Artemisia afra and Artemisia annua Extracts Have Bactericidal Activity against Mycobacterium tuberculosis in Physiologically Relevant Carbon Sources and Hypoxia
Abstract
1. Introduction
2. Methodology
2.1. Plant Materials
2.2. Preparation of Plant Extracts
2.3. Bacterial Strains and Culture Conditions
2.4. Determination of the Minimum Inhibitory Concentration (MIC)
2.5. A. afra and A. annua Bactericidal Activity against Mtb in Different Carbon Sources
2.6. A. afra and A. annua Bactericidal Activity against Mtb under Hypoxia
2.7. Statistical Analysis
3. Results
3.1. Determination of the Minimum Inhibitory Concentration (MIC)
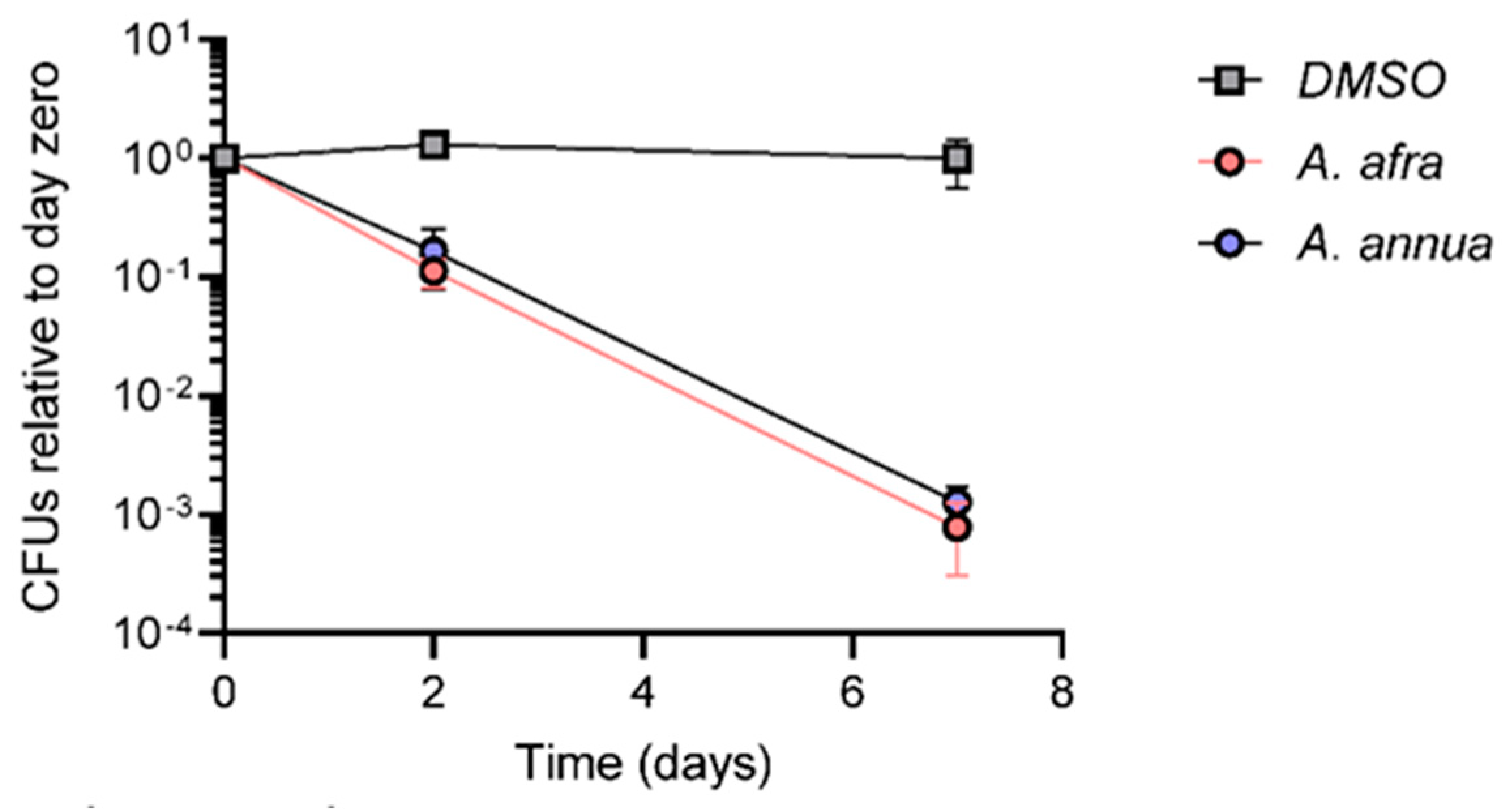
3.2. A. afra and A. annua Bactericidal Activity against Mtb in Different Carbon Sources
3.3. A. afra and A. annua Bactericidal Activity against Mtb under Hypoxia
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | artemisinin |
| DCM | dichloromethane |
| MAL | Malawi cultivar of Artemisia afra |
| PSM | plant specialty metabolites (aka secondary metabolites) |
| SAM | US cultivar of A. annua |
| SEN | Senegal cultivar of A. afra |
References
- WHO. Global Tuberculosis Report 2022. 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 2 December 2022).
- Keam, S.J. Pretomanid: First approval. Drugs 2019, 79, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Mokrousov, I.; Akhmedova, G.; Polev, D.; Molchanov, V.; Vyazovaya, A. Acquisition of bedaquiline resistance by extensively drug-resistant Mycobacterium tuberculosis strain of Central Asian Outbreak clade. Clin. Microbiol. Infect. 2019, 25, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Polsfuss, S.; Hofmann-Thiel, S.; Merker, M.; Krieger, D.; Niemann, S.; Rüssmann, H.; Schönfeld, N.; Hoffmann, H.; Kranzer, K. Emergence of low-level delamanid and bedaquiline resistance during extremely drug-resistant tuberculosis treatment. Clin. Infect. Dis. 2019, 69, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.J.; Lo, J.H. Delamanid: First global approval. Drugs 2014, 74, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.R.; Vijjamarri, A.K.; Sarkar, D. Metabolic switching of Mycobacterium tuberculosis during hypoxia is controlled by the virulence regulator PhoP. J. Bacteriol. 2020, 202, e00705-19. [Google Scholar] [CrossRef]
- Parbhoo, T.; Mouton, J.M.; Sampson, S.L. Phenotypic adaptation of Mycobacterium tuberculosis to host-associated stressors that induce persister formation. Front. Cell. Infect. Microbiol. 2022, 12, 956607. [Google Scholar] [CrossRef]
- Samuels, A.N.; Wang, E.R.; Harrison, G.A.; Valenta, J.C.; Stallings, C.L. Understanding the contribution of metabolism to Mycobacterium tuberculosis drug tolerance. Front. Cell. Infect. Microbiol. 2022, 12, 958555. [Google Scholar] [CrossRef]
