Association of Ambient Temperature and Absolute Humidity with the Effective Reproduction Number of COVID-19 in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design Setting
2.2. Empirical Datasets
2.2.1. Epidemiological Data
2.2.2. Meteorological Data
2.2.3. Mobility Data
2.2.4. Restrictions
2.3. Statistical Analysis
2.3.1. Descriptive Descriptions
2.3.2. Estimation of Time-Varying Transmissibility
2.3.3. Construction of the Time-Series Statistical Model
2.3.4. Sensitivity Analysis
2.4. Ethical Considerations
3. Results
3.1. Descriptive Statistics
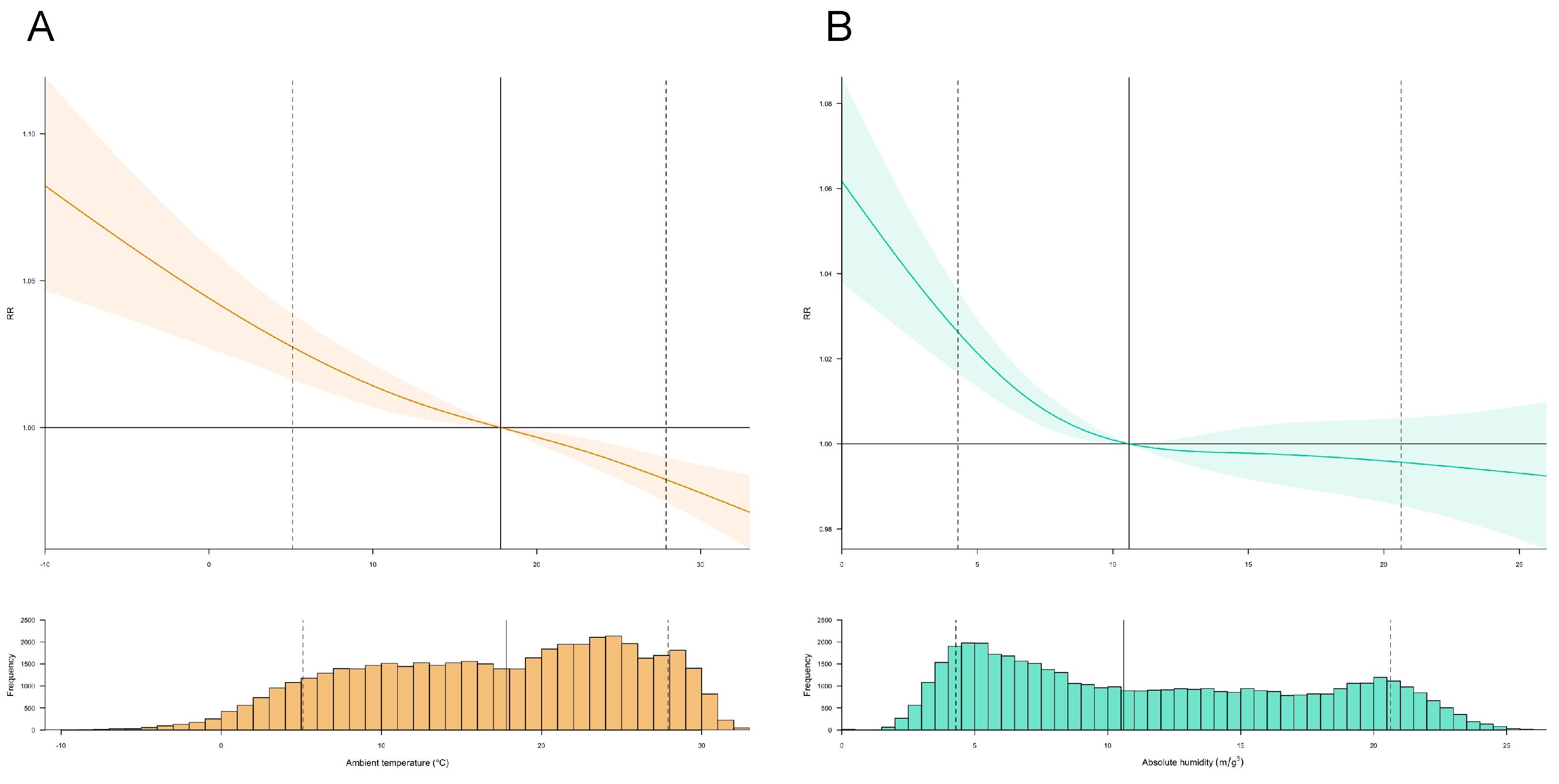
3.2. Exposure–Response Relationships between Meteorological Drivers and Transmissibility
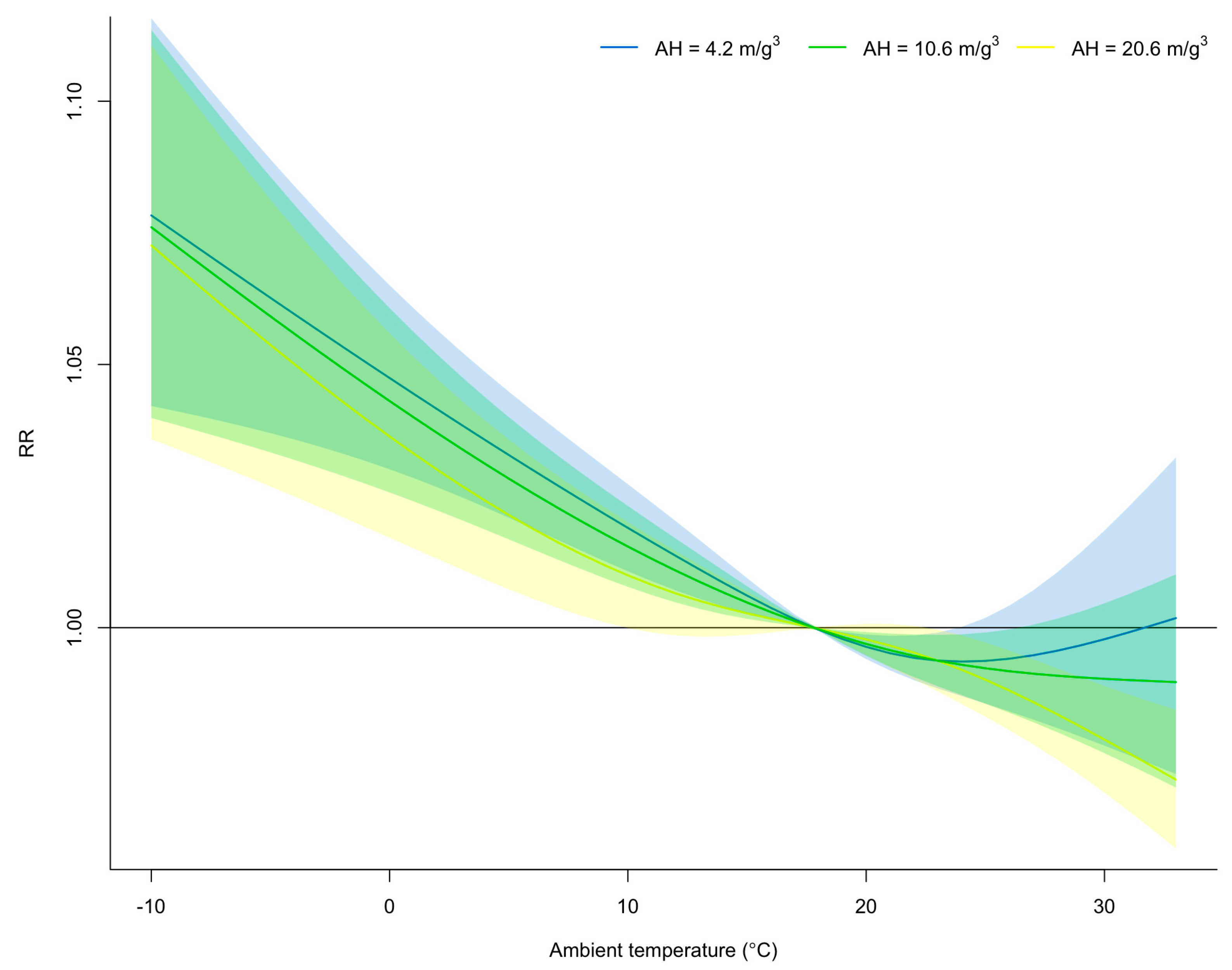
3.3. Effect Modification of Absolute Humidity on the Exposure–Response Relationship between Mean Ambient Temperature and Time-Varying Transmissibility
3.4. Further Investigations
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heymann, D.L.; Shindo, N. COVID-19: What is next for public health? Lancet 2020, 395, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Oshitani, H. COVID lessons from Japan: The right messaging empowers citizens. Nature 2022, 605, 589. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Endo, A.; Kinoshita, R.; Nishiura, H. Projecting a second wave of COVID-19 in Japan with variable interventions in high-risk settings. R. Soc. Open Sci. 2021, 8, 202169. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y.; Sando, E.; Tsuchiya, N.; Miyahara, R.; Yasuda, I.; Ko, Y.K.; Saito, M.; Morimoto, K.; Imamura, T.; Shobugawa, Y.; et al. Clusters of Coronavirus Disease in Communities, Japan, January-April 2020. Emerg. Infect. Dis. 2020, 26, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Endo, A.; Akhmetzhanov, A.R.; Nishiura, H. Predicting the effective reproduction number of COVID-19: Inference using human mobility, temperature, and risk awareness. Int. J. Infect. Dis. 2021, 113, 47–54. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Requests for Reducing Operation Hours from November 2020. Available online: https://www.mhlw.go.jp/stf/newpage_16945.html (accessed on 19 July 2023).
- Cabinet Relations Office. Ongoing Topics. Available online: https://japan.kantei.go.jp/ongoingtopics/_00038.html (accessed on 19 July 2023).
- Ito, K.; Piantham, C.; Nishiura, H. Predicted dominance of variant Delta of SARS-CoV-2 before Tokyo Olympic Games, Japan, July 2021. Eurosurveillance 2021, 26, 2100570. [Google Scholar] [CrossRef]
- Okada, Y.; Yamasaki, S.; Nishida, A.; Shibasaki, R.; Nishiura, H. Night-time population consistently explains the transmission dynamics of coronavirus disease 2019 in three megacities in Japan. Front. Public Health 2023, 11, 1163698. [Google Scholar] [CrossRef]
