Incidence of Human and Free-Ranging Wild Rodent Infections with Leishmania (Viannia) braziliensis, Aetiological Agent of Cutaneous Leishmaniasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Site
2.3. Human Recruitment
2.4. Leishmanin Skin Test (LST)
2.5. ACL Lesions/Scars and Metadata Collection
2.6. Rodent Trapping and Sampling
2.7. Rodent Xenodiagnosis
2.8. Data Analyses
2.9. Infection Estimates
2.10. Statistical Analyses
3. Results
3.1. Humans
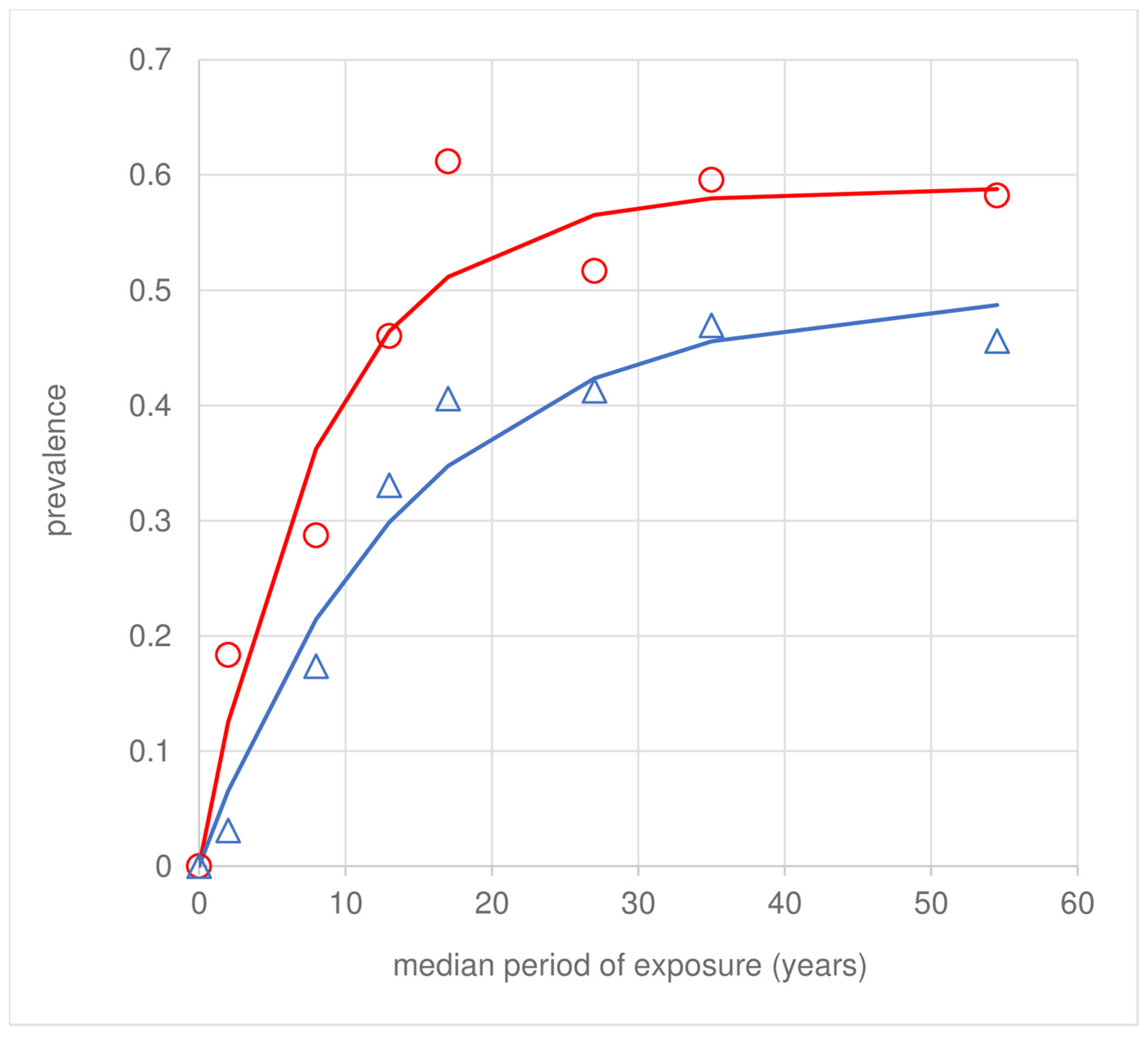
3.2. LST Infection Rates
3.3. ACL Scars
3.4. Effect of Age and Sex on Transmission
3.5. Concordance between LST and Scars
4. Rodents
4.1. Rodent Sample
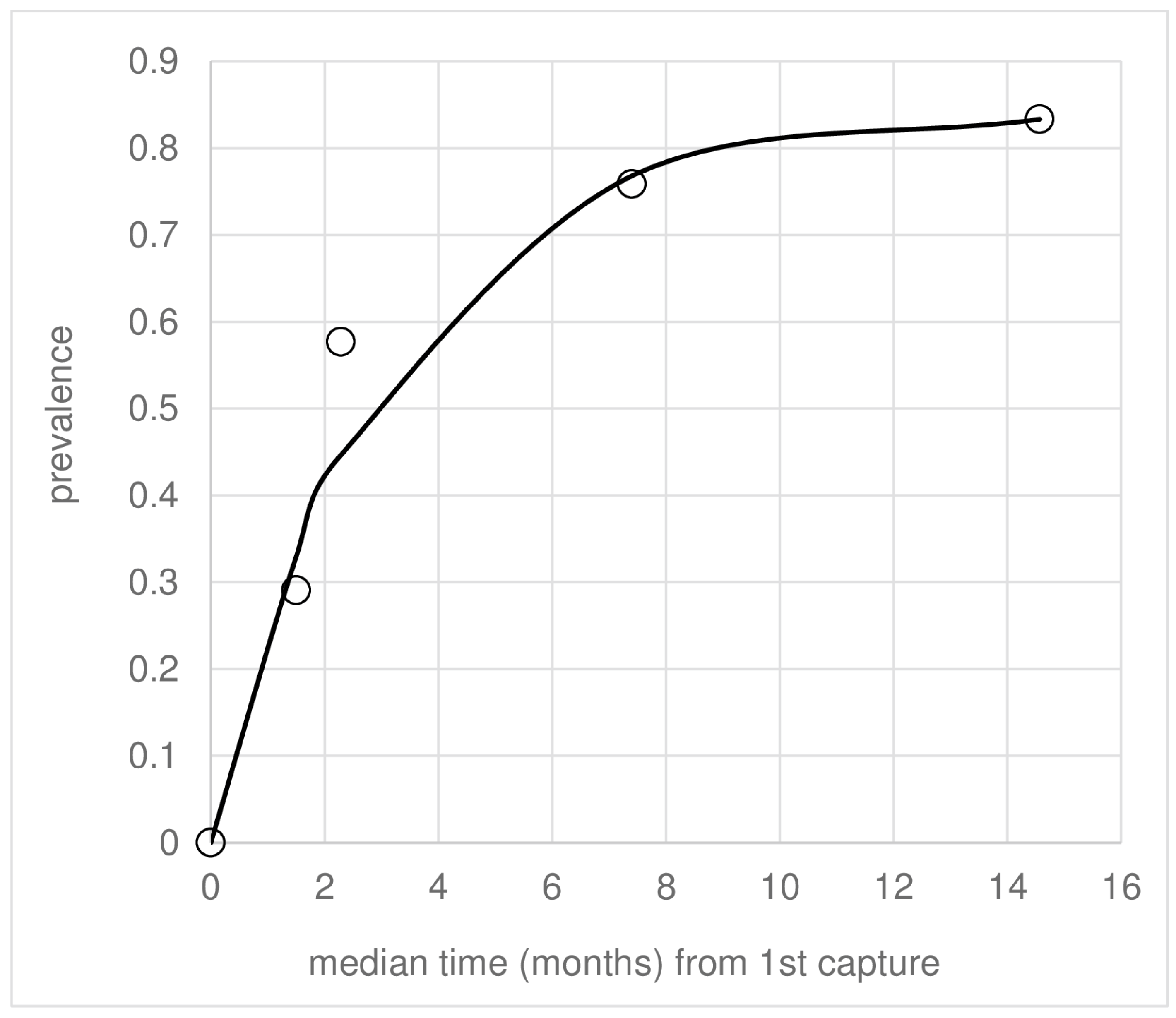
4.2. Rodent Infection
4.3. Loss of Infection
5. The Association between Human and Rodent Leishmania Infection
Xenodiagnosis
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.S.; Courtenay, O.; Brito, M.E.F.; Carvalho, F.G.; Carvalho, A.W.S.; Soares, F.; Carvalho, S.M.; Costa, P.L.; Zampieri, R.; Floeter-Winter, L.M.; et al. Infectiousness of Sylvatic and Synanthropic Small Rodents Implicates a Multi-host Reservoir of Leishmania (Viannia) braziliensis. PLoS Neglected Trop. Dis. 2015, 9, e0004137. [Google Scholar] [CrossRef] [PubMed]
