Differential Contribution of Hydrogen Metabolism to Proteus mirabilis Fitness during Single-Species and Polymicrobial Catheterized Urinary Tract Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Genome Sequencing, and Culture Conditions
2.2. Whole-Cell Hydrogenase Assay
2.3. Growth Curves
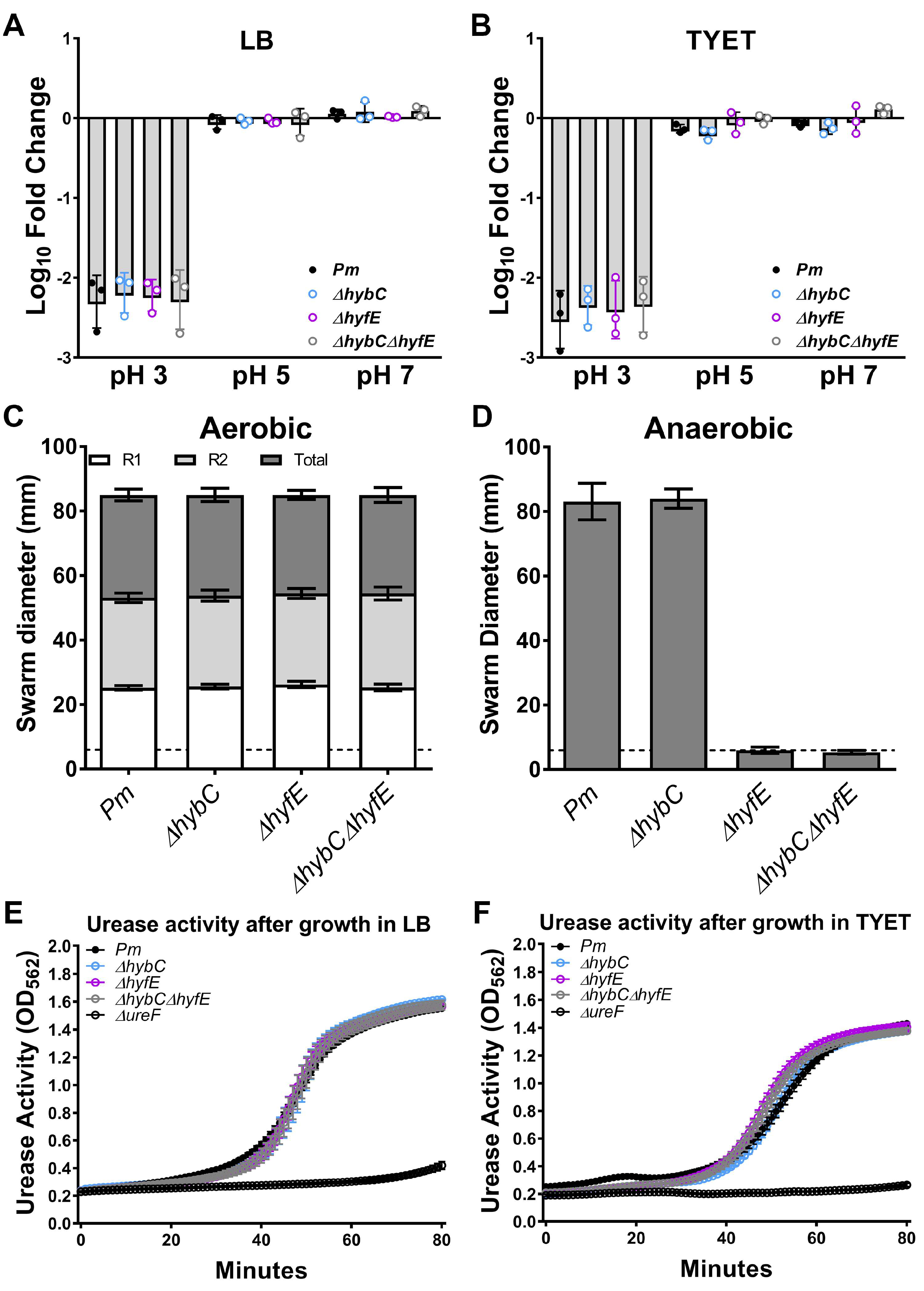
2.4. Acid Tolerance Assay
2.5. Motility Assays
2.6. Urease Assay
2.7. RNA Extraction and qRT-PCR
2.8. Mouse Model of CAUTI
2.9. Statistical Analysis
3. Results
3.1. Genomic Context of the Two [NiFe] Hydrogenases in P. mirabilis HI4320
3.2. HybC and HyfE Both Contribute to Benzyl Viologen Reduction by P. mirabilis HI4320
3.3. H2 Metabolism Is Not Required for P. mirabilis Growth under Standard In Vitro Conditions
3.4. The Hyf-Type Group 4a [NiFe] Hydrogenase Contributes to PMF under Anaerobic Conditions
3.5. HybC and HyfE Are Both Highly Induced during Growth in the TYET Medium
3.6. [NiFe] Hydrogenase Activity Contributes to Competitive Fitness during Growth in the TYET Medium
3.7. Flexible H2 Metabolism Contributes to Competitive Fitness during Experimental CAUTI
3.8. Polymicrobial Interactions Alter the Expression and Contribution of [NiFe] Hydrogenases to P. mirabilis Fitness in TYET Medium
3.9. Polymicrobial Interactions Alter the Contribution of [NiFe] Hydrogenases to P. mirabilis Fitness during Experimental CAUTI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef] [PubMed]
- Catheter-Associated Urinary Tract Infections (CAUTI). Available online: https://www.cdc.gov/hai/ca_uti/uti.html (accessed on 28 February 2023).