- Wu, Y.; Jing, W.; Liu, J.; Ma, Q.; Yuan, J.; Wang, Y.; Du, M.; Liu, M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total. Environ. 2020, 729, 139051. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, Y.; Jing, W.; Liu, J.; Du, M.; Wang, Y.; Liu, M. Association between meteorological factors and daily new cases of COVID-19 in 188 countries: A time series analysis. Sci. Total. Environ. 2021, 780, 146538. [Google Scholar] [CrossRef]
- Guo, C.; Bo, Y.; Lin, C.; Li, H.B.; Zeng, Y.; Zhang, Y.; Hossain, M.S.; Chan, J.W.M.; Yeung, D.W.; Kwok, K.O.; et al. Meteorological factors and COVID-19 incidence in 190 countries: An observational study. Sci. Total. Environ. 2021, 757, 143783. [Google Scholar] [CrossRef]
- Azuma, K.; Kagi, N.; Kim, H.; Hayashi, M. Impact of climate and ambient air pollution on the epidemic growth during COVID-19 outbreak in Japan. Environ. Res. 2020, 190, 110042. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhu, Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020, 724, 138201. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.Q.; Zhang, H.Y.; Zhao, H.; Che, T.L.; Zhang, A.R.; Liu, M.J.; Shi, W.Q.; Guo, J.P.; Zhang, Y.; Liu, W.; et al. Meteorological conditions and nonpharmaceutical interventions jointly determined local transmissibility of COVID-19 in 41 Chinese cities: A retrospective observational study. Lancet Reg. Health West. Pac. 2020, 2, 100020. [Google Scholar] [CrossRef] [PubMed]
- Nottmeyer, L.; Armstrong, B.; Lowe, R.; Abbott, S.; Meakin, S.; O’Reilly, K.; von Borries, R.; Schneider, R.; Royé, D.; Hashizume, M.; et al. The association of COVID-19 incidence with temperature, humidity, and UV radiation—A global multi-city analysis. Sci. Total Environ. 2022, 854, 158636. [Google Scholar] [CrossRef]
- Sarkodie, S.A.; Owusu, P.A. Impact of meteorological factors on COVID-19 pandemic: Evidence from top 20 countries with confirmed cases. Environ. Res. 2020, 191, 110101. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, Y.; Jing, W.; Liu, J.; Du, M.; Wang, Y.; Liu, M. Non-linear correlation between daily new cases of COVID-19 and meteorological factors in 127 countries. Environ. Res. 2021, 193, 110521. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, H.; Strobl, E.; Li, H.; Li, R.; Jensen, S.S.; Zhang, Y. The role of weather conditions in COVID-19 transmission: A study of a global panel of 1236 regions. J. Clean Prod. 2021, 292, 125987. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, K.M.; Auzenbergs, M.; Jafari, Y.; Liu, Y.; Flasche, S.; Lowe, R. Effective transmission across the globe: The role of climate in COVID-19 mitigation strategies. Lancet Planet. Health 2020, 4, e172. [Google Scholar] [CrossRef] [PubMed]