- Kreutzfeldt, K.M.; Jansen, R.S.; Hartman, T.E.; Gouzy, A.; Wang, R.; Krieger, I.V.; Zimmerman, M.D.; Gengenbacher, M.; Sarathy, J.P.; Xie, M.; et al. CinA mediates multidrug tolerance in Mycobacterium tuberculosis. Nat. Commun. 2022, 13, 2203. [Google Scholar] [CrossRef]
- Ehrt, S.; Schnappinger, D.; Rhee, K.Y. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 496–507. [Google Scholar] [CrossRef]
- Olive, A.J.; Sassetti, C.M. Metabolic crosstalk between host and pathogen: Sensing, adapting and competing. Nat. Rev. Microbiol. 2016, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Lovewell, R.R.; Sassetti, C.M.; VanderVen, B.C. Chewing the fat: Lipid metabolism and homeostasis during M. tuberculosis infection. Curr. Opin. Microbiol. 2016, 29, 30–36. [Google Scholar] [CrossRef]
- Serafini, A.; Tan, L.; Horswell, S.; Howell, S.; Greenwood, D.J.; Hunt, D.M.; Phan, M.-D.; Schembri, M.; Monteleone, M.; Montague, C.R.; et al. Mycobacterium tuberculosis requires glyoxylate shunt and reverse methylcitrate cycle for lactate and pyruvate metabolism. Mol. Microbiol. 2019, 112, 1284–1307. [Google Scholar] [CrossRef] [PubMed]
- Noy, T.; Vergnolle, O.; Hartman, T.E.; Rhee, K.Y.; Jacobs, W.R.; Berney, M.; Blanchard, J.S. Central Role of Pyruvate Kinase in Carbon Co-catabolism of Mycobacterium tuberculosis. J. Biol. Chem. 2016, 291, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.P.S.; Fischer, S.M.; Marrero, J.; Nathan, C.; Ehrt, S.; Rhee, K.Y. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010, 17, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-I.; Oluoch, P.O.; Ruecker, N.; Proulx, M.K.; Soni, V.; Murphy, K.C.; Papavinasasundaram, K.; Reames, C.J.; Trujillo, C.; Zaveri, A.; et al. Chemical–genetic interaction mapping links carbon metabolism and cell wall structure to tuberculosis drug efficacy. Proc. Natl. Acad. Sci. USA 2022, 119, e2201632119. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Kogadeeva, M.; Gengenbacher, M.; McEwen, G.; Mollenkopf, H.-J.; Zamboni, N.; Kaufmann, S.H.E.; Sauer, U. Integration of metabolomics and transcriptomics reveals a complex diet of Mycobacterium tuberculosis during early macrophage infection. MSystems 2017, 2, e00057-17. [Google Scholar] [CrossRef]
- Kalia, N.P.; Shi Lee, B.; Ab Rahman, N.B.; Moraski, G.C.; Miller, M.J.; Pethe, K. Carbon metabolism modulates the efficacy of drugs targeting the cytochrome bc1: aa3 in Mycobacterium tuberculosis. Sci. Rep. 2019, 9, 8608. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.; Alves, T.M.d.A.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; De Castro, S.L.; Ferreira, V.F.; De Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part II. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, S.; Khullar, G.; Setia, D.; et al. Phytochemicals from plant foods as potential source of antiviral agents: An overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef]
