Human Papillomavirus Genotypes Infecting the Anal Canal and Cervix in HIV+ Men and Women, Anal Cytology, and Risk Factors for Anal Infection
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Description of the Studied Population
3.2. HPV Detection and Typing in Anal Canal and Cervix
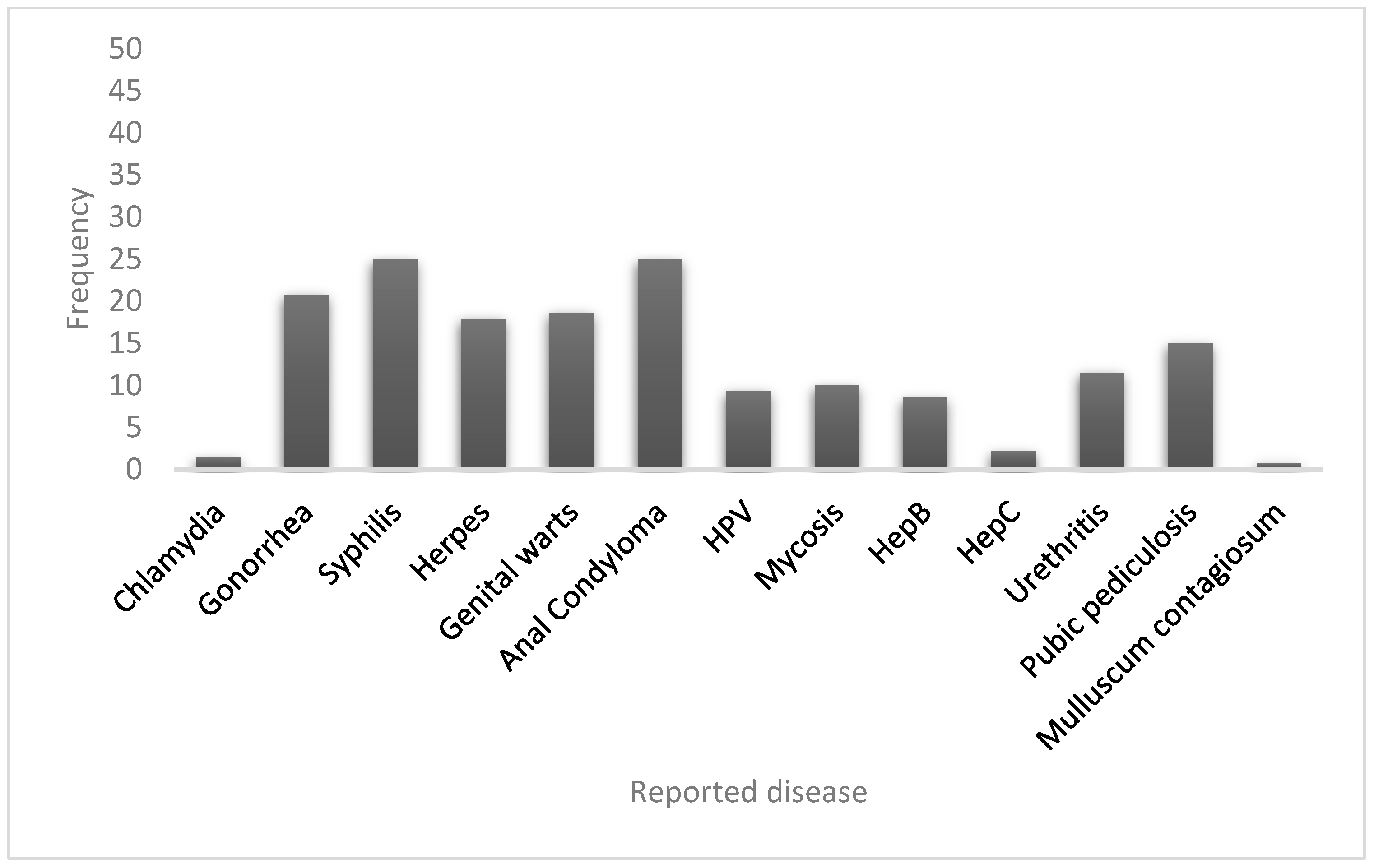
3.3. Anal Cytology
3.4. Epidemiological Determinants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Palefsky, J.M. HPV-associated anal and cervical cancers in HIV-infected individuals: Incidence and prevention in the antiretroviral therapy era. Curr. Opin. HIV AIDS 2017, 12, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Brogden, D.R.; Lupi, M.E.; Warren, O.J.; Kontovounisios, C.; Mills, S.C. Comparing and contrasting clinical consensus and guidelines for anal intraepithelial neoplasia in different geographical regions. Updates Surg. 2021, 73, 2047–2058. [Google Scholar] [CrossRef]
- Palefsky, J.M. Screening to prevent anal cancer: Current thinking and future directions. Cancer Cytopathol. 2015, 123, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Darragh, T.M.; Winkler, B. Anal cancer and cervical cancer screening: Key differences. Cancer Cytopathol. 2011, 119, 5–19. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Peyton, C.L.; Alessi, T.Q.; Wheller, C.M.; Coutleé, F.; Hildesheim, A.; Schiffman, M.H.; Scott, D.R.; Aplle, R.J. Improved Amplification of Genital Human Papillomaviruses. J. Clin. Microbiol. 2000, 38, 357–361. [Google Scholar] [CrossRef]
- Sotlar, K.; Dieme, D.; Dethleffs, A.; Hack, Y.; Stubner, A.; Vollmer, N.; Menton, S.; Menton, M.; Dietz, K.; Wallwiener, D.; et al. Detection and Typing of Human Papillomavirus by E6 Nested PCR. J. Clin. Microbiol. 2004, 42, 3176–3184. [Google Scholar] [CrossRef]
- Centro Nacional Para la Prevención y Control del VIH y el Sida. Available online: https://www.gob.mx/cms/uploads/attachment/file/533424/RN_4o_Trim_2019.pdf (accessed on 30 May 2022).
