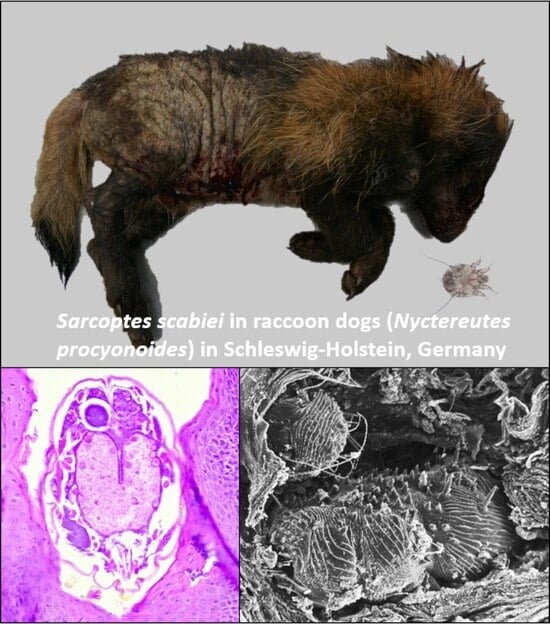
Malicious Mites—Sarcoptes scabiei in Raccoon Dogs (Nyctereutes procyonoides) in Schleswig-Holstein, Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Postmortem Examination, and Sample Preparation
2.2. Parasitology
2.3. Histopathology and Scanning Electron Microscopy (SEM)
2.4. Microbiology
3. Results
3.1. Gross Pathology
3.2. Parasitology
3.3. Histopathology and Scanning Electron Microscopy (SEM)
3.4. Microbiology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowak, E. Ansiedlung und Ausbreitung des Marderhundes (Nyctereutes procyonoides Gray) in Europa. Beitr. Jagd- Und Wildforsch. 1974, 8, 351–384. [Google Scholar]
- Nowak, E. Verbreitungs-und bestandsentwicklung des Marderhundes, Nyctereutes procyonoides (Gray, 1834) in Europa. Z. Für Jagdwiss. 1984, 30, 137–154. [Google Scholar] [CrossRef]
- Mulder, J.L. A review of the ecology of the raccoon dog (Nyctereutes procyonoides) in Europe. Lutra 2012, 55, 101–127. [Google Scholar]
- Tedeschi, L.; Biancolini, D.; Capinha, C.; Rondinini, C.; Essl, F. Introduction, spread, and impacts of invasive alien mammal species in Europe. Mamm. Rev. 2022, 52, 252–266. [Google Scholar] [CrossRef]
- Borkenhagen, P. Die Säugetiere Schleswig-Holsteins; Faunistisch- Ökologische Arbeitsgemeinschaft E. V.: Husum, Germany, 2011. [Google Scholar]
- Sutor, A.; Schwarz, S.; Conraths, F.J. The biological potential of the raccoon dog (Nyctereutes procyonoides, Gray 1834) as an invasive species in Europe—New risks for disease spread? Acta Theriol. 2014, 59, 49–59. [Google Scholar] [CrossRef]
- Drygala, F.; Werner, U.; Zoller, H. Diet composition of the invasive raccoon dog (Nyctereutes procyonoides) and the native red fox (Vulpes vulpes) in north-east Germany. Hystrix-Ital. J. Mammal. 2013, 24, 190–194. [Google Scholar] [CrossRef]
- Kauhala, K. Introduced carnivores in Europe with special reference to central and northern Europe. Wildl. Biol. 1996, 2, 197–204. [Google Scholar] [CrossRef]
- Kauhala, K.; Holmala, K. Contact rate and risk of rabies spread between medium-sized carnivores in southeast Finland. In Proceedings of the Annales Zoologici Fennici, Helsinki, Finland, 28 August 2006; pp. 348–357. [Google Scholar]
- Kauhala, K.; Holmala, K.; Lammers, W.; Schregel, J. Home ranges and densities of medium-sized carnivores in south-east Finland, with special reference to rabies spread. Acta Theriol. 2006, 51, 1–13. [Google Scholar] [CrossRef]
- Deplazes, P. Gattung Sarcoptes. In Parasitologie Für Die Tiermedizin, 4th ed.; Deplazes, P., Joachim, A., Mathis, A., Strube, C., Taubert, A., von Samson-Himmelstjerna, G., Zahner, H., Eds.; Georg Thieme Verlag KG: Stuttgart, Germany, 2020. [Google Scholar]
- Pence, D.B.; Ueckermann, E. Sarcoptic manage in wildlife. Rev. Sci. Tech. 2002, 21, 385–398. [Google Scholar] [CrossRef]
- Fain, A. Etude de la variabilite de Sarcoptes scabiei avec une revision des Sarcoptidae. Acta Zool. Pathol. Antverp. 1968, 47, 1–196. [Google Scholar]