- Bouhdid, S.; Abrini, J.; Amensour, M.; Zhiri, A.; Espuny, M.; Manresa, A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J. Appl. Microbiol. 2010, 109, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.I.; Rahman, M.M.; Gibbons, S.; Piddock, L.J. Medicinal plant extracts with efflux inhibitory activity against Gram-negative bacteria. Int. J. Antimicrob. Agents 2011, 37, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 2007, 153, 3923–3938. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia species with high biological values as a potential source of medicinal and cosmetic raw materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.; Seca, A.M. Research advances on health effects of edible Artemisia species and some sesquiterpene lactones constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Towler, M.; Weathers, P. Artemisia annua L. hot-water extracts show potent activity in vitro against Covid-19 variants including delta. J. Ethnopharmacol. 2022, 284, 114797. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Huang, Y.; Weathers, P. SARS-CoV-2 omicron variants succumb in vitro to Artemisia annua hot water extracts. bioRxiv. 2022, 22, 501141. [Google Scholar] [CrossRef]
- Nie, C.; Trimpert, J.; Moon, S.; Haag, R.; Gilmore, K.; Kaufer, B.B.; Seeberger, P.H. In vitro efficacy of Artemisia extracts against SARS-CoV-2. Virol. J. 2021, 18, 182. [Google Scholar] [CrossRef]
- Cantrell, C.; Fischer, N.; Urbatsch, L.; McGuire, M.; Franzblau, S. Antimycobacterial crude plant extracts from South, Central, and North America. Phytomedicine 1998, 5, 137–145. [Google Scholar] [CrossRef]
- Uba, A.; Ibrahim, K.; Agbo, E.; Makinde, A. In vitro inhibition of Mycobacterium smegmatis and Mycobacterium tuberculosis by some Nigerian Medicinal Plants. East Cent. Afr. J. Pharm. Sci. 2003, 6, 15–19. [Google Scholar] [CrossRef]
- Thring, T.; Weitz, F. Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef]
- Martini, M.; Zhang, T.; Williams, J.; Abramovitch, R.; Weathers, P.; Shell, S. Artemisia annua and Artemisia afra extracts exhibit strong bactericidal activity against Mycobacterium tuberculosis. J. Ethnopharmacol. 2020, 262, 113191. [Google Scholar] [CrossRef]
- Zheng, H.; Colvin, C.J.; Johnson, B.K.; Kirchhoff, P.D.; Wilson, M.; Jorgensen-Muga, K.; Larsen, S.D.; Abramovitch, R.B. Inhibitors of Mycobacterium tuberculosis DosRST signaling and persistence. Nat. Chem. Biol. 2017, 13, 218–225. [Google Scholar] [CrossRef]
- Choi, W.H. Novel Pharmacological Activity of Artesunate and Artemisinin: Their Potential as Anti-Tubercular Agents. J. Clin. Med. 2017, 6, 30. [Google Scholar] [CrossRef]