- Brandão-Filho, S.P.; Brito, M.E.; Carvalho, F.G.; Ishikawa, E.A.; Cupolillo, E.; Floeter-Winter, L.; Shaw, J.J. Wild and synanthropic hosts of Leishmania (Viannia) braziliensis in the endemic cutaneous leishmaniasis locality of Amaraji, Pernambuco State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Brandão-Filho, S.P.; Catvalho, F.G.; Brito, M.E.F.; Almeida, F.A.; Nascimento, L.A. American cutaneous leishmaniasis in Pernambuco, Brazil: Eco-epidemiological aspects in ‘Zona da Mata’ region. Mem. Inst. Oswaldo Cruz 1994, 89, 445–449. [Google Scholar] [CrossRef]
- Lima, B.S.; Dantas-Torres, F.; de Carvalho, M.R.; Marinho-Junior, J.F.; de Almeida, E.L.; Brito, M.E.F.; Gomes, F.; Brandao-Filho, S.P. Small mammals as hosts of Leishmania spp. in a highly endemic area for zoonotic leishmaniasis in north-eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 592–597. [Google Scholar] [CrossRef]
- Marinho-Júnior, J.F.; Monteiro, J.; de Carvalho, A.W.S.; de Carvalho, F.G.; Cavalcanti, M.D.; Shaw, J.; Courtenay, O.; Brandão-Filho, S.P. High levels of infectiousness of asymptomatic Leishmania (Viannia) braziliensis infections in wild rodents highlights their importance in the epidemiology of American Tegumentary Leishmaniasis in Brazil. PLoS Neglected Trop. Dis. 2023, 17, e0010996. [Google Scholar] [CrossRef]
