Prevalence of Hepatocellular Carcinoma in Hepatitis B Population within Southeast Asia: A Systematic Review and Meta-Analysis of 39,050 Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search for Studies
2.2. Eligibility and Data Extraction
2.3. Statistical Analysis and Quality Assessment
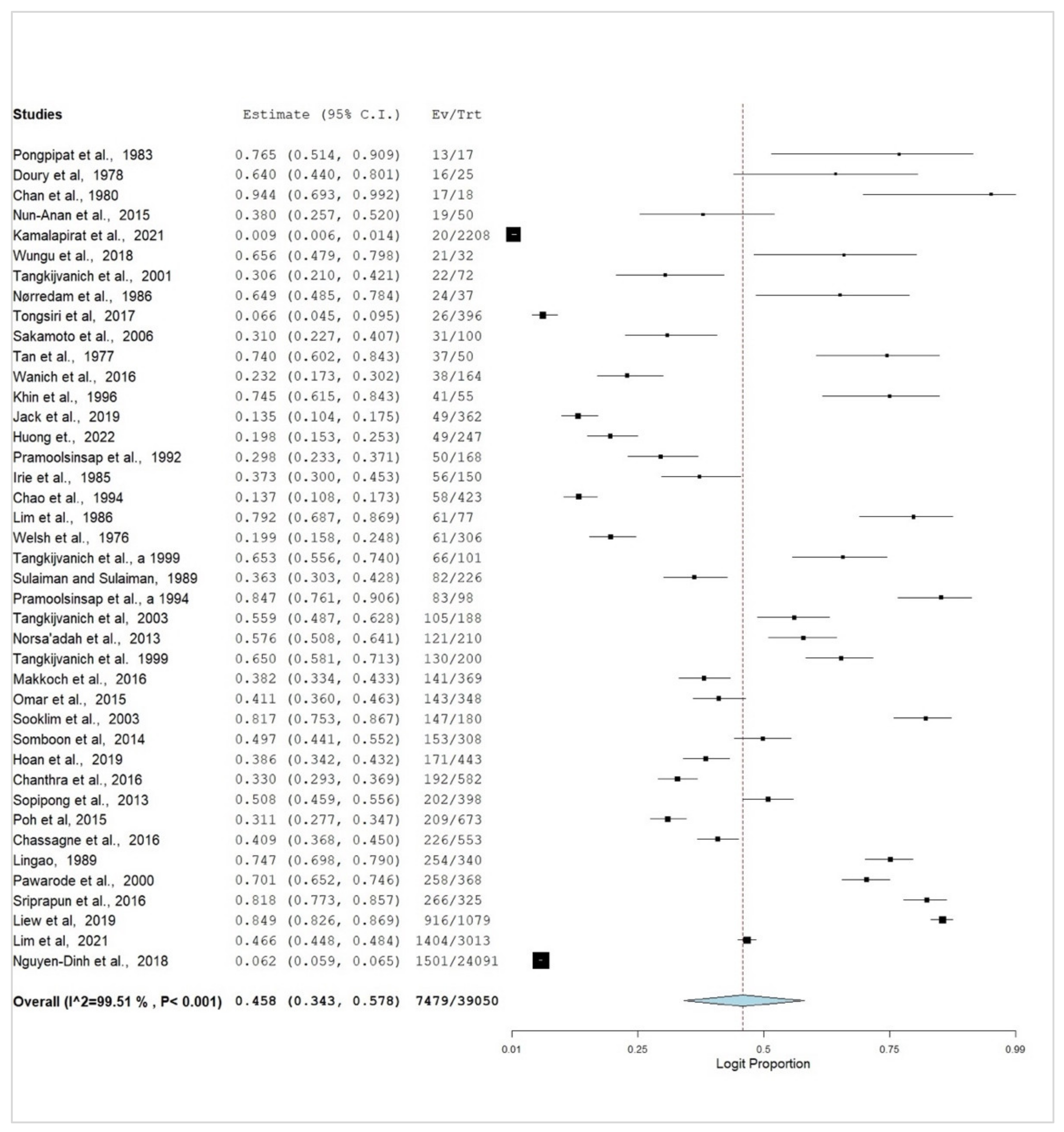
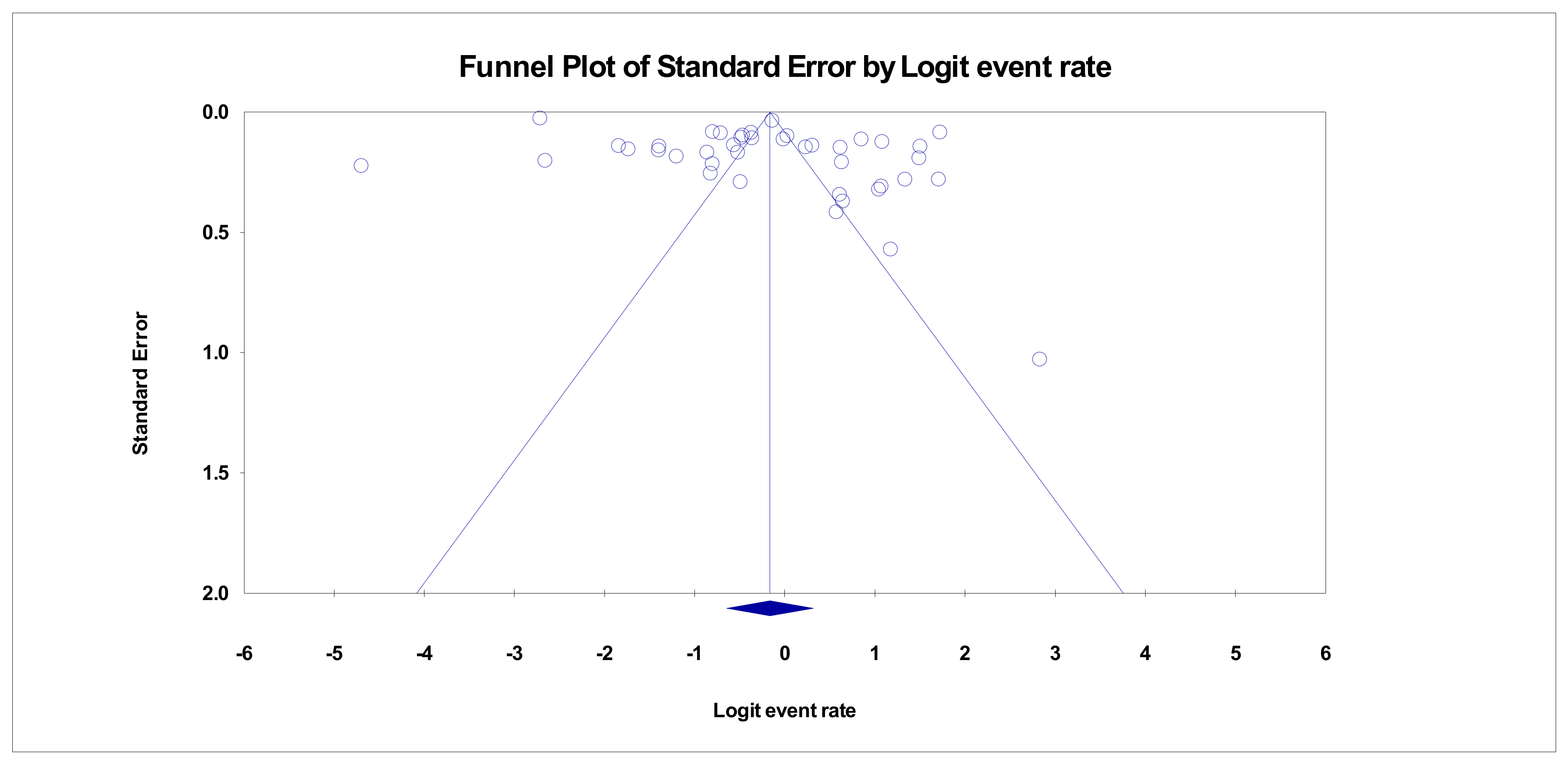
3. Results
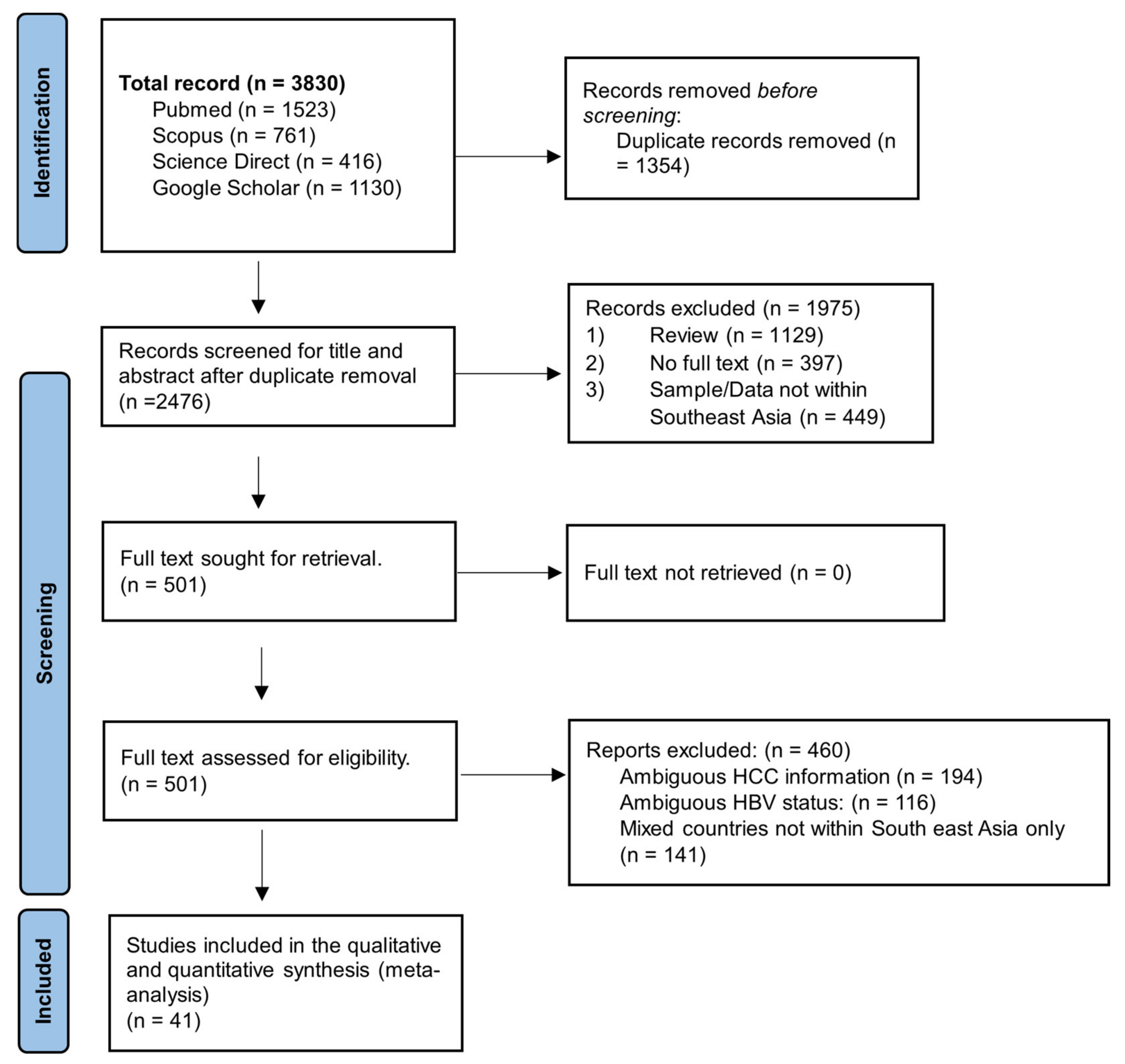
3.1. Search Results and Eligible Studies
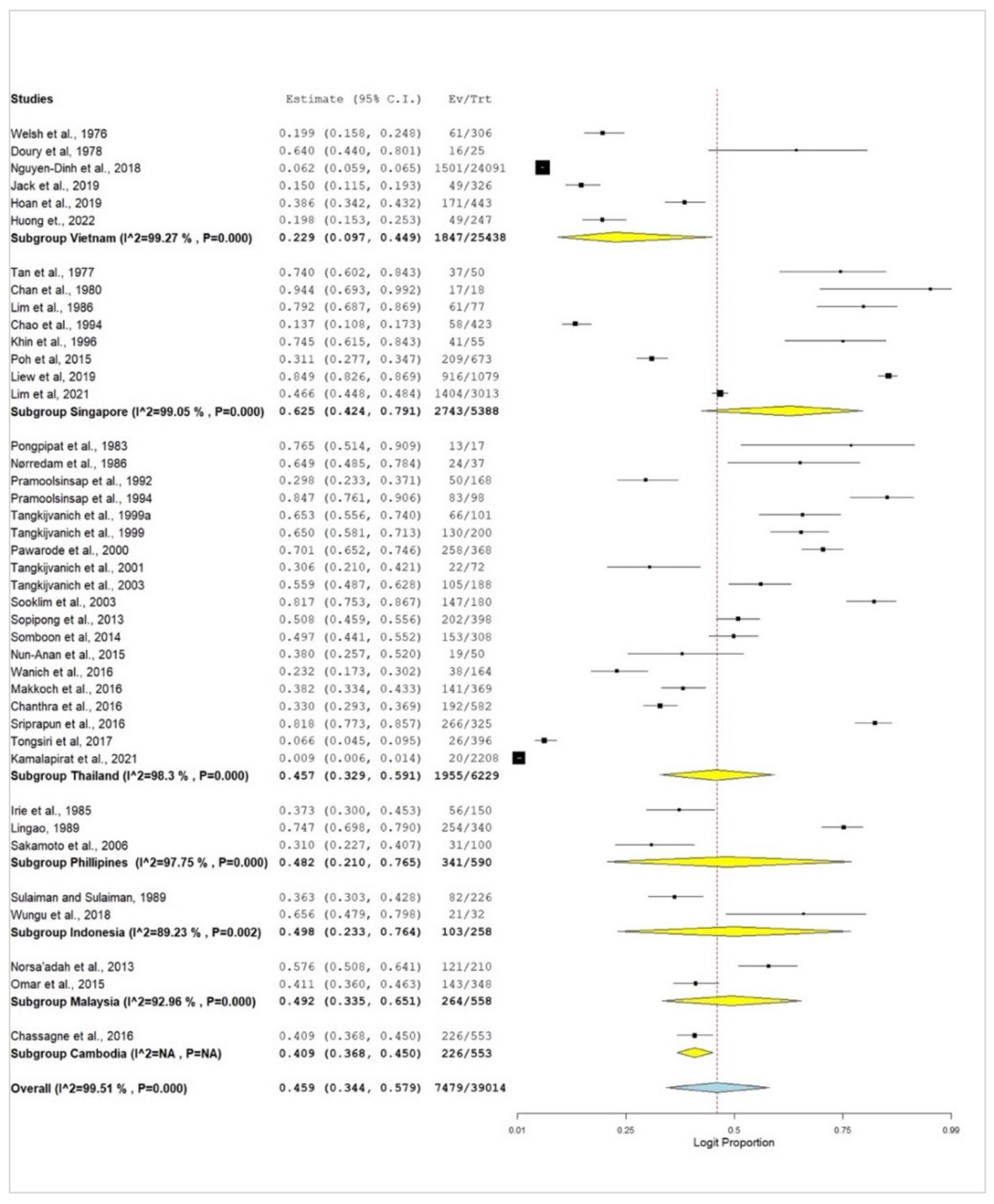
3.2. Subgroup Meta-Analysis
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lordick, F. Hepatocellular carcinoma—United forces against a global killer. Ann. Oncol. 2020, 31, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.-F.; Hou, J.-L.; Chutaputti, A. Hepatocellular carcinoma in the Asia pacific region. J. Gastroenterol. Hepatol. 2009, 24, 346–353. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Yip, T.C.; Ho, H.J.; Wong, V.W.; Huang, Y.T.; El-Serag, H.B.; Lee, T.Y.; Wu, M.S.; Lin, J.T.; Wong, G.L.; et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J. Hepatol. 2018, 69, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ratana-Amornpin, S.; Ratha, V.; Muhammed, M.; Natsuda, A.; Kittipong, K.; Pongjarat, N.A.; Soothrorn, C.; Sith, S.; Patommatatat, B.; Bubpha, P.; et al. Clinical features and overall survival of females with hepatocellular carcinoma: A retrospective study and review of the literature in the association of southeast asian nations. Int. J. Womens Health 2021, 13, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sooklim, K.; Sriplung, H.; Piratvisuth, T. Histologic subtypes of hepatocellular carcinoma in the southern Thai population. Asian Pac. J. Cancer Prev. 2003, 4, 302–306. [Google Scholar]
- Somboon, K.; Siramolpiwat, S.; Vilaichone, R.K. Epidemiology and Survival of Hepatocellular Carcinoma in the Central Region of Thailand. Asian Pac. J. Cancer Prev. 2014, 15, 3567–3570. [Google Scholar] [CrossRef]
- Akarapatima, K.; Chang, A.; Prateepchaiboon, T.; Pungpipattrakul, N.; Songjamrat, A.; Pakdeejit, S.; Rattanasupar, A. Comparison of Overall Survival between Transarterial Chemoembolization and Best Supportive Care in Intermediate- Stage Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2022, 23, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Pramoolsinsap, C.; Sumalnop, K.; Busagorn, N.; Kurathong, S. Prevalence and outcomes of HBV and anti-HCV seropositive patients with chronic liver disease and hepatocellular carcinoma. Southeast Asian J. Trop. Med. Public Health 1992, 23, 6–11. [Google Scholar]
- Hasan, I.; Loho, I.M.; Setiawan, P.B.; Djumhana, A.; Purnomo, H.D.; Siregar, L.; Gani, R.A.; Sulaiman, A.S.; Lesmana, C.R.A. Indonesian consensus on systemic therapies for hepatocellular carcinoma. Asia-Pac. J. Clin. Oncol. 2023, 19, 263–274. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Romeo, S.; Valenti, L. Hepatocellular carcinoma in nonalcoholic fatty liver: Role of environmental and genetic factors. World J. Gastroenterol. 2014, 20, 12945–12955. [Google Scholar] [CrossRef]
- Cohen, D.; Ghosh, S.; Shimakawa, Y.; Ramou, N.; Garcia, P.S.; Dubois, A.; Guillot, C.; Kakwata-Nkor Deluce, N.; Tilloy, V.; Durand, G.; et al. Hepatitis B virus preS2Δ38-55 variants: A newly identified risk factor for hepatocellular carcinoma. JHEP Rep. 2020, 2, 100144. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.H.; Wu, S.Y.; Zuchini, R.; Lin, X.Z.; Su, I.J.; Tsai, T.F.; Lin, Y.J.; Wu, C.T.; Liu, H.S. Autophagy suppresses tumorigenesis of hepatitis B virus-associated hepatocellular carcinoma through degradation of microRNA-224. Hepatology 2014, 59, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yang, Z.; Liu, Y.; Zheng, M. The role of HBV-induced autophagy in HBV replication and HBV related-HCC. Life Sci. 2018, 205, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Cho, Y.Y.; Lee, G.Y.; You, D.; Yoo, Y.D.; Kim, Y.J. A direct role for hepatitis B virus X protein in inducing mitochondrial membrane permeabilization. J. Viral. Hepat. 2018, 25, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef]
- Lupberger, J.; Hildt, E. Hepatitis B virus-induced oncogenesis. World J. Gastroenterol. 2007, 13, 74–81. [Google Scholar] [CrossRef]
