Viremia and Inflammatory Cytokines in Dengue: Interleukin-2 as a Biomarker of Infection, and Interferon-α and -γ as Markers of Primary versus Secondary Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Dengue Virus Isolation and Serotyping
2.3. Viremia Quantification in Serum Samples
2.4. Cytokine Determination in Serum Samples
2.5. Sequencing of the Envelope Gene and Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Patients with Dengue virus and Laboratory Diagnosis
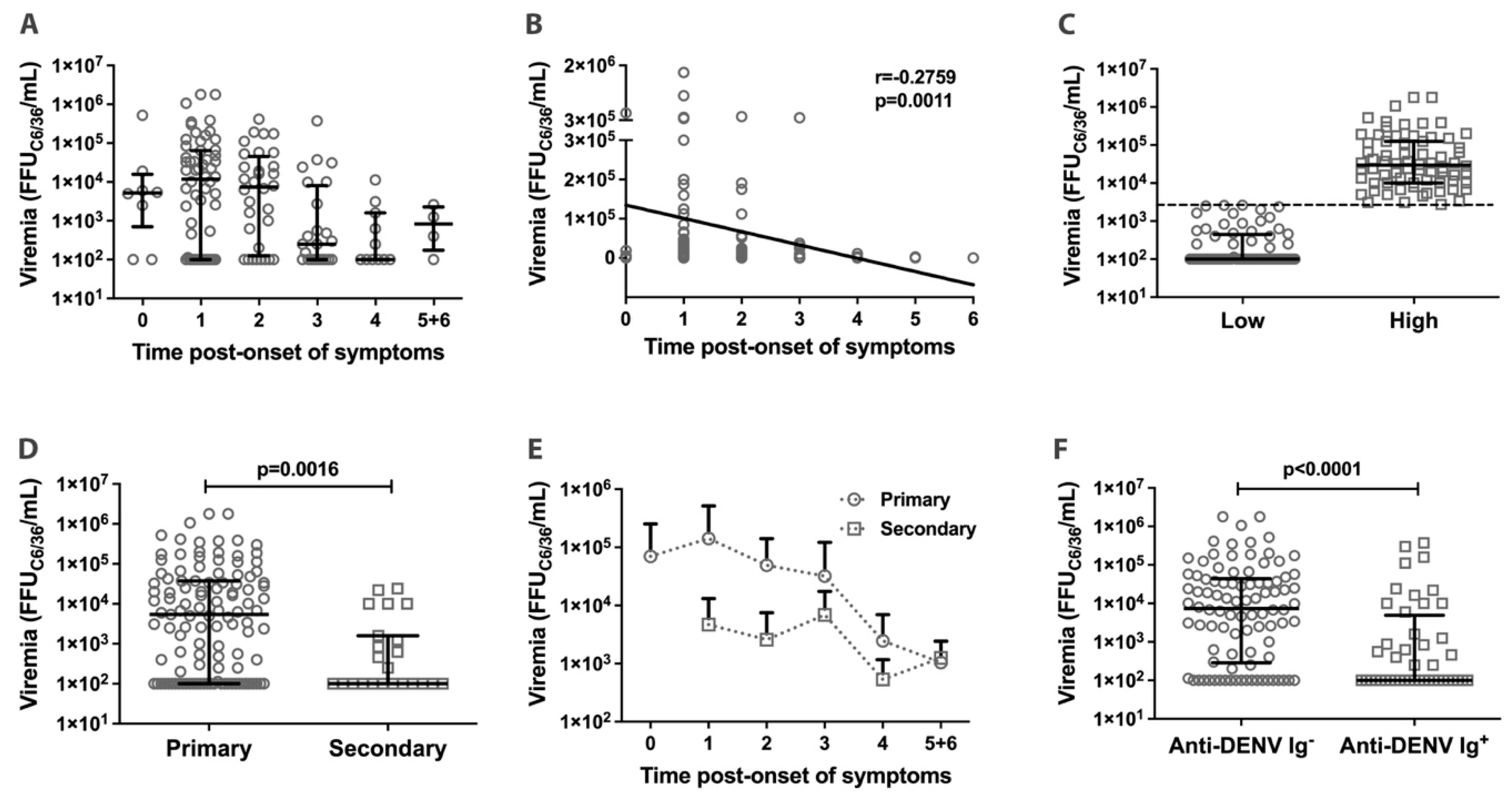
3.2. Dengue Viremia in Acute Phase Patients
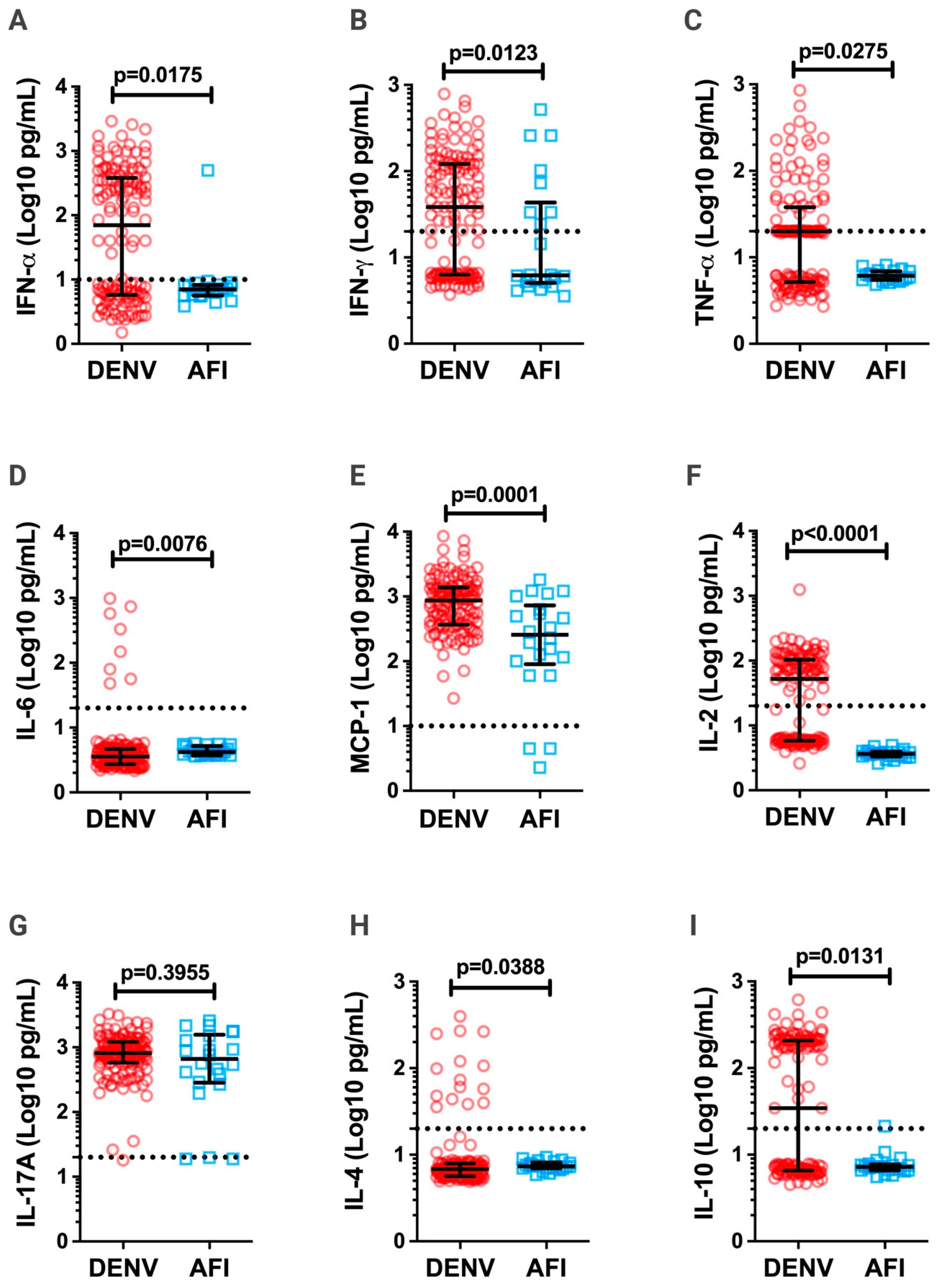
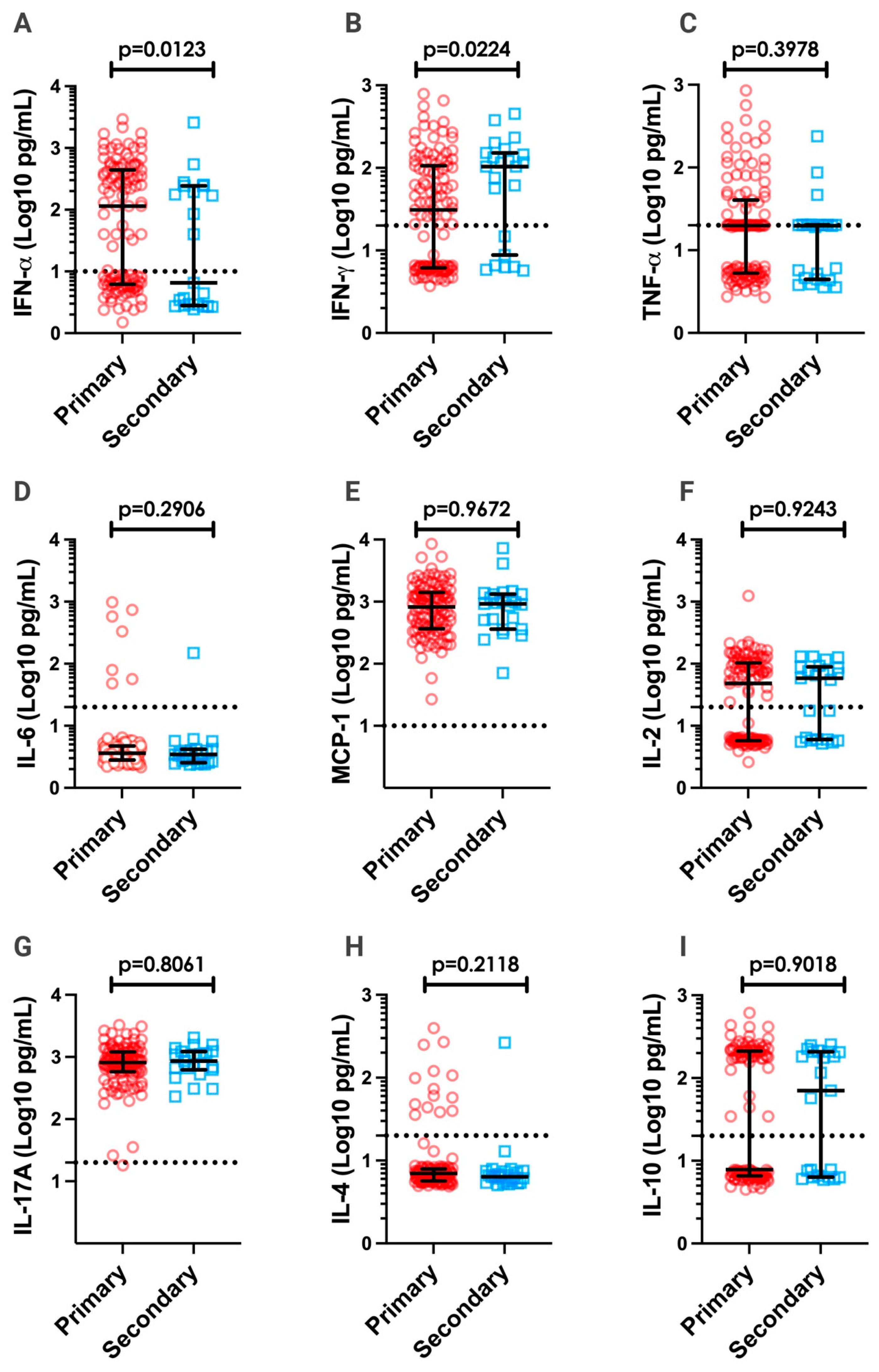
3.3. Inflammatory Cytokines in DV Patients
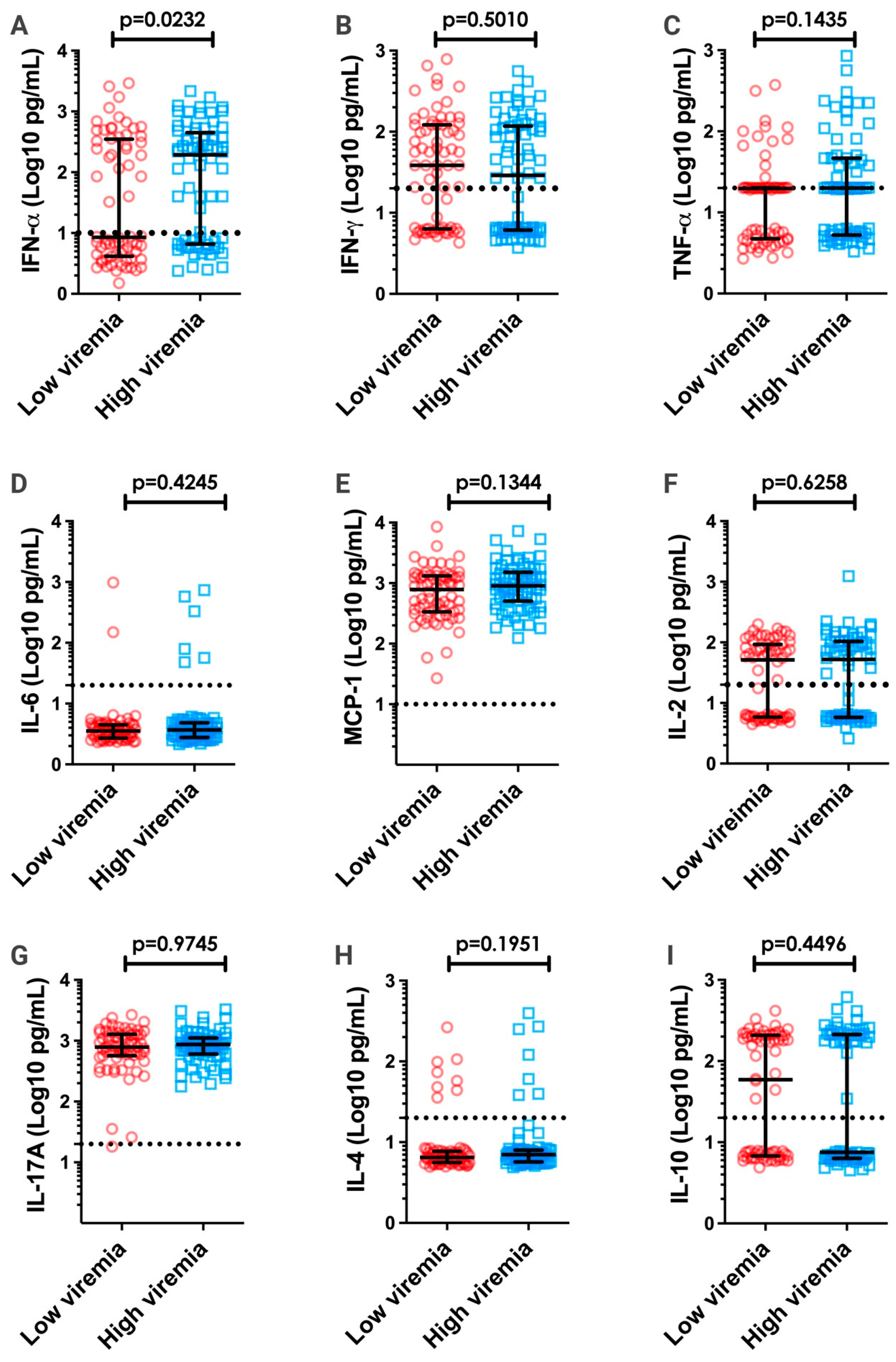