- Pacheco-Fernandez, T.; Volpedo, G.; Gannavaram, S.; Bhattacharya, P.; Dey, R.; Satoskar, A.; Matlashewski, G.; Nakhasi, H.L. Revival of Leishmanization and Leishmanin. Front. Cell. Infect. Microbiol. 2021, 11, 639801. [Google Scholar] [CrossRef]
- Sokal, J.E. Measurement of delayed skin-test responses. N. Engl. J. Med. 1975, 293, 501–502. [Google Scholar] [CrossRef]
- Courtenay, O.; Macdonald, D.W.; Lainson, R.; Shaw, J.J.; Dye, C. Epidemiology of canine leishmaniasis- a comparative serological study of dogs and foxes in Amazon Brazil. Parasitology 1994, 109, 273–279. [Google Scholar] [CrossRef]
- Vynnycky, E.; White, R. An Introduction to Infectious Disease Modelling; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Brandão-Filho, S.P.; Campbell-Lendrum, D.; Brito, M.E.F.; Shaw, J.J.; Davies, C.R. Epidemiological surveys confirm an increasing burden of cutaneous leishmaniasis in north-east Brazil. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 488–494. [Google Scholar] [CrossRef]
- Portella, T.P.; Kraenkel, R.A. Spatial-temporal pattern of cutaneous leishmaniasis in Brazil. Infect. Dis. Poverty 2021, 10, 86. [Google Scholar] [CrossRef]
- PAHO. LEISHMANIASES. Epidemiological Report on the Region of the Americas; Pan American Health Organization: Washington, DC, USA, 2022; pp. 1–12. Available online: https://iris.paho.org/bitstream/handle/10665.2/56831/PAHOCDEVT220021_eng.pdf?sequence=1&isAllowed=y (accessed on 15 October 2023).