- Matsuyama, R.; Yabusaki, T.; Kuninaga, N.; Morimoto, T.; Okano, T.; Suzuki, M.; Asano, M. Coexistence of two different genotypes of Sarcoptes scabiei derived from companion dogs and wild raccoon dogs in Gifu, Japan: The genetic evidence for transmission between domestic and wild canids. Vet. Parasitol. 2015, 212, 356–360. [Google Scholar] [CrossRef]
- Moroni, B.; Rossi, L.; Bernigaud, C.; Guillot, J. Zoonotic Episodes of Scabies: A Global Overview. Pathogens 2022, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E. Nyctereutes procyonoides (Gray, 1834)–Marderhund. In Handbuch der Säugetiere Europas; Stubbe, M., Krapp, F., Eds.; AULA-Verlag: Wiesbaden, Germany, 1993; Volume 5. [Google Scholar]
- Kowalczyk, R.; Zalewski, A. Adaptation to cold and predation—Shelter use by invasive raccoon dogs Nyctereutes procyonoides in Białowieża Primeval Forest (Poland). Eur. J. Wildl. Res. 2011, 57, 133–142. [Google Scholar] [CrossRef]
- Kauhala, K.; Holmala, K.; Schregel, J. Seasonal activity patterns and movements of the raccoon dog, a vector of diseases and parasites, in southern Finland. Mamm. Biol. 2007, 72, 342–353. [Google Scholar] [CrossRef]
- Sutor, A.; Schwarz, S. Home ranges of raccoon dogs (Nyctereutes procyonoides, Gray, 1834) in Southern Brandenburg, Germany. Eur. J. Wildl. Res. 2012, 58, 85–97. [Google Scholar] [CrossRef]
- Osten-Sacken, N.; Slodkowicz-Kowalska, A.; Pacon, J.; Skrzypczak, L.; Werner, A. Intestinal and external parasites of raccoon dogs (Nyctereutes procyonoides) in western Poland. Ann. Parasitol. 2017, 63, 37–44. [Google Scholar] [CrossRef]
- Sutor, A. Dispersal of the alien raccoon dog Nyctereutes procyonoides in Southern Brandenburg, Germany. Eur. J. Wildl. Res. 2008, 54, 321–326. [Google Scholar] [CrossRef]
- Saito, M.U.; Sonoda, Y. Symptomatic Raccoon Dogs and Sarcoptic Mange Along an Urban Gradient. Ecohealth 2017, 14, 318–328. [Google Scholar] [CrossRef]
- Takahashi, M.; Misumi, H.; Nogami, S.; Takahama, M.; Hirayama, K. Severe sarcoptic mange in the raccoon dog, Nyctereutes procyonoides, in Saitama and Gunma Prefectures, Japan. Med. Entomol. Zool. 2001, 52, 67–71. [Google Scholar] [CrossRef]
- Eo, K.Y.; Kwon, O.D.; Shin, N.S.; Shin, T.; Kwak, D. Sarcoptic mange in wild raccoon dogs (Nyctereutes procyonoides) in Korea. J. Zoo Wildl. Med. 2008, 39, 671–673. [Google Scholar] [CrossRef]
- Ninomiya, H.; Ogata, M. Sarcoptic mange in free-ranging raccoon dogs (Nyctereutes procyonoides) in Japan. Vet. Dermatol. 2005, 16, 177–182. [Google Scholar] [CrossRef]
- Nakagawa, T.L.; Takai, Y.; Kubo, M.; Sakai, H.; Masegi, T.; Yanai, T. A pathological study of sepsis associated with sarcoptic mange in raccoon dogs (Nyctereutes procyonoides) in Japan. J. Comp. Pathol. 2009, 141, 177–181. [Google Scholar] [CrossRef]