3.4. Correlation between Viremia and Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halstead, S. Pathogenesis of dengue: Challenges to molecular biology. Science 1988, 239, 476–481. [Google Scholar] [CrossRef]
- Gubler, D.J.; Rosen, L. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am. J. Trop. Med. Hyg. 1976, 25, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Laserna, A.; Barahona-Correa, J.; Baquero, L.; Castañeda-Cardona, C.; Rosseli, D. Economic impact of dengue fever in Latin America and the Caribbean: A systematic review. Rev. Panam. Salud Publica 2018, 42, e111. [Google Scholar] [CrossRef]
- Ministério da Saúde (MS). Boletim Epidemiológico 51. Monitoramento dos Casos de Arboviroses URBANAS transmitidas Pelo Aedes Aegypti (dengue, chikungunya e zila) Semanas Epidemiológicas 1 a 50 de 20200. MS 2020, 51. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2020/boletim_epidemiologico_svs_51.pdf/view (accessed on 9 October 2022).
- Pan American Health Organization (PAHO). Epidemiological Update for Dengue, Chikungunya and Zika in 2022; PAHO: Washington, DC, USA, 2022; Available online: https://ais.paho.org/ha_viz/arbo/pdf/PAHO%20Arbo%20Bulletin%202022.pdf (accessed on 10 October 2022).
- Ministério da Saúde (MS). Boletim Epidemiológico 48. Monitoramento dos Casos de Arboviroses URBANAS transmitidas Pelo Aedes Aegypti (dengue, chikungunya e zila) Semanas Epidemiológicas 1 a 51 de 2021. MS 2021, 52. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2021/boletim-epidemiologico-vol-52-no-48.pdf/view (accessed on 9 October 2022).
- Ministério da Saúde (MS). Boletim Epidemiológico 36. Monitoramento dos Casos de Arboviroses Até a Semana Epidemiológica 37 de 2022. MS 2022, 53. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2022/boletim-epidemiologico-vol-53-no36/view (accessed on 9 October 2022).
- World Health Organization (WHO). Dengue and Severe Dengue; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 27 June 2023).
- World Health Organization (WHO). Chapter 2: Clinical Management and Delivery of Clinical Services. In Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; WHO: Geneva, Switzerland, 2009; pp. 23–55. Available online: https://apps.who.int/iris/handle/10665/44188 (accessed on 27 June 2023).