- Sadeghian, G.; Momeni, A.; Siadat, A.H.; Yousefi, P. Evaluation of leishmanin skin test and its relationship with the clinical form and duration of cutaneous leishmaniasis. Dermatol. Online 2006, 12, 3. [Google Scholar] [CrossRef]
- Weigle, K.A.; Santrich, C.; Martinez, F.; Valderrama, L.; Saravia, N.G. Epidemiology of cutaneous leishmaniasis in colombia—A longitudinal-study of the natural-history, prevalence, and incidence of infection and clinical manifestations. J. Infect. Dis. 1993, 168, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, T. Recurrent cutaneous leishmaniasis—A role for persistent parasites. Parasitol. Today 1994, 10, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Weigle, K.A.; Valderrama, L.; Arias, A.L.; Santrich, C.; Saravia, N.G. Leishmanin skin-test standardization and evaluation of safety, dose, storage, longevity of reaction and sensitization. Am. J. Trop. Med. Hyg. 1991, 44, 260–271. [Google Scholar] [CrossRef]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Souza, N.A.; Andrade-Coelho, C.A.; Peixoto, A.A.; Rangel, E.F. Nocturnal activity rhythms of Lutzomyia intermedia and Lutzomyia whitmani (Diptera: Psychodidae) in a transmission area of American cutaneous leishmaniasis in Rio de Janeiro State, Brazil. J. Med. Entomol. 2005, 42, 986–992. [Google Scholar] [CrossRef]
- Ferreira, E.D.; Cruz, I.; Canavate, C.; de Melo, L.A.; Pereira, A.A.S.; Madeira, F.A.M.; Valerio, S.A.N.; Cunha, H.M.; Paglia, A.P.; Gontijo, C.M.F. Mixed infection of Leishmania infantum and Leishmania braziliensis in rodents from endemic urban area of the New World. BMC Vet. Res. 2015, 11, 71. [Google Scholar] [CrossRef]
- Cardoso, R.M.; de Araujo, N.; Romero, G.A.S.; Souza, T.; Dietrich, A.G.; Mendes, J.D.; Reis, M.L.; Ferreira, J.B.C.; Hecht, M.M.; Gurgel-Goncalves, R. Expanding the knowledge about Leishmania species in wild mammals and dogs in the Brazilian savannah. Parasites Vectors 2015, 8, 171. [Google Scholar] [CrossRef]
- Kassahun, A.; Sadlova, J.; Dvorak, V.; Kostalova, T.; Rohousova, I.; Frynta, D.; Aghova, T.; Yasur-Landau, D.; Lemma, W.; Hailu, A.; et al. Detection of Leishmania donovani and L. tropica in Ethiopian wild rodents. Acta Trop. 2015, 145, 39–44. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Jomaa, I.; Ben Ismail, R.; Ashford, R.W. Leishmania major infection in the fat sand rat Psammomys obesus in Tunisia: Interaction of host and parasite populations. Ann. Trop. Med. Parasitol. 2003, 97, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Shaw, J.J. Leishmaniasis in Brazil: I. Observations on enzootic rodent leishmaniasis—Incrimination of Lutzomyia flaviscutellata (mangabeira) as the vector in the lower amazonian basin. Trans. R. Soc. Trop. Med. Hyg. 1968, 62, 385–395. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Courtenay, O.; Garcez, L.; Dye, C. The epidemiology of canine leishmaniasis: Transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology 1997, 115, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, O.; Quinnell, R.J.; Garcez, L.M.; Dye, C. Low infectiousness of a wildlife host of Leishmania infantum: The crab-eating fox is not important for transmission. Parasitology 2002, 125, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.O.; Pinho, G.M.; Fernandez, F.A.S. Spatial patterns of the semi-aquatic rodent Nectomys squamipes in Atlantic forest streams. J. Nat. Hist. 2016, 50, 497–511. [Google Scholar] [CrossRef]
