Abstract
Human lice, Pediculus humanus, can transmit various pathogens, including Bartonella quintana, Borrelia recurrentis, and Rickettsia prowazekii. Xenosurveillance is an epidemiological approach to assessing human infection risks performed by screening vectors of infectious disease agents. In the proof-of-principle study reported herein, the DNA of 23 human lice was collected from the clothes of 30 homeless Ethiopian individuals. These samples were assessed using 16S rRNA gene-specific pan-eubacterial PCR for screening, followed by Bartonella genus 16S-23S internal transcribed spacer (ITS) sequence-specific PCR, Bartonella genus gltA gene-specific PCR, and 16S rRNA gene PCR with specificity for relapsing-fever-associated Borrelia spp. with subsequent sequencing of the amplicons. In one sample, the pan-eubacterial 16S rRNA gene-specific screening PCR, the Bartonella genus 16S-23S ITS sequence-specific PCR, and the Bartonella genus gltA gene-specific PCR allowed for the sequencing of B. quintana-specific amplicons. In two additional samples, Bartonella genus gltA gene-specific PCR also provided sequences showing 100% sequence identity with B. quintana. In total, 3/23 (13.0%) of the assessed lice were found to be positive for B. quintana. Correlating clinical data were not available; however, the assessment confirmed the presence of B. quintana in the local louse population and thus an associated infection pressure. Larger-sized cross-sectional studies seem advisable to more reliably quantify the infection risk of lice-infested local individuals. The need for prevention by providing opportunities to maintain standard hygiene for Ethiopian homeless individuals is stressed by the reported findings, especially in light of the ongoing migration of refugees.
Keywords:
Ethiopia; xenosurveillance; Pediculus humanus; Bartonella quintana; infection risk; vector 1. Introduction
If standard hygiene precautions cannot be maintained in cases of war, crisis, or displacement, there is an increased risk of the spread of human ectoparasites. Next to causing mechanically induced discomfort, several human ectoparasites have also been associated with the transmission of agents causing infectious diseases. For human lice, their role as vectors for harmful pathogens like Borrelia recurrentis, Rickettsia prowazekii, and Bartonella quintana is considered well established [1,2,3,4,5,6,7,8]. Regarding Yersinia pestis, the causative agent of bubonic plague, the possibility of human lice playing a vector role is still controversial but considered likely based on epidemiological and laboratory-based evidence [1]. Even bacteria primarily linked to nosocomial spread like Acinetobacter baumannii have recently been associated with louse transmission among humans [9,10].
The human louse, Pediculus humanus, comprises the ecotypes head louse and body louse. The molecular discrimination of the subtypes of this louse based on mitochondrial clades A-F is feasible [11]. While it had formerly been assumed that pathogen transmission was basically restricted to body lice, this potential has recently been shown to apply to head lice as well. The effectiveness of preventive medical approaches is hampered by complex epigenetic control of gene expression, which causes a considerable level of flexibility of reaction towards altered environmental conditions, including the acquisition of ivermectin resistance [11,12]. In biting events, a potent vasodilator is known to be injected into the human host [13]. Cold weather and lack of hygiene, e.g., factors applicable to homeless individuals, facilitate the spread of human lice [14,15].
The occasional importation of B. recurrentis, the causative agent of louse-borne relapsing fever [16,17,18], to Europe in the course of recent migration movements has increased the awareness of this otherwise poorly known pathogen in European health centers [19,20]. The frequently reported symptoms comprise fever, headache, jaundice, epistaxis, and hepatosplenomegaly [21]. Recent reports have shown that epidemiological data published on louse-borne relapsing fever are highly variable; in particular, severe outcomes like death either due to the disease itself or a therapy-associated Jarisch–Herxheimer reaction are affected by contextual variance [2]. While B. recurrentis infections were still common in European countries in the 19th century, including in the form of outbreaks in England, Scotland, and Ireland, Afro-Middle Eastern pandemics in the 20th century were followed by a residual area of endemicity in Ethiopia and its neighboring countries within the Horn of Africa [22,23,24,25,26].