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- Libraty, D.H.; Endy, T.P.; Houng, H.-S.H.; Grenn, S.; Kalayanarrooj, S.; Suntayakorn, S.; Chansiriwongs, W.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; et al. Differing Influences of Virus Burden and Immune Activation on Disease Severity in Secondary Dengue-3 Virus Infections. J. Infect. Dis. 2002, 185, 1213–1221. [Google Scholar] [CrossRef]
- Arias, J.; Valero, N.; Mosquera, J.; Montiel, M.; Reyes, E.; Larreal, Y.; Alvarez-Mon, M. Increased expression. Of cytokines, soluble cytokine receptors, soluble apoptosis ligand and apoptosis in dengue. Virology 2014, 452–453, 42–51. [Google Scholar] [CrossRef]
- Coffey, L.L.; Mertens, E.; Brehin, A.-C.; Fernandez-Garcia, M.D.; Amara, A.; Després, P.; Sakuntabhai, A. Human genetic determinants of dengue virus susceptibility. Microbes Infect. 2009, 11, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Oggs, G.S. T cell responses in dengue viral infections. J. Clin. Virol. 2013, 58, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Silveira, G.F.; Strottmann, D.M.; de Borba, L.; Mansur, D.S.; Zanchin, N.I.; Bordignon, J.; Duarte dos Santos, C.N. Single points mutations in the helicase domain of the NS3 protein enhance dengue virus replicative capacity in human monocyte-derived dendritic cells and circumvent the type I IFN response. Clin. Exp. Immunol. 2016, 183, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Cologna, R.; Armstrong, P.M.; Rico-Hesse, R. Selection for virulent dengue virus occurs in human and mosquitoes. J. Virol. 2005, 79, 853–859. [Google Scholar] [CrossRef]
- Whatts, D.M.; Porter, K.R.; Putvatana, P.; Vasquez, B.; Calampa, C.; Hayes, C.G.; Halstead, S.B. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet 1999, 354, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Littaua, R.; Kurane, I.; Ennis, F.A. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 1990, 144, 3183–3186. [Google Scholar] [CrossRef]
- Halstead, S.B.; Nimmannitya, S.; Cohen, S.N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 1970, 42, 311–328. [Google Scholar]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.N.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef]
- Azeredo, E.L.; Zagne, S.M.; Santiago, M.A.; Gouvea, A.S.; Santana, A.A.; Neves-Souza, P.C.; Nogueira, R.M.; Miagostovich, M.P.; Kubelka, C.F. Characterization of lymphocytes response and cytokine patterns in patients with dengue fever. Immunobiology 2001, 204, 494–507. [Google Scholar] [CrossRef]
- Chen, R.-F.; Liu, J.-W.; Yeh, W.-T.; Wang, L.; Chang, J.-C.; Yu, H.-R.; Cheng, J.-T.; Yang, K.D. Altered T helper 1 reaction but not increase of virus load in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microb. 2005, 44, 43–50. [Google Scholar] [CrossRef]
- Chaturvedi, U.C. Shift to Th2 cytokine response in dengue haemorrhagic fever. Indian J. Med. Res. 2009, 129, 1–3. [Google Scholar]
- Atrasheuskaya, A.; Petzelbauer, P.; Fredeking, T.M.; Ignatyev, G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol. Med. Microbiol. 2003, 35, 33–42. [Google Scholar] [CrossRef]
- Yeh, W.-T.; Chen, R.-F.; Wang, L.; Liu, J.-W.; Yang, K.D. Implications of previous subclinical dengue infection but not virus load in dengue hemorrhagic fever. FEMS Immunol. Med. Microb. 2006, 48, 84–90. [Google Scholar] [CrossRef]
- Kuczera, D.; Bavia, L.; Mosimann, A.L.P.; Koishi, A.C.; Mazzarotto, G.A.C.A.; Aoki, M.N.; Mansano, A.M.F.; Tomeleri, E.I.; Costa Junior, W.L.; Miranda, M.M.; et al. Isolation of dengue virus serotype 4 genotype II from a patient with high viral load and a mixed Th1/Th17 inflammatory cytokine profile in South Brazil. Virol. J. 2016, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Sharma, M.; Pujhari, S.K.; Appannanavar, S.B.; Ratho, R.K. Clinical applicability of sinlge-tube multiplex reverse-transcriptase PCR in dengue virus diagnosis and serotyping. J. Clin. Lab. Anal. 2011, 25, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; Arruda, T.B.; Suzukawa, A.A.; Bortot, L.O.; Dias-Baruffi, M.; Verri, W.A., Jr.; et al. The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef]
- Ewing, B.; Hillier, L.; Wendl, M.C.; Green, P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998, 8, 175–185. [Google Scholar] [CrossRef]