- Zeka, A.; Tobias, A.; Leonardi, G.; Bianchi, F.; Lauriola, P.; Crabbe, H.; Vardoulakis, S.; Guo, Y.; Honda, Y.; Gasparrini, A.; et al. Responding to COVID-19 requires strong epidemiological evidence of environmental and societal determining factors. Lancet Planet. Health 2020, 4, e375–e376. [Google Scholar] [CrossRef]
- Yamasaki, L.; Murayama, H.; Hashizume, M. The impact of temperature on the transmissibility potential and virulence of COVID-19 in Tokyo, Japan. Sci. Rep. 2021, 11, 24477. [Google Scholar] [CrossRef]
- Hoang, T.; Tran, T.T.A. Ambient air pollution, meteorology, and COVID-19 infection in Korea. J. Med. Virol. 2021, 93, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Yin, J. Short-Term Effects of Ambient Ozone, PM2.5 and Meteorological Factors on COVID-19 Confirmed Cases and Deaths in Queens, New York. Int. J. Environ. Res. Public Health 2020, 17, 4047. [Google Scholar] [CrossRef]
- Meyer, A.; Sadler, R.; Faverjon, C.; Cameron, A.R.; Bannister-Tyrrell, M. Evidence That Higher Temperatures Are Associated With a Marginally Lower Incidence of COVID-19 Cases. Front. Public Health 2020, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Pequeno, P.; Mendel, B.; Rosa, C.; Bosholn, M.; Souza, J.L.; Baccaro, F.; Barbosa, R.; Magnusson, W. Air transportation, population density and temperature predict the spread of COVID-19 in Brazil. PeerJ 2020, 8, e9322. [Google Scholar] [CrossRef] [PubMed]
- Runkle, J.D.; Sugg, M.M.; Leeper, R.D.; Rao, Y.; Matthews, J.L.; Rennie, J.J. Short-term effects of specific humidity and temperature on COVID-19 morbidity in select US cities. Sci. Total Environ. 2020, 740, 140093. [Google Scholar] [CrossRef]
- Rubin, D.; Huang, J.; Fisher, B.T.; Gasparrini, A.; Tam, V.; Song, L.; Wang, X.; Kaufman, J.; Fitzpatrick, K.; Jain, A.; et al. Association of Social Distancing, Population Density, and Temperature With the Instantaneous Reproduction Number of SARS-CoV-2 in Counties Across the United States. JAMA Netw. Open 2020, 3, e2016099. [Google Scholar] [CrossRef]
- Jüni, P.; Rothenbühler, M.; Bobos, P.; Thorpe, K.E.; da Costa, B.R.; Fisman, D.N.; Slutsky, A.S.; Gesink, D. Impact of climate and public health interventions on the COVID-19 pandemic: A prospective cohort study. CMAJ 2020, 192, e566–e573. [Google Scholar] [CrossRef]
- Carleton, T.; Cornetet, J.; Huybers, P.; Meng, K.C.; Proctor, J. Global evidence for ultraviolet radiation decreasing COVID-19 growth rates. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Han, J.; Yin, J.; Wu, X.; Wang, D.; Li, C. Environment and COVID-19 incidence: A critical review. J. Environ Sci. 2023, 124, 933–951. [Google Scholar] [CrossRef]