- Lim, C.J.; Lee, Y.H.; Pan, L.; Lai, L.; Chua, C.; Wasser, M.; Lim, T.K.H.; Yeong, J.; Toh, H.C.; Lee, S.Y. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019, 68, 916–927. [Google Scholar] [CrossRef]
- Ren, F.; Li, W.; Zhao, S.; Wang, L.; Wang, Q.; Li, M.; Xiang, A.; Guo, Y. A3G-induced mutations show a low prevalence and exhibit plus-strand regional distribution in hepatitis B virus DNA from patients with non-hepatocellular carcinoma (HCC) and HCC. J. Med. Virol. 2021, 93, 3672–3678. [Google Scholar] [CrossRef]
- Bello, K.E.; Mat Jusoh, T.N.A.; Irekeola, A.A.; Abu, N.; Mohd Amin, N.A.Z.; Mustaffa, N.; Shueb, R.H. A Recent Prevalence of Hepatitis B Virus (HBV) Genotypes and Subtypes in Asia: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 1011. [Google Scholar] [CrossRef]
- Kabir, T.; Syn, N.; Ramkumar, M.; Yeo, E.Y.J.; Teo, J.Y.; Koh, Y.X.; Lee, S.Y.; Cheow, P.C.; Chow, P.K.H.; Chung, A.Y.F. Effect of surgical delay on survival outcomes in patients undergoing curative resection for primary hepatocellular carcinoma: Inverse probability of treatment weighting using propensity scores and propensity score adjustment. Surgery 2020, 167, 417–424. [Google Scholar] [CrossRef]
- Stephenson, A.A.; Cao, S.; Taggart, D.J.; Vyavahare, V.P.; Suo, Z. Design, synthesis, and evaluation of liver-specific gemcitabine prodrugs for potential treatment of hepatitis C virus infection and hepatocellular carcinoma. Eur. J. M. Chem. 2021, 213, 113135. [Google Scholar] [CrossRef]
- An, P.; Xu, J.; Yu, Y.; Winkler, C.A. Host and viral genetic variation in HBV-related hepatocellular carcinoma. Front. Genet. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Lesmana, L.A.; Tateishi, R.; Chen, P.J.; Lin, S.M.; Yoshida, H.; Kudo, M.; Lee, J.M.; Choi, B.I.; Poon, R.T.; et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010, 4, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Shao, J.; Wang, Y.; Hu, L.; Zhang, L.; Lyu, C. Lower risk of hepatocellular carcinoma with tenofovir than entecavir in antiviral treatment-naïve chronic hepatitis B patients: A systematic review and meta-analysis involving 90,897 participants. Clin. Exp. Med. 2023, 23, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Ng, C.H.; Tay, P.W.L.; Syn, N.; Muthiah, M.D.; Lim, W.H.; Tang, A.S.P.; Lim, K.E.; Lim, G.E.H.; Tamaki, N. Risk of Hepatocellular Carcinoma with Tenofovir vs Entecavir Treatment for Chronic Hepatitis B Virus: A Reconstructed Individual Patient Data Meta-analysis. JAMA Netw. Open 2022, 5, e2219407. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- George, B.J.; Aban, I.B. An application of meta-analysis based on DerSimonian and Laird method. J. Nucl. Cardiol. 2016, 23, 690–692. [Google Scholar] [CrossRef]
- Fletcher, J. What is heterogeneity and is it important? BMJ 2007, 334, 94–96. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the gap between methodologists and end-users: R as a computational back-end. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Pongpipat, D.; Suvatte, V.; Assateerawatts, A. Hepatitis B surface antigen and alpha-foetoprotein in paediatric hepatocellular carcinoma and hepatoblastoma in Thailand. Asian Pac. J. Allergy Immunol. 1983, 1, 104–106. [Google Scholar] [PubMed]
- Doury, J.C.; Roche, J.C.; Brisou, B. Hepato-cellular carcinoma associated with HBs antigen: A study of 25 cases from Vietnam. Rev. Epidemiol. Sante Publique 1978, 26, 161–170. [Google Scholar]
- Chan, S.H.; Chen, F.; Chio, L.F.; Ho, K.T.; Law, C.H.; Leong, S.F.; Mathew, T.; Ng, P.L.; Oon, C.J.; Seah, C.S.; et al. Hepatitis B virus and alphafetoprotein in liver diseases in Singapore. Singap. Med. J. 1980, 21, 506–508. [Google Scholar]
- Nun-Anan, P.; Chonprasertsuk, S.; Siramolpiwat, S.; Tangaroonsanti, A.; Bhanthumkomol, P.; Pornthisarn, B.; Vilaichone, R.K. CYP2C19 Genotype Could be a Predictive Factor for Aggressive Manifestations of Hepatocellular Carcinoma Related with Chronic Hepatitis B Infection in Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 3253–3256. [Google Scholar] [CrossRef]
- Kamalapirat, T.; Yingcharoen, K.; Ungtrakul, T.; Soonklang, K.; Dechma, J.; Chunnuan, P.; Kusuman, P.; Pothijaroen, C.; Tawpa, J.; Cheirsilpa, K.; et al. Assessing risk scores for predicting hepatocellular carcinoma in Thai patients with chronic hepatitis B. J. Viral. Hepat. 2021, 28, 1034–1041. [Google Scholar] [CrossRef]