- Gordon, D.; Abajian, C.; Green, P. Consed: A graphical tool for sequence finishing. Genome Res. 1998, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Bruycker-Nogueira, F.; Nogueira, R.M.R.; Faria, N.R.C.; Simões, J.B.S.; Nunes, P.C.G.; Filipis, A.M.B.; Dos Santos, F.B. Insights of the genetic diversity of DENV-1 detected in Brazil in 25 years: Analysis of the envelope domain III allows lineages characterization. Infect. Genet. Evol. 2015, 34, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Bavia, L.; Melanda, F.N.; de Arruda, T.B.; Mosimann, A.L.P.; Silveira, G.F.; Aoki, M.N.; Kuczera, D.; Sarzi, M.L.; Junior, W.L.C.; Conchon-Costa, I.; et al. Epidemiological study on dengue in southern Brazil under the perspective of climate and poverty. Sci. Rep. 2020, 10, 2127. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz Hernandez, S.I.; Puerta-Guardo, H.; Flores-Aguilar, H.; González-Mateos, S.; López-Martinez, I.; Ortiz-Navarrete, V.; Ludert, J.E.; Del Angel, R.M. A Strong interferon response correlates with milder dengue clinical condition. J. Clin. Virol. 2014, 60, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Roehrig, J.T. New mouse models for dengue virus vaccine testing. J. Virol. 1999, 73, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Elbishbishi, E.A.; Agarwal, R.; Raghupathy, R.; Nagar, R.; Tandon, R.; Pacsa, A.S.; Younis, O.I.; Azizieh, F. Sequential production of cytokines by dengue virus-infected human peripheral blood leukocyte cultures. J. Med. Virol. 1999, 59, 335–340. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Agarwal, R.; Elbishbishi, E.A.; Mustafa, A.S. Cytokine cascade in dengue hemorrhagic fever: Implications for pathogenesis. FEMS Immunol. Med. Microbiol. 2000, 28, 183–188. [Google Scholar] [CrossRef]
- Abhishek, K.S.; Chakravarti, A.; Baveja, C.P.; Kumar, N.; Siddiqui, O.; Kumar, S. Association of interleukin-2, -4 and -10 with dengue severity. Indian J. Pathol. Microbiol. 2017, 60, 66–69. [Google Scholar] [CrossRef]
- Green, S.; Vaughn, D.W.; Kalayanarooj, S.; Nimmannitya, S.; Suntayakorn, S.; Nisalak, A.; Lew, R.; Innis, B.L.; Kurane, I.; Rothman, A.L.; et al. Early immune activation on acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 1999, 179, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsapaya, J.; Duangchinda, T.; Dejnirattisai, W.; Vasanawathana, S.; Avirutnan, P.; Jairungsri, A.; Khemnu, N.; Tangthawornchaikul, N.; Chotiyarnwong, P.; Sae-Jang, K.; et al. T-cell responses in dengue hemorrhagic fever: Are cross-reactive T cells optimal? J. Immunol. 2006, 176, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Goncalvez, A.P.; Engle, R.E.; St Claire, M.; Purcell, R.H.; Lai, C.J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 2007, 104, 9422–9427. [Google Scholar] [CrossRef]
- Singla, M.; Kar, M.; Sethi, T.; Kabra, S.K.; Lodha, R.; Chandele, A.; Medigeshi, G.R. Immune Response to Dengue Virus Infection in Pediatric Patients in New Delhi, India—Association of Viremia, Inflammatory Mediators and Monocytes with Disease Severity. PLoS Negl. Trop. Dis. 2016, 10, e0004497. [Google Scholar] [CrossRef]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of Vasoactive Cytokines by Antibody-Enhanced Dengue Virus Infection of a Human Mast Cell/Basophil Line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977, 146, 201–217. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J.; Allison, A.C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J. Exp. Med. 1977, 146, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jordán, J.L.; Sánchez-Burgos, G.G.; Laurent-Rolle, M.; García-Sastre, A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef]
- Puc, I.; Ho, T.-C.; Yen, K.-L.; Vats, A.; Tsai, J.-J.; Chen, P.-L.; Chien, Y.-W.; Lo, Y.-C.; Perng, G.C. Cytokine Signature of Dengue Patients at Different Severity of the Disease. Int. J. Mol. Sci. 2021, 22, 2879. [Google Scholar] [CrossRef]
- Schul, W.; Liu, W.; Xu, H.-Y.; Flamand, M.; Vasudevan, S.G. A Dengue Fever Viremia Model in Mice Shows Reduction in Viral Replication and Supression of the Inflammatory Response after Treatment with Antiviral Drugs. J. Infect. Dis. 2007, 195, 665–674. [Google Scholar] [CrossRef]
- Duyen, H.T.L.; Ngoc, T.V.; Ha, D.T.; Hang, V.T.T.; Kieu, N.T.T.; Young, P.R.; Farrar, J.J.; Simmons, C.P.; Wolbers, M.; Wills, B.A. Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: Differential effects according to serotype and immune status. J. Infect. Dis. 2011, 203, 1292–1300. [Google Scholar] [CrossRef]