Bartonella quintana, the causative agent of trench fever, has been associated with disease activity that largely depends on the immune status and the socio-economic status of the patients. Next to individuals infected with the human immunodeficiency virus (HIV), homeless poor people, alcohol, and drug addicts as well as warfighters dwelling in unfavorable front conditions (as witnessed in World War I) are particularly associated with clinical disease. B. quintana infections can comprise a wide spectrum of asymptomatic courses, but also relapsing fever (caused by a periodic bacteremia), endocarditis in HIV-seronegative individuals, and bacillary angiomatosis in immunosuppressed (e.g., AIDS) patients have been described [27,28,29,30,31,32]. Relapses are known to occur after short-course antibiotic therapy [28]. Bactericidal effects on B. quintana have been described for aminoglycosides [28]. In addition, tetracyclines, macrolides, and third-generation cephalosporins have been reported as therapeutically active [29]. An animal reservoir is unknown [33], but transmission of B. quintana via both head and body lice is considered a well-established route [34].
Rickettsia prowazekii, the causative agent of epidemic typhus, can also be spread if louse populations proliferate under poor sanitary conditions. The persistence of this agent is facilitated by delayed disease relapses, called Brill–Zinsser disease, and its natural reservoir host, the flying squirrel (Glaucomys volans) [35,36]. The failure of doxycycline prophylaxis for infection prevention has been reported, in spite of the fact that this antimicrobial serves as the therapy of choice in case of infections [37]. Lately, typhus outbreaks have predominantly been reported to occur in jails and refugee camps, particularly in resource-limited countries [4].
As reviewed in detail elsewhere [38], xenosurveillance in line with the “flying biological syringe” hypothesis constitutes a cost-efficient mode of surveillance for biological agents with relevance for human infections within their natural vectors. In the study presented here, we demonstrated this concept by screening Pediculus humanus lice collected from the clothes of Ethiopian homeless individuals for Bartonella species and Borrelia species.
2. Materials and Methods
2.1. Sample Collection
The sample collection was organized by the Hirsch Institute of Tropical Medicine, which is part of the University Hospital Düsseldorf and located in the Asella referral and teaching hospital compound in Ethiopia. We conducted a program in which new clothes were provided to 30 local homeless individuals under 20 years of age for humanitarian purposes on a voluntary basis in Asella, Ethiopia. Their original clothes were cleaned via autoclaving; prior to this procedure, body lice were collected from the clothes for subsequent molecular assessments. No association between collected lice and participating individuals was recorded to ensure absolute anonymity.
2.2. Molecular Diagnostics
The collected lice were stored and transported in 70% ethanol. Preparation was conducted with single-use consumables in order to avoid cross-contamination. Genomic nucleic acid was extracted via a Maxwell® 16 device using a Maxwell LED DNA kit (Promega, Germany) as described in [39]. A 16S rRNA gene-specific pan-eubacterial PCR was initially performed [40] (Appendix Table A1 for details). Furthermore, Bartonella genus 16S-23S internal transcribed spacer (ITS) sequence-specific nested PCR [41] (Appendix Table A2 for details) and Bartonella genus gltA gene-specific nested PCR [42] (Appendix Table A3 for details) were conducted. Also, a 16S rRNA-gene PCR targeting relapsing-fever-associated Borrelia spp. was performed [43] (Appendix Table A4 for details). Amplicons with the expected sequence length were subjected to Sanger sequencing at Eurofins Scientific (Luxembourg). While results of the pan-eubacterial PCR were considered as preliminary only, any Bartonella spp. and Borrelia spp. sequences obtained with the Bartonella-genus- and Borrelia-genus-specific primers were deposited at NCBI GenBank. All laboratory assessments were conducted in line with standard requirements for molecular diagnostic assessments in laboratory infrastructure accredited according to strictly controlled DIN EN ISO 17025 standards (certificate number D–PL–19082-02-04) [44].
2.3. Ethical Clearance
Ethical clearance was not applicable because no human patients or human-patient-related data were included in the assessment.
3. Results
A total of 23 P. humanus lice were subjected to molecular assessment. A total of 16 positive PCR signals were obtained via pan-eubacterial 16S rRNA gene PCR screening. From the 16 amplicons, DNA was extracted for sequencing from 13 samples with expected amplicon lengths. From a total of eight interpretable sequences, the endosymbiont Candidatus Riesia pediculicola was identified in seven instances, and Bartonella quintana (99% query coverage, 99% sequence identity) was identified in one instance, albeit with relatively poor sequence quality as defined by the ambiguous signals in the trace data from Sanger DNA sequencing.