- Donzelli, G.; Biggeri, A.; Tobias, A.; Nottmeyer, L.N.; Sera, F. Role of meteorological factors on SARS-CoV-2 infection incidence in Italy and Spain before the vaccination campaign. A multi-city time series study. Environ. Res. 2022, 211, 113134. [Google Scholar] [CrossRef]
- Weaver, A.K.; Head, J.R.; Gould, C.F.; Carlton, E.J.; Remais, J.V. Environmental Factors Influencing COVID-19 Incidence and Severity. Annu. Rev. Public Health 2022, 43, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P.J.; Goldberg, M.S. Methodological Considerations for Epidemiological Studies of Air Pollution and the SARS and COVID-19 Coronavirus Outbreaks. Environ. Health Perspect. 2020, 128, 95001. [Google Scholar] [CrossRef]
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Imai, C.; Armstrong, B.; Chalabi, Z.; Mangtani, P.; Hashizume, M. Time series regression model for infectious disease and weather. Environ. Res. 2015, 142, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Nottmeyer, L.N.; Sera, F. Influence of temperature, and of relative and absolute humidity on COVID-19 incidence in England—A multi-city time-series study. Environ. Res. 2021, 196, 110977. [Google Scholar] [CrossRef]
- Ma, Y.; Pei, S.; Shaman, J.; Dubrow, R.; Chen, K. Role of meteorological factors in the transmission of SARS-CoV-2 in the United States. Nat. Commun. 2021, 12, 3602. [Google Scholar] [CrossRef]
- Sera, F.; Armstrong, B.; Abbott, S.; Meakin, S.; O’Reilly, K.; von Borries, R.; Schneider, R.; Royé, D.; Hashizume, M.; Pascal, M.; et al. A cross-sectional analysis of meteorological factors and SARS-CoV-2 transmission in 409 cities across 26 countries. Nat. Commun. 2021, 12, 5968. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Press release. Available online: https://www.mhlw.go.jp/stf/covid-19/open-data.html (accessed on 19 July 2023).
- World Health Organization. COVID-19 Health System Response Monitor Japan. Available online: https://apps.who.int/iris/handle/10665/338399 (accessed on 19 July 2023).
- Japan Meteorological Agency. Meteorological Data Search. Available online: https://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 19 July 2023).
- Ali, S.T.; Cowling, B.J.; Wong, J.Y.; Chen, D.; Shan, S.; Lau, E.H.Y.; He, D.; Tian, L.; Li, Z.; Wu, P. Influenza seasonality and its environmental driving factors in mainland China and Hong Kong. Sci. Total Environ. 2022, 818, 151724. [Google Scholar] [CrossRef]
- Google. Google’s COVID-19 Community Mobility Reports. Available online: https://www.google.com/covid19/mobility/index.html?hl=en (accessed on 19 July 2023).