- Ogale, Y.; Yeh, P.T.; Kennedy, C.E.; Toskin, I.; Narasimhan, M. Self-collection of samples as an additional approach to deliver testing services for sexually transmitted infections: A systematic review and meta-analysis. BMJ Glob. Health 2019, 4, e001349. [Google Scholar] [CrossRef]
- Yared, N.; Horvath, K.; Fashanu, O.; Zhao, R.; Baker, J.; Kulasingam, S. Optimizing Screening for Sexually Transmitted Infections in Men Using Self-Collected Swabs: A Systematic Review. Sex. Transm. Dis. 2018, 45, 294–300. [Google Scholar] [CrossRef]
- Tamalet, C.; Ravaux, I.; Dhiver, C.; Menard, A.; Colson, P.; Stein, A. Feasibility and acceptability of anal self-sampling for Human Papillomavirus Screening in HIV-Infected Patients. Intervirology 2016, 59, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef]
- Villanueva, P.; Diaz, P.; Guido, M.; Rangel, A.; Sotelo, R.; García, R.; Carranca, A. Prevalencia de virus de papiloma humano de alto riesgo en el epitelio anal de hombres VIH positivos. SMB 2002, 27, 94–102. [Google Scholar]
- Hinojos, D.A.; Palma, L.E.; Moreno, V.; Licón, L.; Lora, N.A.; Carrera, N.N.; Santana-Rodríguez, V.M.; Duque, J.; Leal, I. Prevalencia de tipos de virus del papiloma humano en hombres que tienen sexo con hombres, en Chihuahua, México. Acta Univ. 2016, 26, 62–69. [Google Scholar] [CrossRef]
- Sánchez-Aleman, M.; Miranda, L.C.; Medina, C.V.; Vargas, G.; González, A.; Conde, C.J. Concordancia de VPH oncogénico en regiones anal y genital en una muestra de hombres que viven con VIH. Enferm. Infecc. Microbiol. 2012, 32, 139–144. [Google Scholar]
- Torres-Ibarra, L.; Conde-Glez, C.J.; Salmerón, J.; Palefsky, J.; Hernández-Nevares, P.; Sánchez-Alemán, M.A.; Magis, C.L.; Lazcano, E.C. Risk factors for anal HPV-16/18 infection in Mexican HIV-infected men who have sex with men. Prev. Med. 2014, 69, 157–164. [Google Scholar] [CrossRef]
- Szabó, E.; Kósa, C.; Babarczi, E.; Sulyok, M.; Ujhelyi, E.; Bánhegyi, B.; Nagy, I.V. Prevalence of Anal Human Papillomavirus Infection in Hungarian Men Who Have Sex with Men. Pathol. Oncol. Res. 2017, 24, 671–677. [Google Scholar] [CrossRef]