- Wungu, C.D.K.; Mochamad, A.; Ulfa, K.; Gwenny, I.P. The analysis of mutation profile on pre-S1 and pre-S2 region of hepatitis B virus in chronic liver disease. Malays. J. Biochem. Mol. Biol. 2018, 21, 85–92. [Google Scholar]
- Tangkijvanich, P.; Thong-ngam, D.; Mahachai, V.; Kladchareon, N.; Suwangool, P.; Kullavanijaya, P. Long-term effect of interferon therapy on incidence of cirrhosis and hepatocellular carcinoma in Thai patients with chronic hepatitis B. Southeast Asian J. Trop. Med. Public Health 2001, 32, 452–458. [Google Scholar]
- Nørredam, K.; Chainuvati, T.; Aldershvile, J.; Nielsen, J.O.; Viranuvatti, V. Hepatitis b antigens and antibodies in serum from 41 cases of primary carcinoma of the liver. Scand J. Gastroenterol. 1986, 21, 428–432. [Google Scholar] [CrossRef]
- Tongsiri, N.; Subwongcharoen, S.; Treepongkaruna, S.-A. The impact of transarterial chemoembolization for advanced stage hepatocellular carcinoma: A single center experience. J. Med. Assoc. Thail. 2017, 100, 33–41. [Google Scholar]
- Sakamoto, T.; Tanaka, Y.; Orito, E.; Co, J.; Clavio, J.; Sugauchi, F.; Ito, K.; Ozasa, A.; Quino, A.; Ueda, R.; et al. Novel subtypes (subgenotypes) of hepatitis B virus genotypes B and C among chronic liver disease patients in the Philippines. J. Gen. Virol. 2006, 87, 1873–1882. [Google Scholar] [CrossRef]
- Tan, A.Y.O.; Law, C.H.; Lee, Y.S. Hepatitis B antigen in the liver cells in cirrhosis and hepatocellular carcinoma. Pathology 1977, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wanich, N.; Vilaichone, R.-K.; Chotivitayatarakorn, P.; Siramolpiwat, S. High prevalence of hepatocellular carcinoma in patients with chronic hepatitis B infection in Thailand. Asian Pac. J. Cancer Prev. 2016, 17, 2857–2860. [Google Scholar]
- Khin, L.W.; Teo, C.J.; Guan, R. Seroprevalence of hepatitis B and C viral markers in patients with primary hepatocellular carcinoma in Singapore. Singap. Med. J. 1996, 37, 492–496. [Google Scholar]
- Welsh, J.D.; Brown, J.D.; Arnold, K.; Mathews, H.M.; Prince, A.M. Hepatitis Bs Antigen, Malaria Titers, and Primary LiverCancer in South Vietnam. Gastroenterology 1976, 70, 392–396. [Google Scholar] [CrossRef]
- Huan, N.T.C.; Trung, N.Q.; Luong, B.A.; Tram, D.B.; Vu, H.A.; Bui, H.H.; Pham Thi Le, H. Mutations in the HBV PreS/S gene related to hepatocellular carcinoma in Vietnamese chronic HBV-infected patients. PLoS ONE 2022, 17, e0266134. [Google Scholar] [CrossRef]
- Irie, H.; Mori, W.; Zamuco, J.T. Hepatocellular Carcinoma in the Philippines: A Clinicopathological Study of 150 Cases. Jpn. J. Cancer Res. 1985, 76, 297–300. [Google Scholar]
- Chao, T.C.; Lo, D.; Bloodworth, B.C.; Gunasegaram, R.; Koh, T.H.; Ng, H.S. Aflatoxin Exposure in Singapore: Blood Aflatoxin Levels in Normal Subjects, Hepatitis B Virus Carriers and Primary Hepatocellular Carcinoma Patients. Med. Sci. Law 1994, 34, 289–298. [Google Scholar] [CrossRef]
- Lim, T.C.; Teh, L.B.; Kwok, K.C.; Ng, H.S.; Ong, Y.Y.; Seah, C.S. Hepatocellular carcinoma—A clinical study. Ann. Acad. Med. Singap. 1986, 15, 158–161. [Google Scholar]
- Welsh, J.; Brown, J.; Arnold, K.; Chandler, A.; Mau, H.M.; Thuc, T.K. Hepatitis-Associated Antigen in Hepatoma in South Vietnam. Lancet 1972, 299, 592. [Google Scholar] [CrossRef] [PubMed]
- Tangkijvanich, P.; Hirsch, P.; Theamboonlers, A.; Nuchprayoon, I.; Poovorawan, Y. Association of hepatitis viruses with hepatocellular carcinoma in Thailand. J. Gastroenterol. 1999, 34, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.A. Hepatitis B virus infection in liver cirrhosis and hepatocellular carcinoma in Jakarta Indonesia. Gastroenterol. Jpn. 1989, 24, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Pramoolsinsap, C.; Promvanit, N.; Komindr, S.; Lerdverasirikul, P.; Srianujata, S. Serum trace metals in chronic viral hepatitis and hepatocellular carcinoma in Thailand. J. Gastroenterol. 1994, 29, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Tangkijvanich, P.; Suwangool, P.; Mahachai, V. Comparison of clinical features and survival of patients with hepatitis B- and hepatitis C-associated hepatocellular carcinoma in Thailand. J. Med. Assoc. Thail. 2003, 86, S250–S256. [Google Scholar]
- Norsa’adah, B.; Nurhazalini-Zayani, C.G. Epidemiology and survival of hepatocellular carcinoma in north-east Peninsular Malaysia. Asian. Pac. J. Cancer Prev. 2013, 14, 6955–6959. [Google Scholar] [CrossRef] [PubMed]