- Miller, M.J.; Walz, A.J.; Zhu, H.; Wu, C.; Moraski, G.; Mollmann, U.; Tristani, E.M.; Crumbliss, A.L.; Ferdig, M.T.; Checkley, L.; et al. Design, synthesis, and study of a mycobactin-artemisinin conjugate that has selective and potent activity against tuberculosis and malaria. J. Am. Chem. Soc. 2011, 133, 2076–2079. [Google Scholar] [CrossRef]
- Zheng, H.; Williams, J.T.; Aleiwi, B.; Ellsworth, E.; Abramovitch, R.B. Inhibiting Mycobacterium tuberculosis DosRST signaling by targeting response regulator DNA binding and sensor kinase heme. ACS Chem. Biol. 2019, 15, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mativandlela, S.P.N.; Meyer, J.J.M.; Hussein, A.A.; Houghton, P.J.; Hamilton, C.J.; Lall, N. Activity against Mycobacterium smegmatis and M. tuberculosis by extract of South. African medicinal plants. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 841–845. [Google Scholar]
- Ntutela, S.; Smith, P.; Matika, L.; Mukinda, J.; Arendse, H.; Allie, N.; Estes, D.M.; Mabusela, W.; Folb, P.; Steyn, L.; et al. Efficacy of Artemisia afra phytotherapy in experimental tuberculosis. Tuberculosis 2009, 89, S33–S40. [Google Scholar] [CrossRef]
- Weathers, P.J.; Towler, M.J. Changes in key constituents of clonally propagated Artemisia annua L. during preparation of compressed leaf tablets for possible therapeutic use. Ind. Crops Prod. 2014, 62, 173–178. [Google Scholar] [PubMed]
- Desrosiers, M.R.; Mittelman, A.; Weathers, P.J. Dried leaf Artemisia annua improves bioavailability of artemisinin via cytochrome P450 inhibition and enhances artemisinin efficacy downstream. Biomolecules 2020, 10, 254. [Google Scholar] [CrossRef]
- Kane, N.F.; Kiani, B.H.; Desrosiers, M.R.; Towler, M.J.; Weathers, P.J. Artemisia extracts differ from artemisinin effects on human hepatic CYP450s 2B6 and 3A4 in vitro. J. Ethnopharmacol. 2022, 298, 115587. [Google Scholar] [CrossRef]
- Sambandamurthy, V.K.; Derrick, S.C.; Hsu, T.; Chen, B.; Larsen, M.H.; Jalapathy, K.V.; Chen, M.; Kim, J.; Porcelli, S.A.; Chan, J.; et al. Mycobacterium tuberculosis ΔRD1 ΔpanCD: A safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine 2006, 24, 6309–6320. [Google Scholar] [CrossRef]
- Martini, M.C.; Zhou, Y.; Sun, H.; Shell, S.S. Defining the Transcriptional and Post-transcriptional Landscapes of Mycobacterium smegmatis in Aerobic Growth and Hypoxia. Front. Microbiol. 2019, 10, 591. [Google Scholar] [CrossRef]
- Gould, T.A.; Van De Langemheen, H.; Muñoz-Elías, E.J.; McKinney, J.D.; Sacchettini, J.C. Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Mol. Microbiol. 2006, 61, 940–947. [Google Scholar] [CrossRef]
- Marrero, J.; Rhee, K.Y.; Schnappinger, D.; Pethe, K.; Ehrt, S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. USA 2010, 107, 9819–9824. [Google Scholar] [CrossRef]
