antigen Promotes Pro-Inflammatory Cytokines, Nitric Oxide Production and Increases TLR2 Expression. Int. J. Parasitol. 2007, 37, 1243–1254. [Google Scholar] [CrossRef]
- de Oliveira, T.B.; Pedrosa, R.C.; Filho, D.W. Oxidative Stress in Chronic Cardiopathy Associated with Chagas Disease. Int. J. Cardiol. 2007, 116, 357–363. [Google Scholar] [CrossRef]
- Gupta, S.; Wen, J.-J.; Garg, N.J. Oxidative Stress in Chagas Disease. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 190354. [Google Scholar] [CrossRef]
- Marchant, D.J.; Boyd, J.H.; Lin, D.C.; Granville, D.J.; Garmaroudi, F.S.; McManus, B.M. Inflammation in Myocardial Diseases. Circ. Res. 2012, 110, 126–144. [Google Scholar] [CrossRef]
- Carvalho, C.M.E.; Silverio, J.C.; da Silva, A.A.; Pereira, I.R.; Coelho, J.M.C.; Britto, C.C.; Moreira, O.C.; Marchevsky, R.S.; Xavier, S.S.; Gazzinelli, R.T.; et al. Inducible Nitric Oxide Synthase in Heart Tissue and Nitric Oxide in Serum of Trypanosoma Cruzi-Infected Rhesus Monkeys: Association with Heart Injury. PLoS Negl. Trop. Dis. 2012, 6, e1644. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.M.; Bustamante, J.M.; Tarleton, R.L. CD8+ T Cells in Trypanosoma Cruzi Infection. Curr. Opin. Immunol. 2009, 21, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Dutra, W.O.; Menezes, C.A.S.; Villani, F.N.A.; da Costa, G.C.; da Silveira, A.B.M.; Reis, D. d’Ávila; Gollob, K.J. Cellular and Genetic Mechanisms Involved in the Generation of Protective and Pathogenic Immune Responses in Human Chagas Disease. Mem. Inst. Oswaldo Cruz 2009, 104, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Silverio, J.C.; de-Oliveira-Pinto, L.M.; da Silva, A.A.; de Oliveira, G.M.; Lannes-Vieira, J. Perforin-Expressing Cytotoxic Cells Contribute to Chronic Cardiomyopathy in Trypanosoma Cruzi Infection. Int. J. Exp. Pathol. 2010, 91, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A.C. Assessment of Myocardial Fibrosis With Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Viotti, R.; Vigliano, C. Enfermedad de Chagas, un Enfoque Práctico Basado en la Investigación Médica; Panamericana: Buenos Aires, Argentina, 2015; pp. 187–208. ISBN 978-950-06-0557-1. [Google Scholar]
- Salazar-Schettino, P.M.; Cabrera-Bravo, M.; Vazquez-Antona, C.; Zenteno, E.; Alba-Alvarado, M.D.; Gutierrez, E.T.; Gomez, Y.G.; Perera-Salazar, M.G.; de la Torre, G.G.; Bucio-Torres, M.I. Chagas Disease in Mexico: Report of 14 Cases of Chagasic Cardiomyopathy in Children. Tohoku J. Exp. Med. 2016, 240, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac Fibroblasts, Fibrosis and Extracellular Matrix Remodeling in Heart Disease. Fibrogenesis Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.M.; Meuser, M.; Almeida, D.; Meirelles, M.N.L.; Pereira, M.C.S. Trypanosoma Cruzi–Cardiomyocyte Interaction: Role of Fibronectin in the Recognition Process and Extracellular Matrix Expression in Vitro and in Vivo. Exp. Parasitol. 2004, 107, 20–30. [Google Scholar] [CrossRef]
- Higuchi, M.D.L.; Morais, C.F.D.; Sambiase, N.V.; Pereira-Barretto, A.C.; Bellotti, G.; Pileggi, F. Histopathological Criteria of Myocarditis. A Study Based on Normal Heart, Chagasic Heart and Dilated Cardiomyopathy. Jpn. Circ. J. 1990, 54, 391–400. [Google Scholar] [CrossRef]
- Marino, A.P.M.P.; Silva, A.A.; Pinho, R.T.; Lannes-Vieira, J. Trypanosoma Cruzi Infection: A Continuous Invader-Host Cell Cross Talk with Participation of Extracellular Matrix and Adhesion and Chemoattractant Molecules. Braz. J. Med. Biol. Res. 2003, 36, 1121–1133. [Google Scholar] [CrossRef]
- Talvani, A.; Ribeiro, C.S.; Aliberti, J.C.S.; Michailowsky, V.; Santos, P.V.A.; Murta, S.M.F.; Romanha, A.J.; Almeida, I.C.; Farber, J.; Lannes-Vieira, J.; et al. Kinetics of Cytokine Gene Expression in Experimental Chagasic Cardiomyopathy: Tissue Parasitism and Endogenous IFN-γ as Important Determinants of Chemokine MRNA Expression during Infection with Trypanosoma Cruzi. Microbes Infect. 2000, 2, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.S.; Martins, G.A.; Aliberti, J.C.S.; Mestriner, F.L.A.C.; Cunha, F.Q.; Silva, J.S. Trypanosoma Cruzi—Infected Cardiomyocytes Produce Chemokines and Cytokines That Trigger Potent Nitric Oxide–Dependent Trypanocidal Activity. Circulation 2000, 102, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Jorge, T.C.; Waghabi, M.C.; Soeiro, M.; Keramidas, M.; Bailly, S.; Feige, J.-J. Pivotal Role for TGF-β in Infectious Heart Disease: The Case of Trypanosoma Cruzi Infection and Consequent Chagasic Myocardiopathy. Cytokine Growth Factor Rev. 2008, 19, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Abel, L.C.J.; Rizzo, L.V.; Ianni, B.; Albuquerque, F.; Bacal, F.; Carrara, D.; Bocchi, E.A.; Teixeira, H.C.; Mady, C.; Kalil, J.; et al. Chronic Chagas’ Disease Cardiomyopathy Patients Display an Increased IFN-γ Response to Trypanosoma Cruzi Infection. J. Autoimmun. 2001, 17, 99–107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).