- Nouvellet, P.; Bhatia, S.; Cori, A.; Ainslie, K.E.C.; Baguelin, M.; Bhatt, S.; Boonyasiri, A.; Brazeau, N.F.; Cattarino, L.; Cooper, L.V.; et al. Reduction in mobility and COVID-19 transmission. Nat. Commun. 2021, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Cazelles, B.; Comiskey, C.; Nguyen-Van-Yen, B.; Champagne, C.; Roche, B. Parallel trends in the transmission of SARS-CoV-2 and retail/recreation and public transport mobility during non-lockdown periods. Int. J. Infect. Dis. 2021, 104, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Cori, A.; Ferguson, N.M.; Fraser, C.; Cauchemez, S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am. J. Epidemiol. 2013, 178, 1505–1512. [Google Scholar] [CrossRef]
- Wallinga, J.; Teunis, P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am. J. Epidemiol. 2004, 160, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Linton, N.M.; Akhmetzhanov, A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020, 93, 284–286. [Google Scholar] [CrossRef]
- Chan, Y.H. 2003 Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Gasparrini, A. A tutorial on the case time series design for small-area analysis. BMC Med. Res. Methodol. 2022, 22, 129. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Wagatsuma, K.; Koolhof, I.S.; Saito, R. The Relative Roles of Ambient Temperature and Mobility Patterns in Shaping the Transmission Heterogeneity of SARS-CoV-2 in Japan. Viruses 2022, 14, 2232. [Google Scholar] [CrossRef]
- Briz-Redón, Á.; Serrano-Aroca, Á. The effect of climate on the spread of the COVID-19 pandemic: A review of findings, and statistical and modelling techniques. Prog. Phys. Geogr. Earth Environ. 2020, 44, 591–604. [Google Scholar] [CrossRef]
- Tan, L.; Schultz, D.M. How Is COVID-19 Affected by Weather? Metaregression of 158 Studies and Recommendations for Best Practices in Future Research. Weather Clim. Soc. 2022, 14, 237–255. [Google Scholar] [CrossRef]
- Fontal, A.; Bouma, M.J.; San-José, A.; López, L.; Pascual, M.; Rodó, X. Climatic signatures in the different COVID-19 pandemic waves across both hemispheres. Nat. Comput. Sci. 2021, 1, 655–665. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Chan, K.H.; Peiris, J.S.; Lam, S.Y.; Poon, L.L.; Yuen, K.Y.; Seto, W.H. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007, 3, 1470–1476. [Google Scholar] [CrossRef]
- Tang, J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface 2009, 6, S737–S746. [Google Scholar] [CrossRef]
- Rosti, M.E.; Olivieri, S.; Cavaiola, M.; Seminara, A.; Mazzino, A. Fluid dynamics of COVID-19 airborne infection suggests urgent data for a scientific design of social distancing. Sci. Rep. 2020, 10, 22426. [Google Scholar] [CrossRef]
- Tamerius, J.D.; Shaman, J.; Alonso, W.J.; Bloom-Feshbach, K.; Uejio, C.K.; Comrie, A.; Viboud, C. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013, 9, e1003194. [Google Scholar] [CrossRef]