- Gaston, J.R.; Johnson, A.O.; Bair, K.L.; White, A.N.; Armbruster, C.E. Polymicrobial interactions in the urinary tract: Is the enemy of my enemy my friend? Infect. Immun. 2021, 89, e00652-20. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.E.; Brauer, A.L.; Humby, M.S.; Shao, J.; Chakraborty, S. Prospective assessment of catheter-associated bacteriuria clinical presentation, epidemiology, and colonization dynamics in nursing home residents. JCI Insight 2021, 6, e144775. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.; Mobley, H.; Pearson, M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.P.; Musher, D.M.; Itin, C. Urease. The primary cause of infection-induced urinary stones. Investig. Urol. 1976, 13, 346–350. [Google Scholar]
- Li, X.; Zhao, H.; Lockatell, C.V.; Drachenberg, C.B.; Johnson, D.E.; Mobley, H.L.T. Visualization of Proteus mirabilis within the Matrix of Urease-Induced Bladder Stones during Experimental Urinary Tract Infection. Infect. Immun. 2002, 70, 389–394. [Google Scholar] [CrossRef]
- Foxman, B.; Brown, P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect. Dis. Clin. N. Am. 2003, 17, 227–241. [Google Scholar] [CrossRef]
- Kim, B.N.; Kim, N.J.; Kim, M.N.; Kim, Y.S.; Woo, J.H.; Ryu, J. Bacteraemia due to tribe Proteeae: A review of 132 cases during a decade (1991–2000). Scand. J. Infect. Dis. 2003, 35, 98–103. [Google Scholar] [CrossRef]
- Watanakunakorn, C.; Perni, S.C. Proteus mirabilis bacteremia: A review of 176 cases during 1980–1992. Scand. J. Infect. Dis. 1994, 26, 361–367. [Google Scholar] [CrossRef]
- Burall, L.S.; Harro, J.M.; Li, X.; Lockatell, C.V.; Himpsl, S.D.; Hebel, J.R.; Johnson, D.E.; Mobley, H.L.T. Proteus mirabilis Genes That Contribute to Pathogenesis of Urinary Tract Infection: Identification of 25 Signature-Tagged Mutants Attenuated at Least 100-Fold. Infect. Immun. 2004, 72, 2922–2938. [Google Scholar] [CrossRef]
- Himpsl, S.D.; Lockatell, C.V.; Hebel, J.R.; Johnson, D.E.; Mobley, H.L.T. Identification of virulence determinants in uropathogenic Proteus mirabilis using signature-tagged mutagenesis. J. Med. Microbiol. 2008, 57, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, X.; Johnson, D.E.; Mobley, H.L.T. Identification of protease and rpoN-associated genes of uropathogenic Proteus mirabilis by negative selection in a mouse model of ascending urinary tract infection. Microbiology 1999, 145, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.M.; Yep, A.; Smith, S.N.; Mobley, H.L.T. Transcriptome of Proteus mirabilis in the Murine Urinary Tract: Virulence and Nitrogen Assimilation Gene Expression. Infect. Immun. 2011, 79, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.E.; Forsyth-DeOrnellas, V.; Johnson, A.O.; Smith, S.N.; Zhao, L.; Wu, W.; Mobley, H.L.T. Genome-wide transposon mutagenesis of Proteus mirabilis: Essential genes, fitness factors for catheter-associated urinary tract infection, and the impact of polymicrobial infection on fitness requirements. PLoS Pathog. 2017, 13, e1006434. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.W.; Tenney, J.H.; Hoopes, J.M.; Muncie, H.L.; Anthony, W.C. A Prospective Microbiologic Study of Bacteriuria in Patients with Chronic Indwelling Urethral Catheters. J. Infect. Dis. 1982, 146, 719–723. [Google Scholar] [CrossRef]
- Benoit, S.L.; Maier, R.J.; Sawers, R.G.; Greening, C. Molecular Hydrogen Metabolism: A Widespread Trait of Pathogenic Bacteria and Protists. Microbiol. Mol. Biol. Rev. MMBR 2020, 84, e00092-19. [Google Scholar] [CrossRef]
- Vignais, P.M.; Billoud, B. Occurrence, Classification, and Biological Function of Hydrogenases: An Overview. Chem. Rev. 2007, 107, 4206–4272. [Google Scholar] [CrossRef]