- Rovelli, C.; Poli, A.; Galli, L.; Cernuschi, M.; Tamburini, A.M.; Racca, S.; Tambussi, G.; Rolla, S.; Albarello, L.; Rosati, R.; et al. Presence of multiple genotypes in subjects with HPV-16 infection is highly associated with anal squamous intraepithelial lesions in HIV-1 infected males. PLoS ONE 2017, 12, e0186367. [Google Scholar] [CrossRef] [PubMed]
- Machalek, D.A.; Poynten, M.; Jin, F.; Fairley, C.K.; Farnsworth, A.; Garland, S.M.; Hillman, R.J.; Petoumenos, K.; Roberts, J.; Tabrizi, S.N.; et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: A systematic review and meta-analysis. Lancet Oncol. 2012, 13, 487–500. [Google Scholar] [CrossRef]
- Wang, C.C.J.; Sparano, J.; Palefsky, J.M. Human Immunodeficiency Virus/AIDS, Human Papillomavirus, and Anal Cancer. Surg. Oncol. Clin. 2017, 26, 17–31. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lensing, S.Y.; Berry-Lawhorn, J.M.; Jay, N.; Darragh, T.M.; Goldstone, S.E.; Wilkin, T.J.; Stier, E.A.; Einstein, M.; Pugliese, J.C.; et al. Design of the Anal Cancer/HSIL Outcomes Research study (ANCHOR study): A randomized study to prevent anal cancer among persons living with HIV. Contemp. Clin. Trials 2022, 113, 106679. [Google Scholar] [CrossRef]
- Roberts, J.M.; Poynten, I.M.; Molano, M.; Machalek, D.A.; Hillman, R.J.; Guzman, P.; Jin, F.; Templeton, D.J.; Fairley, C.K.; Law, C.; et al. Human Papillomavirus Genotypes in Anal High-Grade Squamous Intraepithelial Lesion (HSIL): Anal Intraepithelial Neoplasia Grades 2 (AIN2) and 3 (AIN3) Are DifferentHPV Genotypes in Subcategories of Anal HSIL. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2078–2083. [Google Scholar] [CrossRef]