- Muñoz-Elías, E.J.; McKinney, J.D. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005, 11, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Pathan, S.K.; Wadher, S.J. In silico PASS analysis and determination of antimycobacterial, antifungal, and antioxidant efficacies of maslinic acid in an extract rich in pentacyclic triterpenoids. Int. J. Mycobacteriol. 2016, 5, 417–425. [Google Scholar] [CrossRef] [PubMed]
- van der Kooy, F.; Verpoorte, R. The content of artemisinin in the Artemisia annua tea infusion. Planta Med. 2011, 77, 1754–1756. [Google Scholar] [CrossRef] [PubMed]
- Abuhammad, A. Cholesterol metabolism: A potential therapeutic target in Mycobacteria. Br. J. Pharmacol. 2017, 174, 2194–2208. [Google Scholar] [CrossRef]
- Chang, D.P.S.; Guan, X.L. Metabolic versatility of Mycobacterium tuberculosis during infection and dormancy. Metabolites 2021, 11, 88. [Google Scholar] [CrossRef]
- Bellerose, M.M.; Baek, S.H.; Huang, C.C.; Moss, C.E.; Koh, E.I.; Proulx, M.K.; Smith, C.M.; Baker, R.E.; Lee, J.S.; Eum, S.; et al. Common Variants in the Glycerol Kinase Gene Reduce Tuberculosis Drug Efficacy. MBio 2019, 10, e00663-19. [Google Scholar] [CrossRef]
- Pethe, K.; Sequeira, P.C.; Agarwalla, S.; Rhee, K.; Kuhen, K.; Phong, W.Y.; Patel, V.; Beer, D.; Walker, J.R.; Duraiswamy, J.; et al. A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat. Commun. 2010, 1, 57. [Google Scholar] [CrossRef]
- Gibson, S.E.; Harrison, J.; Cox, J.A. Modelling a silent epidemic: A review of the in vitro models of latent tuberculosis. Pathogens 2018, 7, 88. [Google Scholar] [CrossRef]
- Sohaskey, C.D.; Voskuil, M.I. In vitro models that utilize hypoxia to induce non-replicating persistence in Mycobacteria. In Mycobacteria Protocols; Springer: Cham, Switzerland, 2015; pp. 201–213. [Google Scholar]
- Adler-Shohet, F.C.; Low, J.; Carson, M.; Girma, H.; Singh, J. Management of latent tuberculosis infection in child contacts of multidrug-resistant tuberculosis. Pediatr. Infect. Dis. J. 2014, 33, 664–666. [Google Scholar] [CrossRef]
- Lim, J.; Lee, J.J.; Lee, S.-K.; Kim, S.; Eum, S.-Y.; Eoh, H. Phosphoenolpyruvate depletion mediates both growth arrest and drug tolerance of Mycobacterium tuberculosis in hypoxia. Proc. Natl. Acad. Sci. USA 2021, 118, e2105800118. [Google Scholar] [CrossRef] [PubMed]
- Raghunandanan, S.; Jose, L.; Kumar, R.A. Dormant Mycobacterium tuberculosis converts isoniazid to the active drug in a Wayne’s model of dormancy. J. Antibiot. 2018, 71, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Iacobino, A.; Piccaro, G.; Giannoni, F.; Mustazzolu, A.; Fattorini, L. Mycobacterium tuberculosis is selectively killed by rifampin and rifapentine in hypoxia at neutral pH. Antimicrob. Agents Chemother. 2016, 61, e02296-16. [Google Scholar] [CrossRef] [PubMed]