- Kullavanijaya, P.; Tangkijvanich, P.; Poovorawan, Y. Current status of infection-related gastrointestinal and hepatobiliary diseases in Thailand. Southeast Asian J. Trop. Med. Public Health 1999, 30, 96–105. [Google Scholar]
- Makkoch, J.; Praianantathavorn, K.; Sopipong, W.; Chuaypen, N.; Tangkijvanich, P.; Payungporn, S. Genetic variations in XRCC4 (rs1805377) and ATF6 (rs2070150) are not associated with hepatocellular carcinoma in thai patients with hepatitis B virus infection. Asian Pac. J. Cancer Prev. 2016, 17, 591–595. [Google Scholar] [CrossRef]
- Omar, H.; Lim, C.R.; Chao, S.; Lee, M.M.; Bong, C.W.; Ooi, E.J.; Yu, C.G.; Tan, S.S.; Abu Hassan, M.R.; Menon, J.; et al. Blood gene signature for early hepatocellular carcinoma detection in patients with chronic hepatitis B. J. Clin. Gastroenterol. 2015, 49, 150–157. [Google Scholar] [CrossRef]
- Hoan, N.X.; Khuyen, N.; Giang, D.P.; Binh, M.T.; Toan, N.L.; Anh, D.T.; Trung, N.T.; Bang, M.H.; Meyer, C.G.; Velavan, T.P.; et al. Vitamin D receptor ApaI polymorphism associated with progression of liver disease in Vietnamese patients chronically infected with hepatitis B virus. BMC Med. Genet. 2019, 20, 201. [Google Scholar] [CrossRef]
- Chanthra, N.; Payungporn, S.; Chuaypen, N.; Piratanantatavorn, K.; Pinjaroen, N.; Poovorawan, Y.; Tangkijvanich, P. Single nucleotide polymorphisms in STAT3 and STAT4 and risk of hepatocellular carcinoma in Thai patients with chronic hepatitis B. Asian Pac. J. Cancer Prev. 2016, 16, 8405–8410. [Google Scholar] [CrossRef] [PubMed]
- Sopipong, W.; Tangkijvanich, P.; Payungporn, S.; Posuwan, N.; Poovorawan, Y. The KIF1B (rs17401966) single nucleotide polymorphism is not associated with the development of HBV-related hepatocellular carcinoma in Thai patients. Asian Pac. J. Cancer Prev. 2013, 14, 2865–2869. [Google Scholar] [CrossRef]
- Poh, Z.; Goh, B.-B.G.; Chang, P.-E.J.; Tan, C.-K. Rates of cirrhosis and hepatocellular carcinoma in chronic hepatitis B and the role of surveillance: A 10-year follow-up of 673 patients. Eur. J. Gastroenterol. Hepatol. 2015, 27, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Rojas Rojas, T.; Bertani, S.; Bourdy, G.; Eav, S.; Ruiz, E.; Pineau, P.; Deharo, E. A 13-Year Retrospective Study on Primary Liver Cancer in Cambodia: A Strikingly High Hepatitis C Occurrence among Hepatocellular Carcinoma Cases. Oncology 2016, 91, 106–116. [Google Scholar] [CrossRef]
- Lingao, A.L. The relationship of hepatocellular carcinoma and liver cirrhosis to hepatitis B virus infection in the Philippines. Gastroenterol. Jpn. 1989, 24, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Pawarode, A.; Tangkijvanich, P.; Voravud, N. Outcomes of primary hepatocellular carcinoma treatment: An 8-year experience with 368 patients in Thailand. J. Gastroenterol. Hepatol. 2000, 15, 860–864. [Google Scholar] [CrossRef]
- Sriprapun, M.; Chuaypen, N.; Khlaiphuengsin, A.; Pinjaroen, N.; Payungporn, S.; Tangkijvanich, P. Association of PINX1 but not TEP1 polymorphisms with progression to hepatocellular carcinoma in thai patients with chronic hepatitis B virus infection. Asian Pac. J. Cancer Prev. 2016, 17, 2019–2025. [Google Scholar] [CrossRef]
- Liew, Z.-H.; Goh, G.B.-B.; Hao, Y.; Chang, P.-E.; Tan, C.-K. Comparison of Hepatocellular Carcinoma in Patients with Cryptogenic Versus Hepatitis B Etiology: A Study of 1079 Cases Over 3 Decades. Dig. Dis. Sci. 2019, 64, 585–590. [Google Scholar] [CrossRef]
- Lim, M.S.; Goh, G.B.; Chang, J.P.; Low, J.K.; Shelat, V.G.; Huey, T.C.; Dan, Y.Y.; Kow, A.; Shridhar, I.; Tan, P.S.; et al. A study of 3013 cases of hepatocellular carcinoma: Etiology and therapy before and during the current decade. JGH Open 2021, 5, 1015–1018. [Google Scholar] [CrossRef]
- Nguyen-Dinh, S.H.; Do, A.; Pham, T.N.D.; Dao, D.Y.; Nguy, T.N.; Chen, M.S., Jr. High burden of hepatocellular carcinoma and viral hepatitis in Southern and Central Vietnam: Experience of a large tertiary referral center, 2010 to 2016. World J. Hepatol. 2018, 10, 116–123. [Google Scholar] [CrossRef]
- Yau, T.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Kang, Y.K.; Hou, M.M.; Numata, K.; Yeo, W.; Chopra, A.; Ikeda, M.; et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J. Hepatol. 2019, 71, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.Y.; Soh, S.Y.; Cheng, F.W.C.; Pang, H.H.; Luk, C.W.; Li, C.H.; Ho, K.K.H.; Chan, E.K.W.; Chan, A.C.Y.; Chung, P.H.Y.; et al. Hepatitis B Virus Seropositivity Is a Poor Prognostic Factor of Pediatric Hepatocellular Carcinoma: A Population-Based Study in Hong Kong and Singapore. Front. Oncol. 2020, 10, 570479. [Google Scholar] [CrossRef] [PubMed]