- González-Hernández, L.A.; Flores-Miramontes, M.G.; Aguilar-Lemarroy, A.; Quintanilla-Peña, K.S.; Martin-Amaya- Barajas, F.L.; Ramos-Solano, M.; Enciso-Gómez, L.F.; Andrade-Villanueva, J.F.; Jave-Suárez, L.F. HPV genotypes detected by linear array and next-generation sequencing in anal samples from HIV positive men who have sex with men in Mexico. Arch. Virol. 2018, 163, 925–935. [Google Scholar] [CrossRef] [PubMed]
- van Heukelom, M.S.; Richel, O.; de Vries, H.; van de Sandt, M.; Beck, S.; Munckhof, H.V.D.; Pirog, E.; de Koning, M.; Prins, J.; Quint, K. Low- and high-risk human papillomavirus genotype infectionsin intra-anal warts in HIV-positive men who have sex with men. Br. J. Dermatol. 2016, 175, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Dube Mandishora, R.S.; Rounge, T.B.; Fitzpatrick, M.; Christiansen, I.K.; Ambur, O.H.; Lagström, S.; Stray-Pedersen, B.; Tommasino, M.; Palefsky, J.; Chirenje, Z.M. Self-collected and clinician-collected anal swabs show modest agreement for HPV genotyping. PLoS ONE 2021, 16, e0250426. [Google Scholar] [CrossRef]
- Kojic, E.M.; Conley, L.; Bush, T.; Cu-Uvin, S.; Unger, E.R.; Henry, K.; Hammer, J.; Escota, G.; Darragh, T.M.; Palefsky, J.M.; et al. Prevalence and Incidence of Anal and Cervical High-Risk Human Papillomavirus (HPV) Types Covered by Current HPV Vaccines Among HIV-Infected Women in the SUN Study. J. Infect. Dis. 2018, 217, 1544–1552. [Google Scholar] [CrossRef]
- Castro, J.V.; Hernández, C.; Madrid, V. La anticoncepción hormonal como factor de riesgo para cáncer cervicouterino: Evidencias biológicas, inmunológicas y epidemiológicas. Ginecol. Obs. Mex. 2011, 79, 533–539. [Google Scholar]
- Gonzalez-Losa, M.R.; Puerto-Solís, M.; Ayora-Talavera, G.; Gómez-Carballo, J.; Euán-López, A.; Cisneros-Cutz, J.I.; Rosado-López, A.; Echeverría Salazar, J.; Conde-Ferráez, L. Prevalence of anal infection due to high-risk human papillomavirus and analysis of E2 gene integrity among women with cervical abnormalities. Enfermedades Infecciosas y Microbiología Clínica, Enfermedades infecciosas y microbiologia clínica. Enferm. Infecc. Microbiol. Clin. 2018, 36, 209–213. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Ramírez-Taboada, J.; Gil-Anguita, C.; Esquivias, J.; Omar-Mohamed-Balgahata, M.; Sam Pedro, A.; Lopez-Ruz, M.; Pasquau, J. Safety and immunogenicity of the quadrivalent human papillomavirus (qHPV) vaccine in HIV-positive Spanish men who have sex with men (MSM). AIDS Res. Ther. 2017, 14, 34. [Google Scholar] [CrossRef]
- Venezuela, F.; Kremer, L.E.; Kiguen, X.; Cuffin, C. HPV Detection and genotyping in males from the city of Córdoba, Argentina. Rev. Argent. Microbiol. 2010, 42, 184–188. [Google Scholar]
- Kelly, H.; Weiss, H.A.; Benavente, Y.; de Sanjose, S.; Mayaud, P.; ART and HPV Review Group. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. Lancet HIV 2018, 5, e45–e58. [Google Scholar] [CrossRef]
- Gonçalves, J.C.N.; Macedo, A.C.L.; Madeira, K.; Bavaresco, D.V.; Dondossola, E.R.; Grande, A.J.; da Rosa, M.I. Accuracy of Anal Cytology for Diagnostic of Precursor Lesions of Anal Cancer: Systematic Review and Meta-analysis. Dis. Colon Rectum 2019, 62, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Centro Nacional para la Prevención y Control del VIH y el Sida (CENSIDA). Available online: https://www.gob.mx/censida/documentos/guia-de-manejo-antirretroviral-de-las-personas-con-vih-mexico-2021-297710?idiom=es (accessed on 30 May 2022).
- Hillman, R.J.; Cuming, T.; Darragh, T.; Nathan, M.; Berry-Lawthorn, M.; Goldstone, S.; Bouchard, C. IANS international guidelines for practice standards in the detection of anal cancer precursors. J. Low. Genit. Tract Dis. 2016, 20, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Cuming, T.; Nathan, M. Anal cancer screening: Techniques and guidelines. Semin. Colon Rectal Surg. 2017, 28, 69–74. [Google Scholar] [CrossRef]
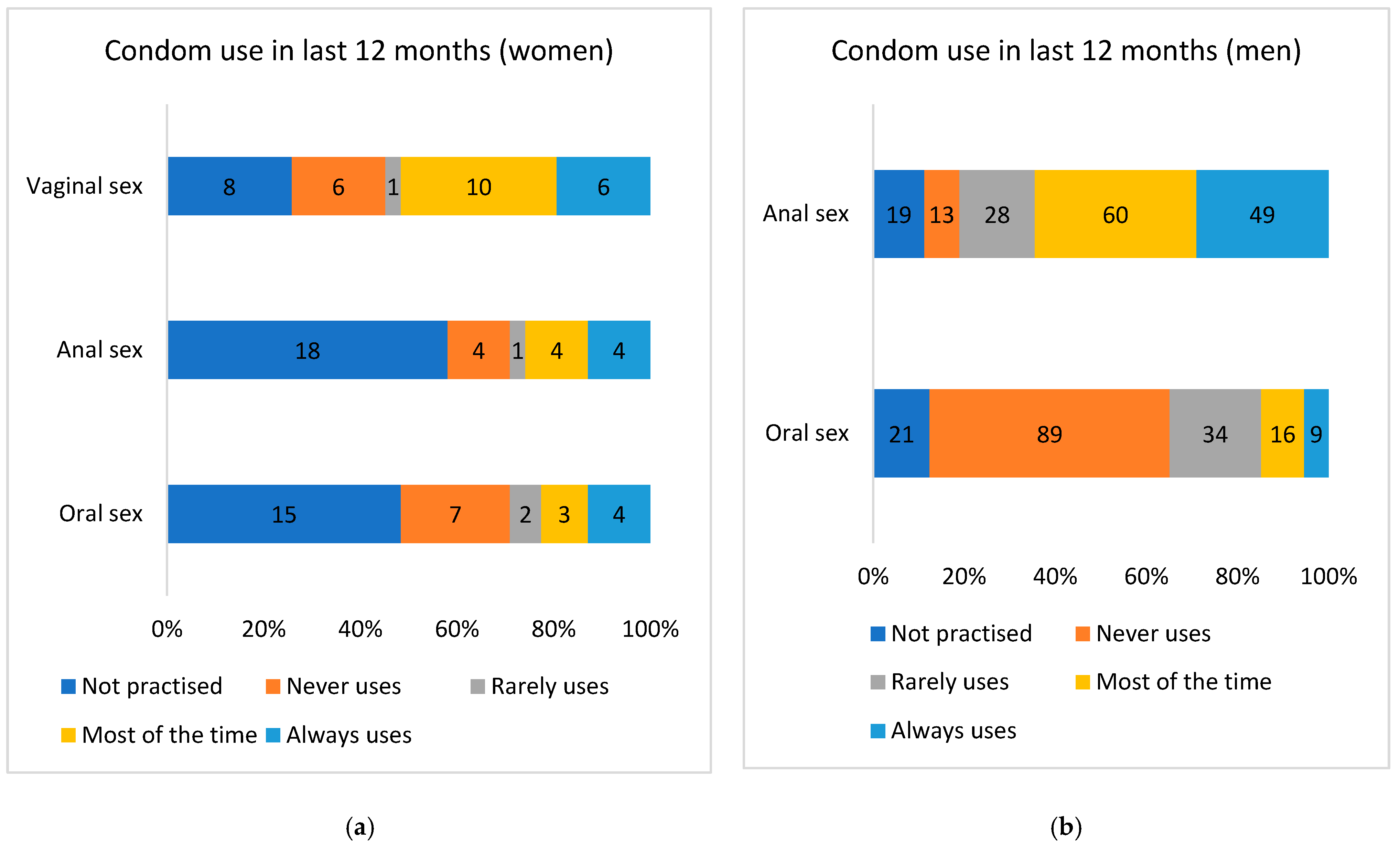
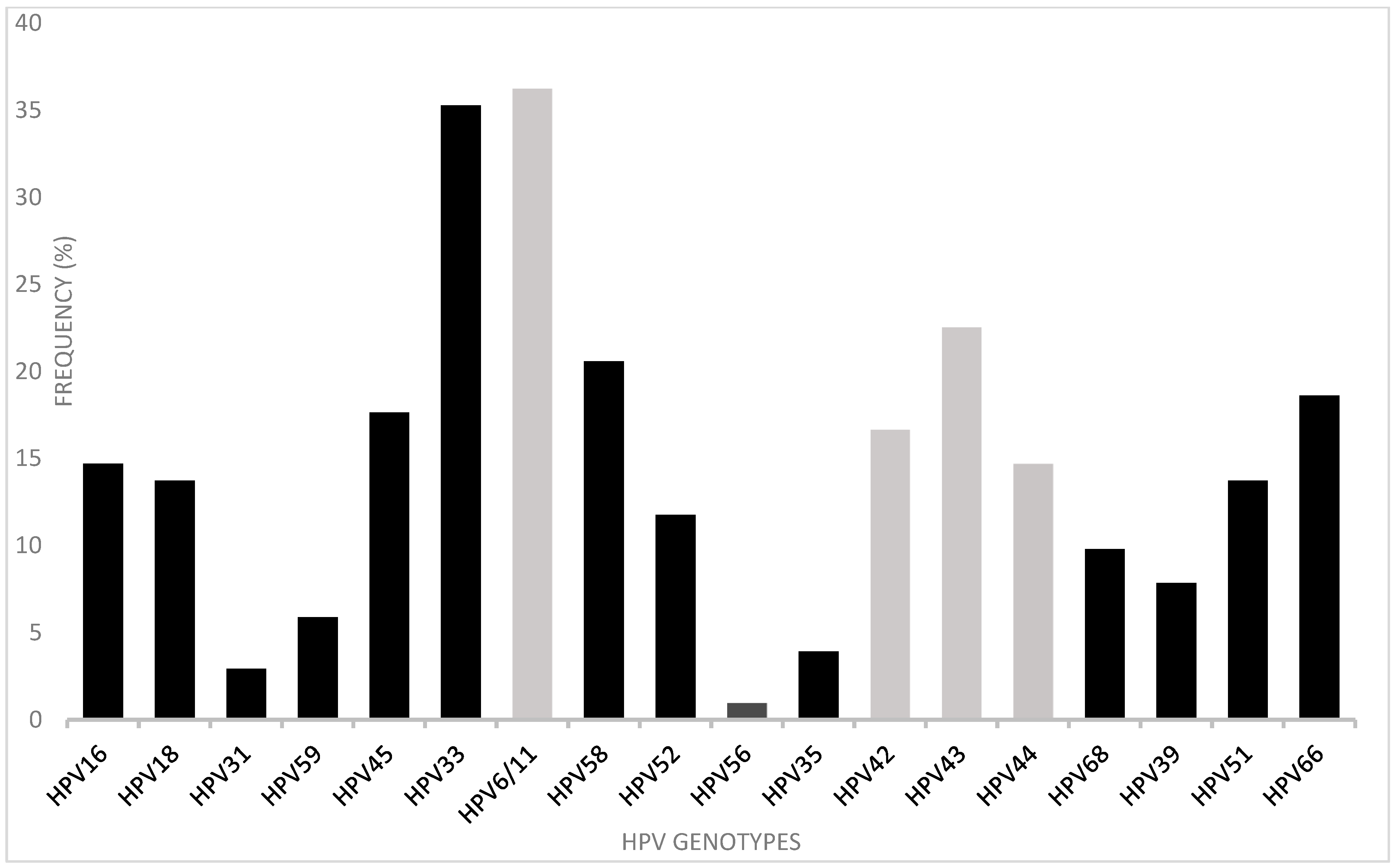
| Participant Code | Cervical HPV | Anal HPV | Cervical Genotypes | Anal Genotypes | Genotype Agreement * |
|---|---|---|---|---|---|
| VVH11 | + | + | 16, 59, 52, 33 | 16, 6/11, 66, 68 | Yes |