- Schoelmerich, M.C.; Müller, V. Energy-converting hydrogenases: The link between H2 metabolism and energy conservation. Cell. Mol. Life Sci. 2020, 77, 1461–1481. [Google Scholar] [CrossRef]
- Krab, K.; Oltmann, L.F.; Stouthamer, A.H. Linkage of formate hydrogenlyase with anaerobic respiration in Proteus mirabilis. Biochim. Et Biophys. Acta (BBA)–Bioenerg. 1982, 679, 51–59. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Liaw, S.-J. Deacidification by FhlA-dependent hydrogenase is involved in urease activity and urinary stone formation in uropathogenic Proteus mirabilis. Sci. Rep. 2020, 10, 19546. [Google Scholar] [CrossRef]
- McDowall, J.S.; Murphy, B.J.; Haumann, M.; Palmer, T.; Armstrong, F.A.; Sargent, F. Bacterial formate hydrogenlyase complex. Proc. Natl. Acad. Sci. USA 2014, 111, E3948–E3956. [Google Scholar] [CrossRef]
- Zbell, A.L.; Maier, R.J. Role of the Hya hydrogenase in recycling of anaerobically produced H2 in Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2009, 75, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Parkin, A.; Roessler, M.M.; Murphy, B.J.; Harmer, J.; Palmer, T.; Sargent, F.; Armstrong, F.A. How Escherichia coli Is Equipped to Oxidize Hydrogen under Different Redox Conditions2. J. Biol. Chem. 2010, 285, 3928–3938. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.; Chippendale, G.R.; Fraiman, M.H.; Tenney, J.H.; Warren, J.W. Variable phenotypes of Providencia stuartii due to plasmid-encoded traits. J. Clin. Microbiol. 1985, 22, 851–853. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Warren, J.W. Urease-Positive Bacteriuria and Obstruction of Long-Term Urinary Catheters. J. Clin. Microbiol. 1987, 25, 2216–2217. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.E.; Smith, S.N.; Johnson, A.O.; DeOrnellas, V.; Eaton, K.A.; Yep, A.; Mody, L.; Wu, W.; Mobley, H.L.T. The Pathogenic Potential of Proteus mirabilis is Enhanced by Other Uropathogens During Polymicrobial Urinary Tract Infection. Infect. Immun. 2017, 85, e00808–e00816. [Google Scholar] [CrossRef]
- Pearson, M.M.; Sebaihia, M.; Churcher, C.; Quail, M.A.; Seshasayee, A.S.; Luscombe, N.M.; Abdellah, Z.; Arrosmith, C.; Atkin, B.; Chillingworth, T.; et al. Complete Genome Sequence of Uropathogenic Proteus mirabilis, a Master of both Adherence and Motility. J. Bacteriol. 2008, 190, 4027–4037. [Google Scholar] [CrossRef]
- Pearson, M.M.; Mobley, H.L.T. The type III secretion system of Proteus mirabilis HI4320 does not contribute to virulence in the mouse model of ascending urinary tract infection. J. Med. Microbiol. 2007, 56, 1277–1283. [Google Scholar] [CrossRef]
- Lacasse, M.J.; Sebastiampillai, S.; Cote, J.P.; Hodkinson, N.; Brown, E.D.; Zamble, D.B. A whole-cell, high-throughput hydrogenase assay to identify factors that modulate [NiFe]-hydrogenase activity. J. Biol. Chem. 2019, 294, 15373–15385. [Google Scholar] [CrossRef]
- Brauer, A.L.; Learman, B.S.; Armbruster, C.E. Ynt is the primary nickel import system used by Proteus mirabilis and specifically contributes to fitness by supplying nickel for urease activity. Mol. Microbiol. 2020, 114, 185–199. [Google Scholar] [CrossRef]
- Guiton, P.S.; Hung, C.S.; Hancock, L.E.; Caparon, M.G.; Hultgren, S.J. Enterococcal Biofilm Formation and Virulence in an Optimized Murine Model of Foreign Body-Associated Urinary Tract Infections. Infect. Immun. 2010, 78, 4166–4175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zamble, D.B. Nickel homeostasis and nickel regulation: An overview. Chem. Rev. 2009, 109, 4617–4643. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.F.; Mandrand-Berthelot, M.-A. Genetic and physiological characterization of new Escherichia coli mutants impaired in hydrogenase activity. Biochimie 1986, 68, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.F.; Navarro, C.; de Pina, K.; Quenard, M.; Mandrand, M.A. Antagonistic effect of nickel on the fermentative growth of Escherichia coli K-12 and comparison of nickel and cobalt toxicity on the aerobic and anaerobic growth. Environ. Health Perspect. 1994, 102 (Suppl. S3), 297–300. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Hodges, S.A.; Smith, S.N.; Alteri, C.J.; Mobley, H.L. Arginine promotes Proteus mirabilis motility and fitness by contributing to conservation of the proton gradient and proton motive force. MicrobiologyOpen 2014, 3, 630–641. [Google Scholar] [CrossRef]