- Rosales-Chilama, M.; Gongora, R.E.; Valderrama, L.; Jojoa, J.; Alexander, N.; Rubiano, L.C.; Cossio, A.; Adams, E.R.; Saravia, N.G.; Gomez, M.A. Parasitological Confirmation and Analysis of Leishmania Diversity in Asymptomatic and Subclinical Infection following Resolution of Cutaneous Leishmaniasis. PLoS Neglected Trop. Dis. 2015, 9, e0004273. [Google Scholar] [CrossRef]
- Castellucci, L.A.; Cheng, L.H.; Araújo, C.; Guimaraes, L.H.; Lessa, H.; Machado, P.; Almeida, M.F.; Oliveira, A.; Ko, A.; Johnson, W.D.; et al. Familial aggregation of mucosal leishmaniasis in Northeast Brazil. Am. J. Trop. Med. Hyg. 2005, 73, 69–73. [Google Scholar] [CrossRef]
- Cupolillo, E.; Brahim, L.R.; Toaldo, C.B.; de Oliveira-Neto, M.P.; de Brito, M.E.; Falqueto, A.; de Farias Naiff, M.; Grimaldi, G., Jr. Genetic polymorphism and molecular epidemiology of Leishmania (Viannia) braziliensis from different hosts and geographic areas in Brazil. J. Clin. Microbiol. 2003, 41, 3126–3132. [Google Scholar] [CrossRef]
- Brito, M.E.; Andrade, M.S.; Mendonca, M.G.; Silva, C.J.; Almeida, E.L.; Lima, B.S.; Felix, S.M.; Abath, F.G.; da Graca, G.C.; Porrozzi, R.; et al. Species diversity of Leishmania (Viannia) parasites circulating in an endemic area for cutaneous leishmaniasis located in the Atlantic rainforest region of northeastern Brazil. Trop. Med. Int. Health 2009, 14, 1278–1286. [Google Scholar] [CrossRef]
- Dantas-Torres, F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 2007, 149, 139–146. [Google Scholar] [CrossRef]
- Lago, J.; Fraga, D.; Coelho, L.; Jesus, M.S.D.; Leite, B.; Werneck, G.L.; Arruda, S.; Lago, E.; Carvalho, E.M.; Bacellar, O. Dogs Harbor Leishmania braziliensis and Participate in the Transmission Cycle of Human Tegumentary Leishmaniasis. Pathogens 2023, 12, 981. [Google Scholar] [CrossRef]
- Souza, N.A.; Andrade-Coelho, C.A.; Vilela, M.L.; Peixoto, A.A.; Rangel, E.F. Seasonality of Lutzomyia intermedia and Lutzomyia whitmani (Diptera: Psychodidae: Phlebotominae), occurring sympatrically in area of cutaneous leishmaniasis in the state of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2002, 97, 759–765. [Google Scholar] [CrossRef]
- Campbell-Lendrum, D.H.; Pinto, M.C.; Brandao, S.P.; De Souza, A.A.; Ready, P.D.; Davies, C.R. Experimental comparison of anthropophily between geographically dispersed populations of Lutzomyia whitmani (Diptera: Psychodidae). Med. Vet. Entomol. 1999, 13, 299–309. [Google Scholar] [CrossRef]
- Diniz, M.; Ovallos, F.G.; Gomes, C.M.D.; Lavitschka, C.D.; Galati, E.A.B. Host-biting rate and susceptibility of some suspected vectors to Leishmania braziliensis. Parasites Vectors 2014, 7, 139. [Google Scholar] [CrossRef]