- Guzmán, M.G. Global voices of science. Deciphering dengue: The Cuban experience. Science 2005, 309, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Kou, Z.; Zhang, F.; Yao, X.; Liu, S.; Ma, J.; Zhou, Y.; Zhao, W.; Tang, X.; Jin, X. Both Viremia and Cytokine Levels Associate with the Lack of Severe Disease in Secondary Dengue 1 Infection among Adult Chinese Patients. PLoS ONE 2010, 5, e15631. [Google Scholar] [CrossRef]
- Voung, N.L.; Quyen, N.T.H.; Tien, N.T.H.; Tuan, N.M.; Kien, D.T.H.; Lam, P.K.; Tam, D.T.H.; Ngoc, T.V.; Yacoub, S.; Jaenisch, T.; et al. Higher Plasma Viremia in the Febrile Phase Is Associated With Adverse Dengue Outcomes Irrespective of Infecting Serotype or Host Immmune Status: An Analisys of 5642 Vietnamese Cases. Clin. Infect. Dis. 2021, 72, 21074–21083. [Google Scholar] [CrossRef]
- Wang, W.K.; Chen, H.L.; Yang, C.F.; Hsieh, S.C.; Juan, C.C.; Chang, S.M.; Yu, C.C.; Lin, L.H.; Huang, J.H.; King, C.C. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin. Infect. Dis. 2006, 43, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Cardosa, M.J.; Guzman, M.G. Of cascades and perfect storms: The immunopathogenesis of dengue hemorrhagic fever-dengue shock syndrome. Immunol. Cell Biol. 2007, 85, 43–45. [Google Scholar] [CrossRef]
- Kurane, I.; Innis, B.L.; Nimmannitya, S.; Nisalak, A.; Meager, A.; Ennis, F.A. High levels of interferon alpha in the sera of children with dengue virus infection. Am. J. Trop. Med. Hyg. 1993, 48, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Bozza, F.A.; Cruz, O.G.; Zagne, S.M.O.; Azeredo, E.L.; Nogueira, R.M.R.; Assis, E.F.; Bozza, P.T.; Kubelka, C.F. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect. Dis. 2008, 8, 86. [Google Scholar] [CrossRef]
- Mangada, M.M.; Rothman, A.L. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J. Immunol. 2005, 175, 2676–2683. [Google Scholar] [CrossRef]
- Mangada, M.M.; Endy, T.P.; Nisalak, A.; Chunsuttiwat, S.; Vaughn, D.W.; Libraty, D.H.; Green, S.; Ennis, F.A.; Rothman, A.L. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J. Infect. Dis. 2002, 185, 1697–1703. [Google Scholar] [CrossRef]
- Hatch, S.; Endy, T.P.; Thomas, S.; Mathew, A.; Potts, J.; Pazoles, P.; Libraty, D.H.; Gibbons, R.; Rothman, A.L. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J. Infect. Dis. 2011, 203, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.H.G.; Figueiredo, M.M.; Queiroz, C.P.; Magalhães, T.V.B.; Mafra, A.; Diniz, L.M.O.; da Costa, U.L.; Gollob, K.J.; Antonelli, L.R.V.; Santiago, H.C. T cells producing multiple combinations of IFN-γ, TNF and IL10 are associated with mild forms of dengue infection. Immunology 2020, 160, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Innis, B.L.; Nimmannitya, S.; Nisalak, A. Activation of T Lymphocytes in Dengue Virus Infections: High levels of Soluble Interleukin 2 Receptor, Soluble CD4, Soluble CD8, Interleukin 2 and Interferon-γ in Sera of Children with Dengue. J. Clin. Investig. 1991, 88, 1473–1480. [Google Scholar] [CrossRef]
- Morgan, D.A.; Ruscetti, F.W.; Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976, 193, 1007–1008. [Google Scholar] [CrossRef]
- Gillis, S.; Baker, P.E.; Ruscetti, F.W.; Smith, K.A. Long-term culture of human antigen-specific cytotoxic T cells lines. J. Exp. Med. 1978, 148, 1093–1098. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Ann. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Zheng, S.G.; Wang, J.; Wang, P.; Dixon, G.; Horwitz, D.A. IL-2 Is Essential for TGF-β to Convert Naïve CD4+CD25+ cells to CD25+Foxp3+ Regulatory T Cells and for Expansion of These Cells. J. Immunol. 2007, 178, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Luhn, K.; Simmons, C.P.; Moran, E.; Dung, N.T.P.; Chau, T.N.B.; Quyen, N.T.H.; Thao, L.T.T.; Ngoc, T.V.; Dung, N.M.; Wills, B.; et al. Increased frequencies of CD4+CD25(high) regulatory T cells in acute dengue infection. J. Exp. Med. 2007, 204, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, J.-X.; Leonard, W.J. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Imunol. 2011, 23, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Imad, H.A.; Phumratanaprapin, W.; Phonrat, B.; Chotivanich, K.; Charunwatthana, P.; Muangnoichareon, S.; Khusmith, S.; Tantawichien, T.; Phadungsombat, J.; Nakayama, E.; et al. Cytokine Expression in Dengue Fever and Dengue Hemorrhagic Fever Patients with Bleedind and Severe Hepatitis. Am. J. Trop. Med. Hyg. 2020, 102, 943–950. [Google Scholar] [CrossRef]