- Yuan, H.; Kramer, S.C.; Lau, E.H.Y.; Cowling, B.J.; Yang, W. Modeling influenza seasonality in the tropics and subtropics. PLoS Comput. Biol. 2021, 17, e1009050. [Google Scholar] [CrossRef] [PubMed]
- Shimmei, K.; Nakamura, T.; Ng, C.F.S.; Hashizume, M.; Murakami, Y.; Maruyama, A.; Misaki, T.; Okabe, N.; Nishiwaki, Y. Association Between Seasonal Influenza and Absolute Humidity: Time-Series Analysis with Daily Surveillance Data in Japan. Sci. Rep. 2020, 10, 7764. [Google Scholar] [CrossRef] [PubMed]
- Mecenas, P.; Bastos, R.; Vallinoto, A.C.R.; Normando, D. Effects of temperature and humidity on the spread of COVID-19: A systematic review. PLoS ONE 2020, 15, e0238339. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.P.; Liu, Y.; Xiao, S.; Zhang, Z. Challenges in the control of COVID-19 outbreaks caused by the delta variant during periods of low humidity: An observational study in Sydney, Australia. Infect. Dis. Poverty 2021, 10, 139. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, Y.; Tong, S.; Ding, C.; Wei, L.; Zhai, M.; Xu, K.; Hao, R.; Wang, X.; Li, N.; et al. Association between meteorological factors and COVID-19 transmission in low- and middle-income countries: A time-stratified case-crossover study. Environ. Res. 2023, 231, 116088. [Google Scholar] [CrossRef]
- Li, H.L.; Yang, B.Y.; Wang, L.J.; Liao, K.; Sun, N.; Liu, Y.C.; Ma, R.F.; Yang, X.D. A meta-analysis result: Uneven influences of season, geo-spatial scale and latitude on relationship between meteorological factors and the COVID-19 transmission. Environ. Res. 2022, 212, 113297. [Google Scholar] [CrossRef]
- Moriyama, M.; Ichinohe, T. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc. Natl. Acad. Sci. USA 2019, 116, 3118–3125. [Google Scholar] [CrossRef]
- Chung, Y.; Yang, D.; Gasparrini, A.; Vicedo-Cabrera, A.M.; Fook Sheng Ng, C.; Kim, Y.; Honda, Y.; Hashizume, M. Changing Susceptibility to Non-Optimum Temperatures in Japan, 1972-2012: The Role of Climate, Demographic, and Socioeconomic Factors. Environ. Health. Perspect. 2018, 126, 057002. [Google Scholar] [CrossRef]
- Karmakar, M.; Lantz, P.M.; Tipirneni, R. Association of Social and Demographic Factors With COVID-19 Incidence and Death Rates in the US. JAMA Netw. Open 2021, 4, e2036462. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Liu, J.; He, X.; Wang, B.; Fu, S.; Yan, J.; Niu, J.; Zhou, J.; Luo, B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020, 724, 138226. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef]