| Cultivar | Part Tested a | Solvent b | Mtb Inhibition c | Extraction Method | Test System d | Ref. e |
|---|---|---|---|---|---|---|
| Artemisia annua | ||||||
| SAM | L | D | 4.5 mg/mL | 1 g/20 mL DCM, 30 min in sonicating water bath, rt, repeat twice, pooled, dry under N2 5 g/L water, boiled 10 min, cooled, filtered, −20 °C storage | I | This study |
| W | 1.9 mg/mL | I | ||||
| NS | L + St | M | 5 mg/mL | 1 kg/2.5 L, 72 h rt, repeat twice, pool, rot. evap. 100 g/500 mL, 72 h rt, then vacuum dry | I | [36] |
| W | 5 mg/mL | I | ||||
| NS | L + St | D | 77% inhib. by 100 µg/mL extract | 500 g/UNK vol solvent, sit at rt 12–48 h | I | [35] |
| Artemisia afra | ||||||
| SEN | L | D | 4.8 mg/mL | 1 g/20 mL DCM, 30 min in sonicating water bath, rt, repeat twice, pooled, dry under N2 | I | [38] |
| SEN | L | D | 5–10 mg/mL | 1 g/20 mL DCM, 30 min in sonicating water bath, rt, repeat twice, pooled, dry under N2 5 g/L water, boiled 10 min, cooled, filtered, −20 °C storage | I | This study |
| MAL | D | 2.5 mg/mL | ||||
| SEN | W | 1.3 mg/mL | ||||
| PAR | W | 1.7 mg/mL | ||||
| LUX | W | 1.5 mg/mL | ||||
| NS | L | D | 290 µg/mL | 1 kg/10 L, rt 3 h stir, sit overnight, filter, rot. evap., repeat twice w 5 L DCM, pool evap. extracts 200 g/4 L, 30 min boil, filter, freeze dry. | I | [44] |
| NA | M | |||||
| W | NA | M | ||||
| NS | L | E | NA | 50 g/500 mL, 24 h rt, repeat twice, pool, rot. evap. | I | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, B.H.; Alonso, M.N.; Weathers, P.J.; Shell, S.S. Artemisia afra and Artemisia annua Extracts Have Bactericidal Activity against Mycobacterium tuberculosis in Physiologically Relevant Carbon Sources and Hypoxia. Pathogens 2023, 12, 227. https://doi.org/10.3390/pathogens12020227
Kiani BH, Alonso MN, Weathers PJ, Shell SS. Artemisia afra and Artemisia annua Extracts Have Bactericidal Activity against Mycobacterium tuberculosis in Physiologically Relevant Carbon Sources and Hypoxia. Pathogens. 2023; 12(2):227. https://doi.org/10.3390/pathogens12020227
Chicago/Turabian StyleKiani, Bushra Hafeez, Maria Natalia Alonso, Pamela J. Weathers, and Scarlet S. Shell. 2023. "Artemisia afra and Artemisia annua Extracts Have Bactericidal Activity against Mycobacterium tuberculosis in Physiologically Relevant Carbon Sources and Hypoxia" Pathogens 12, no. 2: 227. https://doi.org/10.3390/pathogens12020227
APA StyleKiani, B. H., Alonso, M. N., Weathers, P. J., & Shell, S. S. (2023). Artemisia afra and Artemisia annua Extracts Have Bactericidal Activity against Mycobacterium tuberculosis in Physiologically Relevant Carbon Sources and Hypoxia. Pathogens, 12(2), 227. https://doi.org/10.3390/pathogens12020227