- Simpson, S.; Kaislasuo, J.; Guller, S.; Pal, L. Thermal stability of cytokines: A review. Cytokine 2020, 125, 154829. [Google Scholar] [CrossRef] [PubMed]
| Parameters | DENV | AFI | p-Value | |
|---|---|---|---|---|
| Patients | 138 (100%) | 22 (100%) | - | |
| Age (years) mean ± SD | 35.26 ± 17.52 | 28.45 ± 16.07 | 0.0603 | |
| Gender | Female | 68 (49.3%) | 14 (63.6%) | 0.2545 |
| Male | 70 (50.7%) | 8 (36.4%) | ||
| Serology | NS1+/IgM− | 105 (76.1%) | 0 | - |
| NS1−/IgM+ | 9 (6.5%) | 0 | ||
| NS1+/IgM+ | 24 (17.4%) | 0 | ||
| IgG+ | 23 (16.7%) | 5 (22.7%) | 0.5455 | |
| IgG− | 115 (83.3%) | 17 (77.3%) | ||
| Clinical | Fever | 124 (89.8%) | 18 (81.8%) | 0.4697 |
| Symptoms | Headache | 116 (84.1%) | 19 (86.4%) | |
| Myalgia | 106 (76.8%) | 16 (72.7%) | ||
| Prostration | 90 (65.2%) | 9 (40.9%) | ||
| Retro-orbital pain | 70 (50.7%) | 12 (54.5%) | ||
| Nausea | 73 (52.9%) | 11 (50%) | ||
| Arthralgia | 56 (40.6%) | 11 (50%) | ||
| Diarrhea | 14 (10.1%) | 6 (27.2%) | ||
| Exanthema | 9 (6.5%) | 0 | ||
| Others * | 9 (6.5%) | 2 (9.1%) | ||
| Hemorrhagic | Tourniquet test | 6 (4.3%) | 2 (9.1%) | 1.0000 |
| Symptoms | Petechiae | 2 (1.4%) | 0 | |
| Epistaxis | 0 | 0 | ||
| Gum bleeding | 0 | 0 | ||
| Gut bleeding | 0 | 0 | ||
| Cytokine | AUC | Youden Index | Cut off | Sensitivity% | Specificity% |
|---|---|---|---|---|---|
| IFN-α | 0.6581 | 0.5197 | <9.930 | 95.45 | 56.52 |
| IFN-γ | 0.6665 | 0.3445 | <6.300 | 59.09 | 75.36 |
| TNF-α | 0.6467 | 0.5797 | <13.61 | 100.0 | 57.97 |
| IL-6 | 0.6775 | 0.5145 | >3.615 | 100.0 | 51.45 |
| MCP-1 | 0.7546 | 0.4038 | <192.7 | 45.45 | 94.93 |
| IL-2 | 0.9890 | 0.9493 | <4.975 | 100.0 | 94.93 |
| IL-17A | 0.5567 | 0.2754 | <562.8 | 50.00 | 77.54 |
| IL-4 | 0.6375 | 0.3399 | >6.890 | 81.82 | 52.17 |
| IL-10 | 0.6650 | 0.5072 | <27.90 | 100.0 | 50.72 |
| IL-2 vs | Spearman (r) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| IFN-α | 0.3225 | 0.1594 to 0.4685 | 0.0001 |
| IFN-γ | 0.3516 | 0.1912 to 0.4938 | <0.0001 |
| TNF-α | 0.5200 | 0.3822 to 0.6352 | <0.0001 |
| IL-6 | −0.4963 | −0.6157 to −0.3545 | <0.0001 |
| IL-4 | −0.2256 | −0.3827 to −0.05574 | 0.0078 |
| IL-10 | 0.7322 | 0.6410 to 0.8031 | <0.0001 |
| IL-17A | 0.2137 | 0.04330 to 0.3720 | 0.0118 |
| MCP-1 | 0.06995 | −0.1033 to 0.2391 | 0.4150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Arruda, T.B.; Bavia, L.; Mosimann, A.L.P.; Aoki, M.N.; Sarzi, M.L.; Conchon-Costa, I.; Wowk, P.F.; Duarte dos Santos, C.N.; Pavanelli, W.R.; Silveira, G.F.; et al. Viremia and Inflammatory Cytokines in Dengue: Interleukin-2 as a Biomarker of Infection, and Interferon-α and -γ as Markers of Primary versus Secondary Infection. Pathogens 2023, 12, 1362. https://doi.org/10.3390/pathogens12111362
de Arruda TB, Bavia L, Mosimann ALP, Aoki MN, Sarzi ML, Conchon-Costa I, Wowk PF, Duarte dos Santos CN, Pavanelli WR, Silveira GF, et al. Viremia and Inflammatory Cytokines in Dengue: Interleukin-2 as a Biomarker of Infection, and Interferon-α and -γ as Markers of Primary versus Secondary Infection. Pathogens. 2023; 12(11):1362. https://doi.org/10.3390/pathogens12111362
Chicago/Turabian Stylede Arruda, Thaís Bonato, Lorena Bavia, Ana Luiza Pamplona Mosimann, Mateus Nobrega Aoki, Maria Lo Sarzi, Ivete Conchon-Costa, Pryscilla Fanini Wowk, Claudia Nunes Duarte dos Santos, Wander Rogério Pavanelli, Guilherme Ferreira Silveira, and et al. 2023. "Viremia and Inflammatory Cytokines in Dengue: Interleukin-2 as a Biomarker of Infection, and Interferon-α and -γ as Markers of Primary versus Secondary Infection" Pathogens 12, no. 11: 1362. https://doi.org/10.3390/pathogens12111362
APA Stylede Arruda, T. B., Bavia, L., Mosimann, A. L. P., Aoki, M. N., Sarzi, M. L., Conchon-Costa, I., Wowk, P. F., Duarte dos Santos, C. N., Pavanelli, W. R., Silveira, G. F., & Bordignon, J. (2023). Viremia and Inflammatory Cytokines in Dengue: Interleukin-2 as a Biomarker of Infection, and Interferon-α and -γ as Markers of Primary versus Secondary Infection. Pathogens, 12(11), 1362. https://doi.org/10.3390/pathogens12111362