| VVH22 | + | + | 18, 59, 6/11, 44 | 16, 6/11 | Yes |
| VVH42 | + | + | 58, 44, 42 | 33, 42, 43, 44 | Yes |
| VVH50 | + | − | 16 | N/A | N/A |
| VVH51 | + | + | 16 | 16, 44, 68 | Yes |
| VVH54 | + | − | 18, 45, 33, 58 | N/A | N/A |
| VVH77 | + | − | 58, 44, 42 | N/A | N/A |
| VVH114 | + | + | 33, 35, 39 | 33, 56, 66 | Yes |
| VVH116 | − | + | N/A | 58 | N/A |
| VVH117 | + | + | 43, 68 | 18, 43 | Yes |
| VVH118 | + | − | 18, 56, 42, 39 | N/A | N/A |
| VVH125 | + | + | 6/11, 42, 51 | 6/11, 51 | Yes |
| VVH146 | + | − | 45, 44 | N/A | N/A |
| VVH147 | + | + | 44, 66, 68 | 45 | No |
| VVH150 | + | + | 45, 56, 51 | 56, 51 | Yes |
| VVH156 | + | − | 45, 6/11 | N/A | N/A |
| VVH160 | + | − | 6/11, 44 | N/A | N/A |
| VVH183 | inadequate | + | N/A | 33, 42, 44 | N/A |
| VVH187 | + | + | 52, 66 | 45, 52 | Yes |
| VVH188 | + | − | 6/11 | N/A | N/A |
| Anal Cytology Result | Pap Smear | Liquid-Based |
|---|---|---|
| LSIL, HPV infection | 29 (25%) | 33 (28.4%) |
| HSIL, cancer in situ | 4 (3.4%) | 3 (2.6%) |
| Inflammatory changes | 40 (34.5%) | 34 (29.3%) |
| Without abnormalities | 41 (35.3%) | 29 (25.0%) |
| Result not available | 2 (1.7%) | 17 (14.7%) |
| Total | 116 | 116 |
| Variables | HPV+ | HPV− | OR (CI) | p Value (X2) |
|---|---|---|---|---|
| Age at sexual debut | ||||
| ≤16 years | 62/116 (53%) | 43/78 (55%) | 0.934 (0.525–1.663) | 0.817 |
| >16 years | 54/116 (47%) | 35/78 (45%) | 1 | |
| Total | 116 | 78 | ||
| Sexual partners (anoreceptive intercourse, last 12 months) | ||||
| ≥10 | 13/116 (10.3%) | 42/78 (53.9%) | 0.43 (0.19–0.99) | 0.047 |
| 1–10 | 82/116 (70.7%) | 5/78 (6.4%) | 23 (8.04–66.39) | <0.001 |
| None | 22/116 (19%) | 31/78 (39.7%) | 1 | |
| Total | 116 | 78 | ||
| Consistent condom use (anoreceptive intercourse, last 12 months) | ||||
| No | 58/94 (61.7%) | 9/55 (16.4%) | 8.23 (3.60–18.82) | <0.001 |
| Yes | 36/94 (38.3%) | 46/55 (83.6%) | 1 | |
| Total (35 not applied) | 94 | 55 | ||
| HIV viral RNA load | ||||