- Brandao, S.P.; Donalisio, M.R.; da Silva, F.J.; Valenca, H.F.; Costa, P.L.; Shaw, J.J.; Peterson, A.T. Spatial and temporal patterns of occurrence of Lutzomyia sand fly species in an endemic area for cutaneous leishmaniasis in the Atlantic Forest region of northeast Brazil. J. Vector Ecol. 2011, 36, S71–S76. [Google Scholar] [CrossRef]
- da Costa, S.M.; Cechinel, M.; Bandeira, V.; Zannuncio, J.C.; Lainson, R.; Rangel, E.F. Lutzomyia (Nyssomyia) whitmani s.l. (Antunes & Coutinho, 1939) (Diptera: Psychodidae: Phlebotominae): Geographical distribution and the epidemiology of American cutaneous leishmaniasis in Brazil–Mini-review. Mem. Inst. Oswaldo Cruz 2007, 102, 149–153. [Google Scholar]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Ortega, C.N.; Sauer, E.L.; Sehgal, T.; Young, S.; et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8667–8671. [Google Scholar] [CrossRef]
- Kocher, A.; Cornuault, J.; Gantier, J.C.; Manzi, S.; Chavy, A.; Girod, R.; Dusfour, I.; Forget, P.M.; Ginouves, M.; Prévot, G.; et al. Biodiversity and vector-borne diseases: Host dilution and vector amplification occur simultaneously for Amazonian leishmaniases. Mol. Ecol. 2023, 32, 1817–1831. [Google Scholar] [CrossRef]

| Study Population/Foci | Numbers LST Tested (with Age/Exposure Data) | Numbers Examined for Scars (with Time of Occurrence Data) | Numbers with Records for Both LST and ACL Scars; (with Exposure Occurrence Data) | Median Age in Years (Q1, Q3) | M/F Sex Ratio |
|---|---|---|---|---|---|
| Amaraji | 70 | 70 | 70 | 24 (5.6, 58.0) | 0.75 |
| Moreno | 170 | 180 | 170 | 15 (3.0, 61.0) | 1.22 |
| Vicência | 263 (261) | 274 (272) | 263 (261) | 23 (6.7, 60.4) | 1.62 |
| All | 503 (501) | 524 (522) | 503 (501) | 20 (5.0, 60.0) | 1.32 |
| Study Population/Location Foci | Number LST+ve/Number Tested (Proportion) | λ (95% C.L.) | ρ (95% C.L.) | Median (Q1, Q3) Period of Residential Exposure in Years |
|---|---|---|---|---|
| Amaraji | 24/70 (0.343) | 0.03 (0.003, 0.054) | 0.02 (0.000, 0.084) | 15 (2.0, 41.4) |
| Moreno | 57/170 (0.335) | 0.04 (0.015, 0.061) | 0.04 (0.000, 0.090) | 12 (2.0, 54.9) |
| Vicência | 148/263 (0.563) | 0.15 (0.055, 0.243) | 0.11 (0.028, 0.186) | 20 (5.0, 60.0) |
| All combined | 229/503 (0.455) | 0.07 (0.048, 0.093) | 0.05 (0.020, 0.078) | 15 (3.0, 57.0) |
| Location | Number Leishmania Scar Positive/Number Tested (Proportion) | λ (95% C.L.) | ρ (95% C.L.) | Median Age (IQR) at the Time of Active Lesion Appearance in Years |
|---|---|---|---|---|
| Amaraji | 15/70 (0.214) | 0.02 (0.006, 0.024) | - | 20 (1.5, 46.0) |
| Moreno | 22/180 (0.122) | 0.02 (0.001, 0.031) | 0.04 (0.000, 0.147) | 20 (7.0, 62.0) |
| Vicência | 130/274 (0.475) | 0.07 (0.032, 0.116) | 0.06 (0.010, 0.114) | 13 (3.3, 50.7) |
| Total | 167/524 (0.319) | 0.04 (0.024, 0.046) | 0.04 (0.011, 0.059) | 14 (4.0, 52.0) |
| Infection Status (n) | Median (Q1, Q3) Period of Residential Exposure in Years Prior to Appearance of ACL Active Lesion |
|---|---|
| LST+ve scar+ve (158) | 20 (8.0, 60.0) |
| LST+ve scar-ve (71) | 15 (2.0, 57.0) |
| LST-ve scar+ve (8) | 16 (6.0, 55.0) |
| LST-ve scar-ve (266) | 13 (2.0, 54.7) |
| Rodent Species | Period Prevalence (Number Infected Animals/Total Tested) | Incidence/Month (Number of New Infections/Uninfected at First Capture) | Force of Infection λ/Month (SD) 1 | Median (IQR) Days to Infection from First Capture |