- Takahashi, M.; Nogami, S.; Misumi, H.; Maruyama, S.; Shiibashi, T.; Yamamoto, Y.; Sakai, T. Mange caused by Sarcoptes scabiei (Acari: Sarcoptidae) in wild raccoon dogs, Nyctereutes procyonoides, in Kanagawa Prefecture, Japan. J. Vet. Med. Sci. 2001, 63, 457–460. [Google Scholar] [CrossRef]
- Sugiura, N.; Doi, K.; Kato, T.; Morita, T.; Hayama, S.I. Epizootic of sarcoptic mange in raccoon dogs (Nyctereutes procyonoides) in relation to population density. J. Vet. Med. Sci. 2018, 80, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Noviana, D.; Harjanti, D.W.; Otsuka, Y.; Horii, Y. Proliferation of protease-enriched mast cells in sarcoptic skin lesions of raccoon dogs. J. Comp. Pathol. 2004, 131, 28–37. [Google Scholar] [CrossRef]
- Faehndrich, M.; Klink, J.C.; Roller, M.; Wohlsein, P.; Raue, K.; Strube, C.; Prenger-Berninghoff, E.; Ewers, C.; Capucci, L.; Lavazza, A.; et al. Status of Infectious Diseases in Free-Ranging European Brown Hares (Lepus europaeus) Found Dead between 2017 and 2020 in Schleswig-Holstein, Germany. Pathogens 2023, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Niiranen, L.; Makela, K.A.; Mutt, S.J.; Viitanen, R.; Kaisanlahti, A.; Vicente, D.; Noponen, T.; Autio, A.; Roivainen, A.; Nuutila, P.; et al. Role of Brown and Beige Adipose Tissues in Seasonal Adaptation in the Raccoon Dog (Nyctereutes procyonoides). Int. J. Mol. Sci. 2021, 22, 9623. [Google Scholar] [CrossRef] [PubMed]
- Arlian, L.G. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annu. Rev. Entomol. 1989, 34, 139–161. [Google Scholar] [CrossRef]
- Mullen, G.R.; Oconnor, B.M. MITES (Acari). In Medical and Veterinary Entomology; Mullen, G., Durden, L., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 449–516. [Google Scholar]
- Pence, D.B.; Windberg, L.A.; Pence, B.C.; Sprowls, R. The epizootiology and pathology of sarcoptic mange in coyotes, Canis latrans, from south Texas. J. Parasitol. 1983, 69, 1100–1115. [Google Scholar] [CrossRef]
- Ballweber, L. Arthropods. In Veterinary Parasitology; Ballweber, L.R., Messonnier, S.P., Eds.; Butterworth-Heinemann: Oxford, UK, 2001; pp. 5–51. [Google Scholar]
- Arlian, L.G.; Morgan, M.S. A review of Sarcoptes scabiei: Past, present and future. Parasites Vectors 2017, 10, 297. [Google Scholar] [CrossRef]
- Arlian, L.G.; Vyszenski-Moher, D.L. Response of Sarcoptes scabiei var. canis (Acari: Sarcoptidae) to lipids of mammalian skin. J. Med. Entomol. 1995, 32, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; O’Dowd, G.; Woodford, P. Wheater’s Functional Histology E-Book: A Text and Colour Atlas; Elsevier Health Sciences: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bentley, J.K.; Hershenson, M.B. Airway smooth muscle growth in asthma: Proliferation, hypertrophy, and migration. Proc. Am. Thorac. Soc. 2008, 5, 89–96. [Google Scholar] [CrossRef]
- James, A.L.; Elliot, J.G.; Jones, R.L.; Carroll, M.L.; Mauad, T.; Bai, T.R.; Abramson, M.J.; McKay, K.O.; Green, F.H. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Arkwright, P.D.; Bruggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef] [PubMed]