- Quak, S.H. Hepatitis B vaccination programme in Singapore. Singap. Paediatr. J. 2001, 43, 124–125. [Google Scholar]
- Taylor, V.M.; Jackson, J.C.; Chan, N.; Kuniyuki, A.; Yasui, Y. Hepatitis B knowledge and practices among Cambodian American women in Seattle, Washington. J. Community Health 2002, 27, 151–163. [Google Scholar] [CrossRef]
- Taylor, V.M.; Burke, N.J.; Sos, C.; Do, H.H.; Liu, Q.; Yasui, Y. Community health worker hepatitis B education for Cambodian American men and women. Asian Pac. J. Cancer Prev. 2013, 14, 4705–4709. [Google Scholar] [CrossRef]
- Rattanasupar, A.; Chartleeraha, S.; Akarapatima, K.; Chang, A. Factors that Affect the Surveillance and Late-Stage Detection of a Newly Diagnosed Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 3293–3298. [Google Scholar] [CrossRef]
- Reeves, H.L.; Zaki, M.Y.W.; Day, C.P. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig. Dis. Sci. 2016, 61, 1234–1245. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Chen, Y.Y.; Chen, P.C. Statins were not associated with hepatocellular carcinoma after controlling for time-varying confounders in patients with diabetes. J. Clin. Epidemiol. 2022, 150, 98–105. [Google Scholar] [CrossRef]
- Borentain, P.; Colson, P.; Bolon, E.; Gauchez, P.; Coso, D.; Gérolami, R. Hepatocellular carcinoma complicating hepatitis E virus–related cirrhosis. Hepatology 2018, 67, 446–448. [Google Scholar] [CrossRef]
- Gumilas, N.S.A.; Harini, I.M.; Ernawati, D.A.; Indriani, V.; Novrial, D.; Kurniawan, D.W. Potential of Apolipoprotein A1 (ApoA1) for Detecting Liver Cirrhosis and Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2022, 23, 2001–2008. [Google Scholar] [CrossRef]
- Llovet, J.; Zucman-Rossi, J.; Pikarsky, E. Hepatocellular carcinoma. Nat. Rev. Dis Primers 2021, 7, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Bello, K.E.; Irekeola, A.A.; Al-Mhanna, S.B.; Oseni, O.M.; Omebije, A.P.; Shueb, R.H.; Mustaffa, N. Prevalence of Spontaneous Bacterial Peritonitis (SBP) in Hepatitis B (HBV), and Hepatitis C (HCV) Liver Cirrhosis: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, X.; Wei, S.; Ma, X.; Zhao, Y. Research Progress and Treatment Status of Liver Cirrhosis with Hypoproteinemia. Evid. Based Complement. Altern. Med. 2022, 2022, 2245491. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, X.L.; Fan, R.; Bai, J.; Wen, H.; Du, L.T.; Jiang, G.Q.; Wang, C.Y.; Fan, X.T.; Ye, Y.N.; et al. The landscape of cell-free HBV integrations and mutations in cirrhosis and hepatocellular carcinoma patients. Clin. Cancer Res. 2021, 27, 3772–3783. [Google Scholar] [CrossRef]
- Saha, S.K.; Saha, S.; Shakur, S.; Hanif, M.; Habib, M.A.; Datta, S.K.; Bock, H.L. Community-based cross-sectional seroprevalence study of hepatitis A in Bangladesh. World J. Gastroenterol. 2009, 15, 4932–4937. [Google Scholar] [CrossRef]
- Rajamoorthy, Y.; Taib, N.M.; Mudatsir, M.; Harapan, H.; Wagner, A.L.; Munusammy, S.; Rahim, K.A.; Radam, A. Risk behaviours related to hepatitis B virus infection among adults in Malaysia: A cross-sectional household survey. Clin. Epidemiol. Glob. Health 2020, 8, 76–82. [Google Scholar] [CrossRef]
- Zhu, R.X.; Seto, W.-K.; Lai, C.-L.; Yuen, M.-F. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef]
- Wiangnon, S.; Kamsa-ard, S.; Suwanrungruang, K.; Promthet, S.; Kamsa-ard, S.; Mahaweerawat, S.; Khuntikeo, N. Trends in incidence of hepatocellular carcinoma, 1990–2009, Khon Kaen, Thailand. Asian Pac. J. Cancer Prev. 2012, 13, 1065–1068. [Google Scholar] [CrossRef]
- Thomas, C.E.; Adibi, J.J.; Kuipers, A.L.; Diergaarde, B.; Luu, H.N.; Jin, A.; Koh, W.P.; Gao, Y.T.; Adams-Haduch, J.; Wang, R.; et al. Soluble CD137 and risk of hepatocellular carcinoma: Nested case–control studies in cohorts in Shanghai and Singapore. Br. J. Cancer 2023, 128, 2081–2088. [Google Scholar] [CrossRef]