In the same sample, the Bartonella genus 16S-23S internal transcribed spacer (ITS) sequence-specific PCR showed a positive result. The associated B. quintana sequence was deposited with the NCBI GenBank accession number OR504472. Once more from this sample and from two others as well, three B. quintana gltA sequences were obtained and deposited at NCBI GenBank. The associated accession numbers are OR499104, OR499105, and OR499106. A photograph of the electrophoretic separation of positive amplification products, also comprising amplicons from which Bartonella spp.-specific sequences could not be confirmed via Sanger sequencing, are provided in Appendix A (Figure A1).
Three positive signals were seen using the PCR targeting the 16S rRNA locus of relapsing-fever-associated Borrelia spp. Interpretable sequences were obtained from two out of the three amplicons corresponding to the endosymbiont Candidatus Riesia pediculicola. Borrelia spp.-specific sequences were not recorded.
4. Discussion
The limited xenosurveillance approach applied indicated the presence of Bartonella quintana in three out of twenty-three (13.0%) body lice collected from the clothes of homeless individuals in Ethiopia. The occurrence of B. quintana in Ethiopian lice and patients has been repeatedly described [45,46,47,48]. Insofar, the results observed in this assessment are not surprising. However, the fact that positive findings were achieved even with a very low number of assessed P. humanus lice indicates either random matching or, more likely, a substantial infection pressure for lice-infested homeless local individuals. Due to the design of the study reported here, it remains unknown whether any of the individuals whose clothes were assessed showed any clinical symptoms. However, due to the heterogenicity of symptoms in B. quintana infections [27,28,29,30,31,32], even a lack of symptoms would not have excluded an infection.
As the endemicity of B. recurrentis in Ethiopia is considered well established [22,23,24,25,26], a Borrelia-specific PCR was added to the pan-bacterial 16S rRNA gene PCR. However, both approaches failed to provide a Borrelia-specific sequence. Additionally, Rickettsia prowazekii DNA was not detected in any of the lice.
This study has a number of limitations. The low number of investigated lice provided only superficial insight into the local epidemiology of the lice-transmitted agents of infectious diseases. To provide more reliable data in a cross-sectional assessment, a larger sample size would be necessary. Collecting such a sample was, unfortunately, unfeasible due to financial restraints in this investigator-initiated assessment. Finally, the fact that the cultivation of Bartonella quintana was not attempted in this assessment must be considered a limitation.
5. Conclusions
In spite of the above-mentioned limitations, the described xenosurveillance approach once more confirmed that B. quintana is an infection risk for lice-infested individuals in Ethiopia. This finding stresses the need for further Bartonella spp. surveillance and for providing basic hygiene options for local homeless individuals for infection control and prevention purposes. Also, Bartonalla spp. infection needs to be considered in migrants from Ethiopia. Such infections can both endanger migrants’ health and contribute to the spread of bacterial pathogens in the host countries.
Author Contributions
Conceptualization, S.P., T.B.T. and T.F.; methodology, G.M., V.F. and C.H.; software, G.M., V.F. and C.H. validation, G.M., V.F. and C.H.; formal analysis, G.M., V.F. and C.H.; investigation, T.B.T., G.M., V.F. and C.H.; resources, T.B.T., G.M., V.F., C.H. and T.F.; data curation, G.M., V.F. and C.H.; writing—original draft preparation, H.F.; writing—review and editing, G.M., V.F., C.H., S.P., R.J.B., V.A.J.K., P.K., H.F., T.B.T. and T.F.; visualization, G.M., V.F and C.H.; supervision, S.P. and T.F.; project administration, S.P.; funding acquisition, T.F. All authors have read and agreed to the published version of the manuscript.
Funding
The work of V.A.J.K. and P.K. was supported partially by the Robert Koch-Institute, Berlin, Germany (Bartonella conciliary laboratory, 1369-354), and the LOEWE Center DRUID (Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases).
Institutional Review Board Statement
Not applicable. Neither human patients nor human data were used.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data have been provided within the manuscript. Raw data can be made available at reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Details of the 16S rRNA gene-specific eubacterial screening PCR.
Table A1.