- Staphylokokkeninfektionen bei Hund und Katze. In Tiermedizinische Mikrobiologie, Infektions- und Seuchenlehre, 11th ed.; Selbitz, H.-J.; Truyen, U.; Valentin-Weigand, P. (Eds.) Georg Thieme Verlag KG: Stuttgart, Germany, 2023. [Google Scholar]
- Streptokokkeninfektionen bei Hund und Katze. In Tiermedizinische Mikrobiologie, Infektions- und Seuchenlehre, 11th ed.; Selbitz, H.-J.; Truyen, U.; Valentin-Weigand, P. (Eds.) Georg Thieme Verlag KG: Stuttgart, Germany, 2023. [Google Scholar]
- Aalbaek, B.; Bemis, D.A.; Schjaerff, M.; Kania, S.A.; Frank, L.A.; Guardabassi, L. Coryneform bacteria associated with canine otitis externa. Vet. Microbiol. 2010, 145, 292–298. [Google Scholar] [CrossRef]
- Boynosky, N.A.; Stokking, L.B. Retrospective Evaluation of Canine Dermatitis Secondary to Corynebacterium spp. J. Am. Anim. Hosp. Assoc. 2015, 51, 372–379. [Google Scholar] [CrossRef]
- Collins, M.D.; Hoyles, L.; Lawson, P.A.; Falsen, E.; Robson, R.L.; Foster, G. Phenotypic and phylogenetic characterization of a new Corynebacterium species from dogs: Description of Corynebacterium auriscanis sp. nov. J. Clin. Microbiol. 1999, 37, 3443–3447. [Google Scholar] [CrossRef]
- Moses, I.B.; Santos, F.F.; Gales, A.C. Human Colonization and Infection by Staphylococcus pseudintermedius: An Emerging and Underestimated Zoonotic Pathogen. Microorganisms 2023, 11, 581. [Google Scholar] [CrossRef]
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23, 253-e52. [Google Scholar] [CrossRef]
- Galperine, T.; Cazorla, C.; Blanchard, E.; Boineau, F.; Ragnaud, J.M.; Neau, D. Streptococcus canis infections in humans: Retrospective study of 54 patients. J. Infect. 2007, 55, 23–26. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Kemp, D.J.; Walton, S.F.; Currie, B.J. Scabies: More than just an irritation. Postgrad. Med. J. 2004, 80, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, J.; Guo, K.; Qiu, J.; Cheng, Z.; Liu, F.; Zhao, Y.; Zhang, D.; Guo, H.; Li, H. Outbreak of a novel disease caused by Staphylococcus pseudintermedius in raccoon dogs (Nyctereutes procyonoides). Transbound. Emerg. Dis. 2021, 68, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Tarui, H.; Kido, N.; Imaizumi, K.; Hikosaka, K.; Abe, T.; Minegishi, D.; Tada, Y.; Nakagawa, M.; Tanaka, S.; et al. The complete mitochondrial genome of Sarcoptes scabiei var. nyctereutis from the Japanese raccoon dog: Prediction and detection of two transfer RNAs (tRNA-A and tRNA-Y). Genomics 2019, 111, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Retería-Solís, Z.; Min, A.; Alasaad, S.; Müller, K.; Michler, F.U.; Schmäschke, R.; Wittstatt, U.; Rossi, L.; Wibbelt, G. Genetic epidemiology and pathology of raccoon-derived Sarcoptes mites from urban areas of Germany. Med. Vet. Entomol. 2014, 28, 98–103. [Google Scholar] [CrossRef]