- Losic, B.; Craig, A.J.; Villacorta-Martin, C.; Martins-Filho, S.N.; Akers, N.; Chen, X.; Ahsen, M.E.; von Felden, J.; Labgaa, I.; DʹAvola, D.; et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 2020, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- De Maddalena, C.; Giambelli, C.; Tanzi, E.; Colzani, D.; Schiavini, M.; Milazzo, L.; Bernini, F.; Ebranati, E.; Cargnel, A.; Bruno, R.; et al. High level of genetic heterogeneity in S and P genes of genotype D hepatitis B virus. Virology 2007, 365, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Robotin, M.C.; Masgoret, X.; Porwal, M.; Goldsbury, D.; Khoo, C.; George, J. Using a chronic hepatitis b registry to support population-level liver cancer prevention in sydney, Australia. Clin. Epidemiol. 2018, 10, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.Y.; Wong, D.K.H.; Pollicino, T.; Raimondo, G.; Hollinger, F.B.; Yuen, M.F. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J. Hepatol. 2020, 73, 952–964. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Piewbang, C.; Dankaona, W.; Poonsin, P.; Yostawonkul, J.; Lacharoje, S.; Sirivisoot, S.; Kasantikul, T.; Tummaruk, P.; Techangamsuwan, S. Domestic cat hepadnavirus associated with hepatopathy in cats: A retrospective study. J. Vet. Intern. Med. 2022, 36, 1648–1659. [Google Scholar] [CrossRef]
- Ponzetto, A.; Srinivasan, R.S. Risk factors for hepatocellular carcinoma. Hepatology 2017, 65, 1074. [Google Scholar] [CrossRef]
- Yuen, M.F.; Tanaka, Y.; Fong, D.Y.; Fung, J.; Wong, D.K.; Yuen, J.C.; But, D.Y.; Chan, A.O.; Wong, B.C.; Mizokami, M.; et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J. Hepatol. 2009, 50, 80–88. [Google Scholar] [CrossRef]
- Ahmed, N.; Arshad, S.; Basheer, S.N.; Karobari, M.I.; Marya, A.; Marya, C.M.; Taneja, P.; Messina, P.; Yean, C.Y.; Scardina, G.A. Smoking a Dangerous Addiction: A Systematic Review on an Underrated Risk Factor for Oral Diseases. Int. J. Environ. Res. Public Health 2021, 18, 11003. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; Wan Ghazali, W.S.; Mohamed, M.; Rabaan, A.A.; Santali, E.Y.; Alestad, H.J.; Santali, E.Y.; Arshad, S.; Ahmed, N.; Afolabi, H.A. Effectiveness of physical activity on immunity markers and quality of life in cancer patient: A systematic review. PeerJ 2022, 10, 13664. [Google Scholar] [CrossRef]
- Yusof, W.; Irekeola, A.A.; Wada, Y.; Engku Abd Rahman, E.N.S.; Ahmed, N.; Musa, N.; Khalid, M.F.; Rahman, Z.A.; Hassan, R.; Yusof, N.Y.; et al. A Global Mutational Profile of SARS-CoV-2: A Systematic Review and Meta-Analysis of 368,316 COVID-19 Patients. Life 2021, 11, 1224. [Google Scholar] [CrossRef]
- Sizaret, P.; Tuyns, A.; Martel, N.; Jouvenceaux, A.; Levin, A.; Ong, Y.W.; Rive, J. α-Fetoprotein Levels in Normal Males from Seven Ethnic Groups with Different Hepatocellular Carcinoma Risks. Ann. N. Y. Acad. Sci. 1975, 259, 136–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Long, X.; Jia, W.L.; Wu, H.J.; Zhao, J.; Liang, H.F.; Laurence, A.; Zhu, J.; Dong, D.; Chen, Y.; et al. Viral integration drives multifocal HCC during the occult HBV infection. J. Exp. Clin. Cancer Res. 2019, 38, 261. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Onorato, L.; Iodice, V.; Starace, M.; Minichini, C.; Farella, N.; Liorre, G.; Filippini, P.; Sagnelli, E.; de Stefano, G. Occult HBV infection in HCC and cirrhotic tissue of HBsAgnegative patients: A virological and clinical study. Oncotarget 2016, 7, 62706–62714. [Google Scholar] [CrossRef]
- Velázquez, R.F.; Rodríguez, M.; Navascués, C.A.; Linares, A.; Pérez, R.; Sotorríos, N.G.; Martínez, I.; Rodrigo, L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 2003, 37, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.N.; Hou, J.; Lin, Q.X.; Li, S.M.; Chen, R.; Xie, K.P. Hepatitis E virus re-infection accelerates hepatocellular carcinoma development and relapse in a patient with liver cirrhosis: A case report and review of literature. World J. Hepatol. 2020, 12, 1358–1366. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
| Study ID | Year | Country | Total Number of HBV Cases | Positive HCC | Type of Study | Average Age (Range) | Gender | Positive HBV Genotypes | Cirrhosis | Stage of HCC | Treatment Status | AFP Detectable Level | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | A | B | C | Yes | No | Early | Intermediate | Late | Treated | Naïve | Yes | No | |||||||
| Pongpipat et al. [33] | 1983 | Thailand | 17 | 13 | Cross-sectional | 7.4 (1–14) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 8 | 5 | 8 | 5 |