- Brauer, A.L.; Learman, B.S.; Taddei, S.M.; Deka, N.; Hunt, B.C.; Armbruster, C.E. Preferential catabolism of l- vs. d-serine by Proteus mirabilis contributes to pathogenesis and catheter-associated urinary tract infection. Mol. Microbiol. 2022, 118, 125–144. [Google Scholar] [CrossRef]
- Gabel, C.V.; Berg, H.C. The speed of the flagellar rotary motor of Escherichia coli varies linearly with proton motive force. Proc. Natl. Acad. Sci. USA 2003, 100, 8748–8751. [Google Scholar] [CrossRef]
- Alteri, C.J.; Himpsl, S.D.; Engstrom, M.D.; Mobley, H.L. Anaerobic Respiration Using a Complete Oxidative TCA Cycle Drives Multicellular Swarming in Proteus mirabilis. MBio 2012, 3, e00365-12. [Google Scholar] [CrossRef]
- Learman, B.S.; Brauer, A.L.; Eaton, K.A.; Armbruster, C.E. A Rare Opportunist, Morganella morganii, Decreases Severity of Polymicrobial Catheter-Associated Urinary Tract Infection. Infect. Immun. 2019, 88, e00691-19. [Google Scholar] [CrossRef]
- Johnson, A.O.; Forsyth, V.; Smith, S.N.; Learman, B.S.; Brauer, A.L.; White, A.N.; Zhao, L.; Wu, W.; Mobley, H.L.T.; Armbruster, C.E. Transposon Insertion Site Sequencing of Providencia stuartii: Essential Genes, Fitness Factors for Catheter-Associated Urinary Tract Infection, and the Impact of Polymicrobial Infection on Fitness Requirements. mSphere 2020, 5, e00412-20. [Google Scholar] [CrossRef]
- Gaston, J.R.; Andersen, M.J.; Johnson, A.O.; Bair, K.L.; Sullivan, C.M.; Guterman, L.B.; White, A.N.; Brauer, A.L.; Learman, B.S.; Flores-Mireles, A.L.; et al. Enterococcus faecalis Polymicrobial Interactions Facilitate Biofilm Formation, Antibiotic Recalcitrance, and Persistent Colonization of the Catheterized Urinary Tract. Pathogens 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Guiton, P.S.; Hannan, T.J.; Ford, B.; Caparon, M.G.; Hultgren, S.J. Enterococcus faecalis Overcomes Foreign Body-Mediated Inflammation To Establish Urinary Tract Infections. Infect. Immun. 2013, 81, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.U. Response of bladder and urethral mucosa to catheterization. JAMA 1979, 242, 451–453. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauer, A.L.; Learman, B.S.; Armbruster, C.E. Differential Contribution of Hydrogen Metabolism to Proteus mirabilis Fitness during Single-Species and Polymicrobial Catheterized Urinary Tract Infection. Pathogens 2023, 12, 1377. https://doi.org/10.3390/pathogens12121377
Brauer AL, Learman BS, Armbruster CE. Differential Contribution of Hydrogen Metabolism to Proteus mirabilis Fitness during Single-Species and Polymicrobial Catheterized Urinary Tract Infection. Pathogens. 2023; 12(12):1377. https://doi.org/10.3390/pathogens12121377
Chicago/Turabian StyleBrauer, Aimee L., Brian S. Learman, and Chelsie E. Armbruster. 2023. "Differential Contribution of Hydrogen Metabolism to Proteus mirabilis Fitness during Single-Species and Polymicrobial Catheterized Urinary Tract Infection" Pathogens 12, no. 12: 1377. https://doi.org/10.3390/pathogens12121377
APA StyleBrauer, A. L., Learman, B. S., & Armbruster, C. E. (2023). Differential Contribution of Hydrogen Metabolism to Proteus mirabilis Fitness during Single-Species and Polymicrobial Catheterized Urinary Tract Infection. Pathogens, 12(12), 1377. https://doi.org/10.3390/pathogens12121377