Details of the 16S rRNA gene-specific eubacterial screening PCR.
| 16S rRNA Gene-Specific Pan-Eubacterial PCR | |
|---|---|
| Forward primer | 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) |
| Reverse primer | 1392r (5′-ACGGGCGGTGTGTRC-3′) |
| Reaction mix | 30 µL volumes containing 15 µL of Qiagen (Hilden, Germany) Hot Start Mix, 6 µL of PCR-grade H2O, 3 µL of each primer (final concentration 10 pmol), 5 µL of DNA eluate |
| Initial denaturation | 15 min at 95 °C |
| Denaturation | 30 s at 94 °C |
| Annealing | 30 s at 54 °C |
| Amplification | 1.5 min at 72 °C |
| Cycle numbers | 30 cycles |
| Final extension | 7 min at 72 °C |
| Gel electrophoresis | Application of 6µL PCR product + 2 µL loading dye to a 1.5% agarose gel with 0.065% Gel Red nucleic acid stain |

Table A2.
Details of the Bartonella genus 16S-23S internal transcribed spacer (ITS) sequence-specific, two-round PCR.
Table A2.
Details of the Bartonella genus 16S-23S internal transcribed spacer (ITS) sequence-specific, two-round PCR.
| Bartonella Genus 16S-23S-ITS-Specific PCR (First Round) | Bartonella Genus 16S-23S-ITS-Specific PCR (Second Round) | |
|---|---|---|
| Forward primer | new-bigF (5′-GGAAGGTTTTCCGGTTTATC-3′) | new-bigF (5′-GGAAGGTTTTCCGGTTTATC-3′) |
| Reverse primer | new-bogR (5′-GTCTGAATATA(C/T)CTTCTCTTCAC-3′) | bigR (5′-TCCCAGCTGAGCTACG-3′) |
| Reaction mix | 25 µL volumes containing 12.5 µL of Qiagen (Hilden, Germany) Hot Start Mix, 9.5 µL of PCR-grade H2O, 1 µL of each primer (final concentration 10 pmol), 1 µL of DNA eluate | |
| Initial denaturation | 15 min at 95 °C | |
| Denaturation | 30 s at 95 °C | |
| Annealing | 30 s at 55 °C | |
| Amplification | 1.0 min at 72 °C | |
| Cycle numbers | 35 cycles | |
| Final extension | 5 min at 72 °C | |
| Gel electrophoresis | Application of 6 µL PCR product + 2 µL loading dye to a 1.5% agarose gel with 0.065% Gel Red nucleic acid stain | |

Table A3.
Details of the Bartonella genus gltA gene-specific, two-round PCR.
Table A3.
Details of the Bartonella genus gltA gene-specific, two-round PCR.
| Bartonella Genus gltA Gene, First Round | Bartonella Genus gltA Gene, Second Round | |
|---|---|---|
| Forward primer | 443f (5′-GCTATGTCTGCATTCTATCA-3′) | 781f (5′-GGGGACCAGCTCATGGTGG-3′) |
| Reverse primer | 1137r (5′-AATGCAAAAAGAACAGTAAACA-3′) | 1137r (5′-AATGCAAAAAGAACAGTAAACA-3′) |
| Reaction mix | 50 µL volumes containing 25 µL of Qiagen (Hilden, Germany) Hot Start Mix, 20 µL of PCR-grade H2O, 2 µL of each primer (final concentration 10 pmol), 1 µL of DNA eluate | |
| Initial denaturation | 15 min at 95 °C | |
| Denaturation | 30 s at 95 °C | |
| Annealing | 30 s at 55 °C | |
| Amplification | 1.0 min at 72 °C | |
| Cycle numbers | 35 cycles | |
| Final extension | 5 min at 72 °C | |
| Gel electrophoresis | Application of 6µL PCR product + 2 µL loading dye to a 1.5% agarose gel with 0.065% Gel Red nucleic acid stain | |

Table A4.
Details of PCR targeting relapsing-fever-associated Borrelia spp.
Table A4.