|---|---|---|---|---|
| Akodon cursor | 0.125 (4/32) | 0.147 (2/3) | 0.24 (0.195) | 70 (26–124) |
| Holochillus sciureus | 0.425 (17/40) | 0.139 (5/8) | 0.22 (0.124) | 41 (41–63) |
| Necromys lasiurus | 0.337 (28/83) | 0.283 (10/15) | 0.47 (0.208) | 46 (41–50) |
| Nectomys squamipes | 0.615 (150/244) | 0.130 (43/62) | 0.22 (0.055) | 52 (32–114) |
| Oxymycterus dasytrichus | 0.327 (16/49) | 0.190 (8/13) | 0.29 (0.138) | 93 (44–141) |
| Rattus rattus | 0.277 (41/148) | 0.110 (11/25) | 0.15 (0.053) | 53 (41–133) |
| Totals a/mean (95% C.I.s) b | 0.430 (256/596) a | 0.141 (79/126) a | 0.27 (0.049, 0.481) b | 59 (21–97) b |
| Age-Class 1 Assigned at First Capture | Crude Incidence/Month (Number of Incident Cases/Number Uninfected at First Capture) 2 | FOI λ/Month (SD) 2 | Median (IQR) Days to Infection from First Capture |
|---|---|---|---|
| JJ | 0.116 (10/14) | 0.203 (0.0945) | 114 (53–135) |
| JA | 0.123 (28/37) | 0.231 (0.0756) | 47 (31–120) |
| AA | 0.166 (41/75) | 0.240 (0.0524) | 50 (41–77) |
| Humans: Number +ve/n Tested 1 | Rodents: Number +ve/n Tested (Proportion) 2 | |||||
|---|---|---|---|---|---|---|
| Peridomestic Locations | Plantation Locations | |||||
| Home-stead ID | LST | ACL scars | Individual rodents | Capture events | Individual rodents | Capture events |
| 1 | 5/7 | 3/7 | Nd | Nd | 32/63 (0.51) | 49/119 (0.41) |
| 2 | 1/4 | 0/4 | 1/3 (0.33) | 1/3 (0.33) | 6/17 (0.35) | 6/20 (0.30) |
| 3 | 0/3 | 0/3 | 4/26 (0.15) | 4/30 (0.13) | 55/116 (0.47) | 98/201 (0.49) |
| 4 | 2/2 | 2/2 | 1/3 (0.33) | 1/3 (0.33) | 17/33 (0.52) | 20/39 (0.51) |
| 5 | 0/1 | 0/1 | 0/1 (0.00) | 0/1 (0.00) | 31/56 (0.55) | 55/110 (0.50) |
| 6 | 1/1 | 1/1 | 6/10 (0.60) | 6/10 (0.60) | 48/86 (0.56) | 66/125 (0.53) |
| 7 b | 0/1 | 0/1 | Nd | Nd | 6/32 (0.19) | 7/39 (0.18) |
| 8 b | 1/2 | 1/2 | 1/1 (1.00) | 1/1 (1.00) | 8/20 (0.40) | 11/30 (0.37) |
| sum | 10/21 | 7/21 | 13/44 (0.30) | 13/48 (0.27) | 203/423 (0.48) | 312/683 (0.46) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courtenay, O.; Marinho-Júnior, J.F.; Brito, M.E.F.; Monteiro, J.F.C.L.S.; Shaw, J.J.; Brandão-Filho, S.P. Incidence of Human and Free-Ranging Wild Rodent Infections with Leishmania (Viannia) braziliensis, Aetiological Agent of Cutaneous Leishmaniasis. Pathogens 2023, 12, 1395. https://doi.org/10.3390/pathogens12121395
Courtenay O, Marinho-Júnior JF, Brito MEF, Monteiro JFCLS, Shaw JJ, Brandão-Filho SP. Incidence of Human and Free-Ranging Wild Rodent Infections with Leishmania (Viannia) braziliensis, Aetiological Agent of Cutaneous Leishmaniasis. Pathogens. 2023; 12(12):1395. https://doi.org/10.3390/pathogens12121395
Chicago/Turabian StyleCourtenay, Orin, José F. Marinho-Júnior, Maria Edileuza F. Brito, Juliana F. C. L. S. Monteiro, Jeffrey J. Shaw, and Sinval P. Brandão-Filho. 2023. "Incidence of Human and Free-Ranging Wild Rodent Infections with Leishmania (Viannia) braziliensis, Aetiological Agent of Cutaneous Leishmaniasis" Pathogens 12, no. 12: 1395. https://doi.org/10.3390/pathogens12121395