- Sheridan, C.; Klompmaker, J.; Cummins, S.; James, P.; Fecht, D.; Roscoe, C. Associations of air pollution with COVID-19 positivity, hospitalisations, and mortality: Observational evidence from UK Biobank. Environ. Pollut. 2022, 308, 119686. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.A.; Eggink, D.; Andeweg, S.P.; Veldhuijzen, I.K.; van Maarseveen, N.; Vermaas, K.; Vlaemynck, B.; Schepers, R.; van den Hof, S.; Reusken, C.B.; et al. Shorter serial intervals in SARS-CoV-2 cases with Omicron BA.1 variant compared with Delta variant, the Netherlands, 13 to 26 December 2021. Euro Surveill. 2022, 27, 2200042. [Google Scholar] [CrossRef] [PubMed]
| Potential Drivers | Mean | Min | P25 | P50 | P75 | Max |
|---|---|---|---|---|---|---|
| Daily newly confirmed cases | 469 | 0 | 1 | 18 | 239 | 40,406 |
| Effective reproduction number | 1.24 | 0.04 | 0.84 | 1.02 | 1.31 | 78.04 |
| Mean ambient temperature (°C) | 17.02 | −10.40 | 10.20 | 17.80 | 24.20 | 33.00 |
| Absolute humidity (g/m3) | 11.67 | 0.00 | 6.10 | 10.60 | 17.10 | 26.22 |
| Precipitation (mm) | 5.11 | 0.00 | 0.00 | 0.00 | 2.50 | 306.00 |
| Wind speed (m/s) | 2.87 | 0.50 | 2.00 | 2.60 | 3.40 | 17.90 |
| Retail and recreation mobility (%) | −17.44 | −89.00 | −13.00 | −7.00 | −2.00 | 66.00 |
| GSI from the OxCGRT (%) | 42.59 | 19.44 | 37.04 | 45.37 | 47.22 | 55.09 |
| Potential Drivers | Lag (Days) | |||
|---|---|---|---|---|
| 0 | 7 | 14 | 0−14 | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Mena ambient temperature (°C) | ||||
| 5.1 °C | 1.009 (1.004−1.015) | 1.004 (1.001−1.007) | 1.002 (0.996−1.008) | 1.027 (1.016−1.038) |
| 27.9 °C | 1.008 (1.003−1.013) | 1.004 (1.001−1.007) | 1.002 (0.997−1.007) | 0.982 (0.974−0.989) |
| Absolute humidity (m/g3) | ||||
| 4.2 m/g3 | 1.007(1.000−1.007) | 1.006 (1.002−1.006) | 1.006 (0.999−1.006) | 1.026 (1.017−1.036) |
| 20.6 m/g3 | 1.002 (1.000−1.005) | 1.003 (1.001−1.004) | 1.001 (0.999−1.004) | 0.995 (0.985−1.006) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagatsuma, K. Association of Ambient Temperature and Absolute Humidity with the Effective Reproduction Number of COVID-19 in Japan. Pathogens 2023, 12, 1307. https://doi.org/10.3390/pathogens12111307
Wagatsuma K. Association of Ambient Temperature and Absolute Humidity with the Effective Reproduction Number of COVID-19 in Japan. Pathogens. 2023; 12(11):1307. https://doi.org/10.3390/pathogens12111307
Chicago/Turabian StyleWagatsuma, Keita. 2023. "Association of Ambient Temperature and Absolute Humidity with the Effective Reproduction Number of COVID-19 in Japan" Pathogens 12, no. 11: 1307. https://doi.org/10.3390/pathogens12111307
APA StyleWagatsuma, K. (2023). Association of Ambient Temperature and Absolute Humidity with the Effective Reproduction Number of COVID-19 in Japan. Pathogens, 12(11), 1307. https://doi.org/10.3390/pathogens12111307