Details of PCR targeting relapsing-fever-associated Borrelia spp.
| 16S rRNA Gene PCR Targeting Relapsing-Fever-Associated Borrelia spp. | |
|---|---|
| Forward primer | 5′-GGCTTAGAACTAACGCTGGCAGTGC-3′ |
| Reverse primer | 5′-CCCTTTACGCCCAATAATCCCGA-3′ |
| Reaction mix | 30 µL volumes containing 15 µL of Qiagen (Hilden, Germany) Hot Start Mix, 6 µL of PCR-grade H2O, 3 µL of each primer (final concentration 10 pmol), 5 µL of DNA eluate |
| Initial denaturation | 15 min at 95 °C |
| Denaturation | 15 s at 94 °C |
| Annealing | 30 s at 54 °C |
| Amplification | 1 min at 72 °C |
| Cycle numbers | 40 cycles |
| Final extension | 7 min at 72 °C |
| Gel electrophoresis | Application of 6µL PCR product + 2 µL loading dye to a 1.5% agarose gel with 0.065% Gel Red nucleic acid stain |
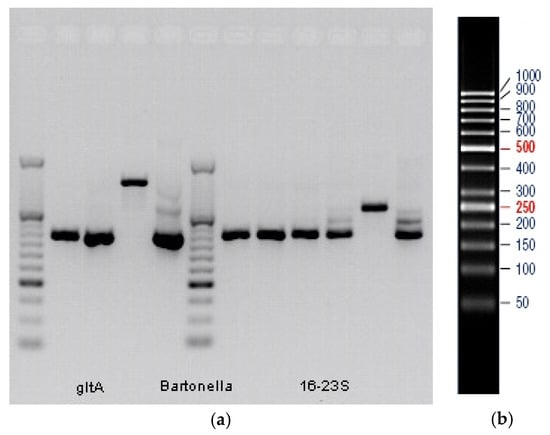
Figure A1.
(a) Electrophoretic separation of positive amplification products of the Bartonella spp.-specific PCRs. Amplicons, from which Bartonella spp.-specific sequences could not be confirmed via Sanger sequencing, are shown as well. First and sixth columns: DNA mass standards. From second to fourth columns: first round of the Bartonella spp.-specific gltA gene PCR (356 bp amplicons in columns two and three, and 694 bp amplicon in column four). Fifth column: second round of the Bartonella spp.-specific gltA gene PCR (356 bp amplicon). Seventh to eleventh columns: first round of the Bartonella spp.-specific 16S-23S rRNA gene PCR (400 bp amplicons in columns seven to ten, and 539 bp amplicon in column eleven). Twelfth column: second round of the Bartonella spp.-specific 16S-23S rRNA gene PCR (400 bp amplicon). Sample HL10: second and ninth columns. Sample BL06: third and tenth columns. Sample BL12: fourth, fifth, eleventh, and twelfth columns. Sample L03: seventh column. Sample L05: eighth column. (b) Pattern of the applied DNA standard. Numbers indicate base pair (bp) counts.
References
- Barbieri, R.; Drancourt, M.; Raoult, D. The role of louse-transmitted diseases in historical plague pandemics. Lancet Infect. Dis. 2021, 21, e17–e25. [Google Scholar] [CrossRef]
- Kahlig, P.; Neumayr, A.; Paris, D.H. Louse-borne relapsing fever-A systematic review and analysis of the literature: Part 2-Mortality, Jarisch-Herxheimer reaction, impact on pregnancy. PLoS Negl. Trop. Dis. 2021, 15, e0008656. [Google Scholar] [CrossRef]
- Brouqui, P.; Raoult, D. Arthropod-borne diseases in homeless. Ann. N. Y. Acad. Sci. 2006, 1078, 223–235. [Google Scholar] [CrossRef]
- Billeter, S.A.; Levy, M.G.; Chomel, B.B.; Breitschwerdt, E.B. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med. Vet. Entomol. 2008, 22, 1–15. [Google Scholar] [CrossRef]
- Azad, A.F.; Beard, C.B. Rickettsial pathogens and their arthropod vectors. Emerg. Infect. Dis. 1998, 4, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.J.; Ammerman, N.C.; Beier-Sexton, M.; Sobral, B.S.; Azad, A.F. Louse- and flea-borne rickettsioses: Biological and genomic analyses. Vet. Res. 2009, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Leo, N.; Prociv, P.; Barker, S.C. Potential role of head lice, Pediculus humanus capitis, as vectors of Rickettsia prowazekii. Parasitol. Res. 2003, 90, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Chang, C.C.; Chuang, S.T.; Chomel, B.B. Bartonella species and their ectoparasites: Selective host adaptation or strain selection between the vector and the mammalian host? Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Badiaga, S.; Brouqui, P. Human louse-transmitted infectious diseases. Clin. Microbiol. Infect. 2012, 18, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P. Arthropod-borne diseases associated with political and social disorder. Annu. Rev. Entomol. 2011, 56, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Amanzougaghene, N.; Fenollar, F.; Raoult, D.; Mediannikov, O. Where Are We With Human Lice? A Review of the Current State of Knowledge. Front. Cell. Infect. Microbiol. 2020, 9, 474. [Google Scholar] [CrossRef]