| Doury et al. [34] | 1978 | Vietnam | 25 | 16 | Cross-sectional | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Chan et al. [35] | 1980 | Singapore | 18 | 17 | Cross-sectional | NR | NR | NR | NR | NR | NR | 1 | 16 | NR | NR | NR | NR | NR | 11 | 6 |
| Nun-Anan et al. [36] | 2015 | Thailand | 50 | 19 | Cross-sectional | 56.4 (18–78) | 13 | 6 | NR | NR | NR | 1 | 18 | 11 | 6 | 2 | NR | NR | NR | NR |
| Kamalapirat et al. [37] | 2021 | Thailand | 2208 | 20 | Cross-sectional | 41.36 (18–82) | 11 | 9 | NR | NR | NR | 3 | 17 | 7 | 9 | 4 | NR | NR | NR | NR |
| Wungu et al. [38] | 2018 | Indonesia | 32 | 21 | Cross-sectional | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Tangkijvanich et al. [39] | 2001 | Thailand | 72 | 22 | Cross-sectional | NR | NR | NR | NR | NR | NR | 11 | 11 | 6 | 13 | 3 | 7 | 15 | NR | NR |
| Nørredam et al. [40] | 1986 | Thailand | 37 | 24 | Cross-sectional | 50.3 (18–81) | 11 | 13 | NR | NR | NR | 16 | 8 | NR | NR | NR | NR | NR | NR | NR |
| Tongsiri et al. [41] | 2017 | Thailand | 396 | 26 | Retrospective | 56.3 (18–76) | 18 | 8 | NR | NR | NR | 9 | 17 | 14 | 5 | 7 | NR | NR | 21 | 5 |
| Sakamoto et al. [42] | 2006 | Philippines | 100 | 31 | Cross-sectional | 53.7 (18–73) | 26 | 5 | 11 | 11 | 9 | 25 | 6 | 12 | 9 | 10 | 27 | 4 | NR | NR |
| Tan et al. [43] | 1977 | Singapore | 50 | 37 | Cross-sectional | NR | NR | NR | NR | NR | NR | 27 | 10 | NR | NR | NR | NR | NR | 26 | 11 |
| Wanich et al. [44] | 2016 | Thailand | 164 | 38 | Cross-sectional | 59.6 (18–87) | 31 | 7 | NR | NR | NR | 28 | 10 | 20 | 6 | 12 | 29 | 9 | 23 | 15 |
| Khin et al. [45] | 1996 | Singapore | 55 | 41 | Case control | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Jack et al. [46] | 2019 | Vietnam | 362 | 49 | Retrospective | NR | NR | NR | NR | NR | NR | 38 | 11 | 15 | 20 | 14 | 37 | 12 | 41 | 8 |
| Huong et al. [47] | 2022 | Vietnam | 247 | 49 | Retrospective | 57.9 (18–81) | 34 | 15 | 1 | 42 | 5 | 32 | 17 | NR | NR | NR | NR | NR | NR | NR |
| Pramoolsinsap et al. [8] | 1992 | Thailand | 168 | 50 | Case control | 50.8 (19–79) | 31 | 19 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Irie et al. [48] | 1985 | Philippines | 150 | 56 | Retrospective | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Chao et al. [49] | 1994 | Singapore | 423 | 58 | Retrospective | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lim et al. [50] | 1986 | Singapore | 77 | 61 | Cross-sectional | NR | 55 | 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 61 | 0 |
| Welsh et al. [51] | 1976 | Vietnam | 306 | 61 | Cross-sectional | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Tangkijvanich et al. [52] | 1999 | Thailand | 101 | 66 | Cross-sectional | 54.4 (18–79) | 48 | 18 | NR | NR | NR | 19 | 47 | NR | NR | NR | NR | NR | NR | NR |
| Sulaiman and Sulaiman [53] | 1989 | Indonesia | 226 | 82 | Retrospective | 51.4 (21–78) | 68 | 14 | NR | NR | NR | 29 | 53 | NR | NR | NR | 20 | 62 | NR | NR |
| Pramoolsinsap et al. [54] | 1994 | Thailand | 98 | 83 | Cross-sectional | 49.2 (18–81) | 51 | 32 | NR | NR | NR | 21 | 62 | NR | NR | NR | NR | NR | NR | NR |
| Tangkijvanich et al. [55] | 2003 | Thailand | 188 | 105 | Retrospective | 42.9 (18–73) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Norsa’adah et al. [56] | 2013 | Malaysia | 210 | 121 | Cross-sectional | 55 (16–82) | 98 | 23 | NR | NR | NR | 42 | 79 | 40 | 58 | 23 | 102 | 19 | 121 | 0 |
| Tangkijvanich et al. [57] | 1999 | Thailand | 200 | 130 | Cross-sectional | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Makkoch et al. [58] | 2016 | Thailand | 369 | 141 | Cross-sectional | 51.8 (18–82) | 98 | 43 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Omar et al. [59] | 2015 | Malaysia | 348 | 143 | Cross-sectional | 56.2 (17–85) | 99 | 44 | NR | NR | NR | NR | NR | 52 | 47 | 44 | NR | NR | NR | NR |
| Sooklim et al. [5] | 2003 | Thailand | 180 | 147 | Retrospective | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Somboonet et al. [6] | 2014 | Thailand | 308 | 153 | Retrospective | 57.4 (18–75) | 99 | 54 | NR | NR | NR | 146 | 7 | 50 | 49 | 54 | 97 | 56 | NR | NR |
| Hoan et al. [60] | 2019 | Vietnam | 443 | 171 | Case control | 51 (18–90) | 129 | 42 | NR | NR | NR | 117 | 54 | NR | NR | NR | NR | NR | NR | NR |