| ≥50 cc/mL | 31/93 (33.33%) | 9/64 (14%) | 3.05 (1.33–6.98) | 0.006 |
| <50 cc/mL | 62/93 (66.66%) | 55/64 (85.9%) | 1 | |
| Total (43 missing data) | 93 | 64 | ||
| CD4+ lymphocyte count | ||||
| CD4 < 200 cells/mm3 | 23/93 (24.7%) | 10/64 (15.6%) | 1.77 (0.78–4.04) | 0.168 |
| CD4 > 200 cells/mm3 | 70/93 (75.3%) | 54/64 (84.4%) | 1 | |
| Total (43 missing) | 93 | 64 | ||
| Active tobacco smoker | ||||
| Yes | 46/116 (39.65%) | 20/78 | 1.90 (1.015–3.57) | 0.043 |
| No | 70/116 (60.34%) | 58/78 | 1 | |
| Total | 116 | 78 | ||
| Injectable drug user | ||||
| Yes | 6 | 2 | 2.07 (0.40–10.54) | 0.370 |
| No | 110 | 76 | 1 | |
| Total | 116 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde-Ferráez, L.; Chan-Mezeta, A.; Gómez-Carballo, J.G.; Ayora-Talavera, G.; González-Losa, M.d.R. Human Papillomavirus Genotypes Infecting the Anal Canal and Cervix in HIV+ Men and Women, Anal Cytology, and Risk Factors for Anal Infection. Pathogens 2023, 12, 252. https://doi.org/10.3390/pathogens12020252
Conde-Ferráez L, Chan-Mezeta A, Gómez-Carballo JG, Ayora-Talavera G, González-Losa MdR. Human Papillomavirus Genotypes Infecting the Anal Canal and Cervix in HIV+ Men and Women, Anal Cytology, and Risk Factors for Anal Infection. Pathogens. 2023; 12(2):252. https://doi.org/10.3390/pathogens12020252
Chicago/Turabian StyleConde-Ferráez, Laura, Alberto Chan-Mezeta, Jesús Gilberto Gómez-Carballo, Guadalupe Ayora-Talavera, and María del Refugio González-Losa. 2023. "Human Papillomavirus Genotypes Infecting the Anal Canal and Cervix in HIV+ Men and Women, Anal Cytology, and Risk Factors for Anal Infection" Pathogens 12, no. 2: 252. https://doi.org/10.3390/pathogens12020252
APA StyleConde-Ferráez, L., Chan-Mezeta, A., Gómez-Carballo, J. G., Ayora-Talavera, G., & González-Losa, M. d. R. (2023). Human Papillomavirus Genotypes Infecting the Anal Canal and Cervix in HIV+ Men and Women, Anal Cytology, and Risk Factors for Anal Infection. Pathogens, 12(2), 252. https://doi.org/10.3390/pathogens12020252