- Boutellis, A.; Abi-Rached, L.; Raoult, D. The origin and distribution of human lice in the world. Infect. Genet. Evol. 2014, 23, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. The neglected saliva: Medically important toxins in the saliva of human lice. Parasitology 1998, 116, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Roux, V. The body louse as a vector of reemerging human diseases. Clin. Infect. Dis. 1999, 29, 888–911. [Google Scholar] [CrossRef]
- Estrada, B. Ectoparasitic infestations in homeless children. Semin. Pediatr. Infect. Dis. 2003, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Hovius, J.W.; Bergström, S. Pathogenesis of Relapsing Fever. Curr. Issues Mol. Biol. 2021, 42, 519–550. [Google Scholar]
- Cutler, S.J. Relapsing Fever Borreliae: A Global Review. Clin. Lab. Med. 2015, 35, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Abdissa, A.; Trape, J.F. New concepts for the old challenge of African relapsing fever borreliosis. Clin. Microbiol. Infect. 2009, 15, 400–406. [Google Scholar] [CrossRef]
- Antinori, S.; Mediannikov, O.; Corbellino, M.; Raoult, D. Louse-borne relapsing fever among East African refugees in Europe. Travel Med. Infect. Dis. 2016, 14, 110–114. [Google Scholar] [CrossRef]
- Eiset, A.H.; Wejse, C. Review of infectious diseases in refugees and asylum seekers-current status and going forward. Public Health Rev. 2017, 38, 22. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abdel Wahab, S.M.; Abdel Malik, M.O.; Abdel Gadir, A.M.; Salih, S.Y.; Omer, A.; Al Hassan, A.M. Louse-borne relapsing fever in the Sudan. A historical review and a clinico-pathological study. Trop. Geogr. Med. 1980, 32, 106–211. [Google Scholar] [PubMed]
- Warrell, D.A. Louse-borne relapsing fever (Borrelia recurrentis infection). Epidemiol. Infect. 2019, 147, e106. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J. Relapsing fever—A forgotten disease revealed. J. Appl. Microbiol. 2010, 108, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- El-Bahnsawy, M.M.; Labib, N.A.; Abdel-Fattah, M.A.; Ibrahim, A.M.; Morsy, T.A. Louse and tick borne relapsing fevers. J. Egypt. Soc. Parasitol. 2012, 42, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, T.; Feldt, T.; Bosselmann, M.; Tufa, T.B.; Lemma, G.; Holtfreter, M.; Häussinger, D. Outbreak of Louse-Borne Relapsing Fever among Urban Dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am. J. Trop. Med. Hyg. 2018, 98, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Raoult, D. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 1996, 9, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Leibler, J.H.; Zakhour, C.M.; Gadhoke, P.; Gaeta, J.M. Zoonotic and Vector-Borne Infections Among Urban Homeless and Marginalized People in the United States and Europe, 1990-2014. Vector Borne Zoonotic Dis. 2016, 16, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ohl, M.E.; Spach, D.H. Bartonella quintana and urban trench fever. Clin. Infect. Dis. 2000, 31, 131–135. [Google Scholar] [CrossRef]
- Ruiz, J. Bartonella quintana, past, present, and future of the scourge of World War I. APMIS. 2018, 126, 831–837. [Google Scholar] [CrossRef]
- Anstead, G.M. The centenary of the discovery of trench fever, an emerging infectious disease of World War 1. Lancet Infect. Dis. 2016, 16, e164–e172. [Google Scholar] [CrossRef]