- Sanno, A.; Ander, M.; Agren, E.; Troell, K. Sarcoptic mange in the wild boar, Sus. scrofa, in Sweden. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100060. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Jedrzejewska, B.; Zalewski, A.; Jedrzejewski, W. Facilitative interactions between the Eurasian badger (Meles meles), the red fox (Vulpes vulpes), and the invasive raccoon dog (Nyctereutes procyonoides) in Bialowieza Primeval Forest, Poland. Can. J. Zool. 2008, 86, 1389–1396. [Google Scholar] [CrossRef]
- Shibata, F.; Kawamichi, T. Decline of raccoon dog populations resulting from sarcoptic mange epizootics. Mammalia 1999, 63, 281–290. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M.; Zalewski, A.; Kowalczyk, R. Sarcoptic mange vulnerability in carnivores of the Białowieża Primeval Forest, Poland: Underlying determinant factors. Ecol. Res. 2014, 29, 237–244. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Zalewski, A.; Jędrzejewska, B.; Ansorge, H.; Bunevich, A.N. Reproduction and mortality of invasive raccoon dogs (Nyctereutes procyonoides) in the Białowieża Primeval Forest (eastern Poland). Ann. Zool. Fenn. 2009, 46, 291–301. [Google Scholar] [CrossRef]
- Mulder, J.L. The raccoon dog (Nyctereutes procyonoides) in the Netherlands-its present status and a risk assessment. Lutra 2013, 56, 23–43. [Google Scholar]
- Kido, N.; Itabashi, M.; Takahashi, M.; Futami, M. Epidemiology of sarcoptic mange in free-ranging raccoon dogs (Nyctereutes procyonoides) in Yokohama, Japan. Vet. Parasitol. 2013, 191, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ministry for Energy Transition, Climate Protection, Environment and Nature. Jahresbericht 2022 Zur Biologischen Vielfalt Jagd und Artenschutz; Schleswig-Holstein: Kiel, Germany, 2022. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klink, J.C.; Rieger, A.; Ansorge, H.; Aurich, S.; Hoffmann, C.; Ewers, C.; Raulf, M.-K.; Strube, C.; Siebert, U. Malicious Mites—Sarcoptes scabiei in Raccoon Dogs (Nyctereutes procyonoides) in Schleswig-Holstein, Germany. Pathogens 2023, 12, 1379. https://doi.org/10.3390/pathogens12121379
Klink JC, Rieger A, Ansorge H, Aurich S, Hoffmann C, Ewers C, Raulf M-K, Strube C, Siebert U. Malicious Mites—Sarcoptes scabiei in Raccoon Dogs (Nyctereutes procyonoides) in Schleswig-Holstein, Germany. Pathogens. 2023; 12(12):1379. https://doi.org/10.3390/pathogens12121379
Chicago/Turabian StyleKlink, Jana C., Alexandra Rieger, Hermann Ansorge, Sophie Aurich, Christiane Hoffmann, Christa Ewers, Marie-Kristin Raulf, Christina Strube, and Ursula Siebert. 2023. "Malicious Mites—Sarcoptes scabiei in Raccoon Dogs (Nyctereutes procyonoides) in Schleswig-Holstein, Germany" Pathogens 12, no. 12: 1379. https://doi.org/10.3390/pathogens12121379
APA StyleKlink, J. C., Rieger, A., Ansorge, H., Aurich, S., Hoffmann, C., Ewers, C., Raulf, M.-K., Strube, C., & Siebert, U. (2023). Malicious Mites—Sarcoptes scabiei in Raccoon Dogs (Nyctereutes procyonoides) in Schleswig-Holstein, Germany. Pathogens, 12(12), 1379. https://doi.org/10.3390/pathogens12121379