- Lam, J.C.; Fonseca, K.; Pabbaraju, K.; Meatherall, B.L. Case Report: Bartonella quintana Endocarditis Outside of the Europe-African Gradient: Comprehensive Review of Cases within North America. Am. J. Trop. Med. Hyg. 2019, 100, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Spach, D.H.; Kanter, A.S.; Dougherty, M.J.; Larson, A.M.; Coyle, M.B.; Brenner, D.J.; Swaminathan, B.; Matar, G.M.; Welch, D.F.; Root, R.K.; et al. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N. Engl. J. Med. 1995, 332, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Birtles, R.; Raoult, D. Current knowledge of Bartonella species. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Veracx, A.; Raoult, D. Biology and genetics of human head and body lice. Trends Parasitol. 2012, 28, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Bechah, Y.; Capo, C.; Mege, J.L.; Raoult, D. Epidemic typhus. Lancet Infect Dis. 2008, 8, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Raoult, D.; Parola, P. Insects and the Transmission of Bacterial Agents. Microbiol. Spectr. 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Kazár, J.; Brezina, R. Control of rickettsial diseases. Eur. J. Epidemiol. 1991, 7, 282–286. [Google Scholar]
- Brinkmann, A.; Nitsche, A.; Kohl, C. Viral Metagenomics on Blood-Feeding Arthropods as a Tool for Human Disease Surveillance. Int. J. Mol. Sci. 2016, 17, 1743. [Google Scholar] [CrossRef]
- Margos, G.; Hepner, S.; Mang, C.; Marosevic, D.; Reynolds, S.E.; Krebs, S.; Sing, A.; Derdakova, M.; Reiter, M.A.; Fingerle, V. Lost in plasmids: Next generation sequencing and the complex genome of the tick-borne pathogen Borrelia burgdorferi. BMC Genom. 2017, 18, 422. [Google Scholar] [CrossRef]
- Ruegger, P.M.; Della Vedova, G.; Jiang, T.; Borneman, J. Improving probe set selection for microbial community analysis by leveraging taxonomic information of training sequences. BMC Bioinform. 2011, 12, 394. [Google Scholar] [CrossRef]
- Telfer, S.; Bown, K.J.; Sekules, R.; Begon, M.; Hayden, T.; Birtles, R. Disruption of a host-parasite system following the introduction of an exotic host species. Parasitology 2005, 130 Pt 6, 661–668. [Google Scholar] [CrossRef]
- Logan, J.M.J.; Hall, J.L.; Chalker, V.J.; O’Connell, B.; Birtles, R.J. Bartonella clarridgeiae infection in a patient with aortic root abscess and endocarditis. Access Microbiol. 2019, 1, e000064. [Google Scholar] [CrossRef]
- Regier, Y.; Komma, K.; Weigel, M.; Kraiczy, P.; Laisi, A.; Pulliainen, A.T.; Hain, T.; Kempf, V.A.J. Combination of microbiome analysis and serodiagnostics to assess the risk of pathogen transmission by ticks to humans and animals in central Germany. Parasit Vectors 2019, 12, 11. [Google Scholar] [CrossRef]
- DIN EN ISO/IEC 17025; Allgemeine Anforderungen an die Kompetenz von Prüf- und Kalibrierlaboratorien. Deutsche Industrienorm (DIN): Berlin, Germany, 2018.
- Pérez-Tanoira, R.; Ramos-Rincón, J.M.; Martín-Martín, I.; Prieto-Pérez, L.; Tefasmariam, A.; Tiziano, G.; Anda, P.; González-Martín-Niño, R.M.; Rodríguez-Vargas, M.; Górgolas, M.; et al. Molecular Survey of Rickettsia spp., Anaplasma spp., Ehrlichia spp., Bartonella spp., and Borrelia spp. in Fleas and Lice in Ethiopia. Vector Borne Zoonotic Dis. 2020, 20, 10–14. [Google Scholar] [CrossRef]
- Cutler, S.; Abdissa, A.; Adamu, H.; Tolosa, T.; Gashaw, A. Bartonella quintana in Ethiopian lice. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 17–21. [Google Scholar] [CrossRef]
- Angelakis, E.; Diatta, G.; Abdissa, A.; Trape, J.F.; Mediannikov, O.; Richet, H.; Raoult, D. Altitude-dependent Bartonella quintana genotype C in head lice, Ethiopia. Emerg. Infect. Dis. 2011, 17, 2357–2359. [Google Scholar] [CrossRef]
- Tasher, D.; Raucher-Sternfeld, A.; Tamir, A.; Giladi, M.; Somekh, E. Bartonella quintana, an Unrecognized Cause of Infective Endocarditis in Children in Ethiopia. Emerg. Infect. Dis. 2017, 23, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).