| Chanthra et al. [61] | 2016 | Thailand | 582 | 192 | Case control | 57.6 (18–83) | 164 | 28 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sopipong et al. [62] | 2013 | Thailand | 398 | 202 | Case control | 59.8 (18–81) | 158 | 44 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Poh et al. [63] | 2015 | Singapore | 673 | 209 | Case control | 56.3 (17–82) | 134 | 75 | NR | NR | NR | 154 | 55 | NR | NR | NR | NR | NR | NR | NR |
| Chassagne et al. [64] | 2016 | Cambodia | 553 | 226 | Retrospective | 58.1 (28–91) | 184 | 42 | NR | NR | NR | 196 | 30 | NR | NR | NR | NR | NR | 201 | 25 |
| Lingao [65] | 1989 | Phillipines | 340 | 254 | Cross-sectional | 46.1 (18–78) | 198 | 56 | NR | NR | NR | 99 | 155 | NR | NR | NR | NR | NR | 249 | 5 |
| Pawarode et al. [66] | 2000 | Thailand | 368 | 258 | Retrospective | 52.1 (2–85) | 190 | 68 | NR | NR | NR | 211 | 47 | NR | NR | NR | 248 | 10 | NR | NR |
| Sriprapun et al. [67] | 2016 | Thailand | 325 | 266 | Cross-sectional | 50.28 (18–91) | 177 | 89 | NR | NR | NR | 80 | 186 | 94 | 120 | 52 | NR | NR | NR | NR |
| Liew et al. [68] | 2019 | Singapore | 1079 | 916 | Retrospective | 59.4 (2–93) | 768 | 148 | NR | NR | NR | NR | NR | 197 | 556 | 163 | NR | NR | NR | NR |
| Lim et al. [69] | 2021 | Singapore | 3013 | 1404 | Retrospective | 63.8 (18–89) | 1028 | 376 | NR | NR | NR | NR | NR | NR | NR | NR | 787 | 617 | NR | NR |
| Nguyen-Dinh et al. [70] | 2018 | Vietnam | 24,091 | 1501 | Retrospective | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Subgroup Meta-Analysis | Number of Studies | Prevalence of HCC (%) | 95% CI | I2 (%) | Q | Heterogeneity Test | |
|---|---|---|---|---|---|---|---|
| D.F | p | ||||||
| Country | |||||||
| Vietnam | 6 | 22.9 | 9.7–44.9 | 99.27 | 682.60 | 5 | <0.001 |
| Singapore | 8 | 62.5 | 42.4–79.1 | 99.05 | 739.37 | 7 | <0.001 |
| Thailand | 19 | 45.7 | 32.9–59.1 | 98.3 | 1058.90 | 18 | <0.001 |
| Philippines | 3 | 48.2 | 21.0–76.5 | 97.75 | 88.72 | 2 | <0.001 |
| Indonesia | 2 | 49.8 | 23.3–76.4 | 89.23 | 9.28 | 1 | 0.002 |
| Malaysia | 2 | 49.2 | 33.5–65.1 | 92.96 | 14.20 | 1 | <0.001 |
| Cambodia | 1 | 40.9 | 36.8–45.0 | - | - | - | - |
| Study design | |||||||
| Cross-sectional | 21 | 53.0 | 39.1–66.5 | 97.86 | 935.81 | 20 | <0.001 |
| Retrospective | 14 | 36.5 | 18.7–59.0 | 99.79 | 6117.67 | 13 | <0.001 |
| Case-control | 6 | 41.2 | 32.9–50.0 | 99.51 | 76.68 | 5 | <0.001 |
| Year of publication | |||||||
| 1975–1985 | 7 | 58.0 | 37.1–76.4 | 97.66 | 256.53 | 6 | <0.001 |
| 1986–1995 | 6 | 47.1 | 30.4–64.5 | 96.87 | 159.99 | 5 | <0.001 |
| 1996–2005 | 7 | 48.9 | 39.7–58.3 | 92.1 | 75.95 | 6 | <0.001 |
| 2006–2015 | 7 | 44.1 | 22.4–68.2 | 98.08 | 312.99 | 6 | <0.001 |
| 2016–2023 | 14 | 37.6 | 19.2–60.5 | 99.78 | 6039.49 | 6 | <0.001 |
| Gender | |||||||
| Male | 26 | 18.6 | 14.3–23.9 | 97.33 | 562.31 | 25 | <0.001 |
| Female | 26 | 6.9 | 5.4–8.8 | 91.06 | 496.64 | 25 | <0.001 |
| HBV genotypes | |||||||
| A | 2 | 0.3 | 0.1–0.6 | 72.04 | 158.38 | 1 | <0.001 |
| B | 2 | 0.3 | 0.1–0.7 | 86.74 | 212.96 | 1 | <0.001 |
| C | 2 | 0.3 | 0.1–0.6 | 68.12 | 135.27 | 1 | <0.001 |
| Cirrhosis | |||||||
| Yes | 22 | 6.7 | 4.7–9.5 | 95.17 | 763.82 | 21 | <0.001 |
| No | 22 | 4.1 | 2.5–6.8 | 96.96 | 647.47 | 21 | <0.001 |
| Stages of HCC | |||||||
| Early | 12 | 4.1 | 2.5–6.8 | 96.96 | 339.72 | 11 | <0.001 |
| Intermediate | 12 | 1.1 | 0.6–2.0 | 96.41 | 281.57 | 11 | <0.001 |
| Late | 12 | 1.3 | 0.8–2.0 | 90.18 | 407.15 | 11 | <0.001 |
| AFP detectabble level | |||||||
| Yes | 10 | 0.7 | 0.5–1.0 | 98.03 | 312.17 | 9 | <0.001 |
| No | 10 | 0.6 | 0.3–1.2 | 88.07 | 269.04 | 9 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaan, A.A.; Bello, K.E.; Irekeola, A.A.; Kaabi, N.A.A.; Halwani, M.A.; Yousuf, A.A.; Alshengeti, A.; Alfaraj, A.H.; Khamis, F.; Al-Subaie, M.F.; et al. Prevalence of Hepatocellular Carcinoma in Hepatitis B Population within Southeast Asia: A Systematic Review and Meta-Analysis of 39,050 Participants. Pathogens 2023, 12, 1220. https://doi.org/10.3390/pathogens12101220
Rabaan AA, Bello KE, Irekeola AA, Kaabi NAA, Halwani MA, Yousuf AA, Alshengeti A, Alfaraj AH, Khamis F, Al-Subaie MF, et al. Prevalence of Hepatocellular Carcinoma in Hepatitis B Population within Southeast Asia: A Systematic Review and Meta-Analysis of 39,050 Participants. Pathogens. 2023; 12(10):1220. https://doi.org/10.3390/pathogens12101220
Chicago/Turabian StyleRabaan, Ali A., Kizito Eneye Bello, Ahmad Adebayo Irekeola, Nawal A. Al Kaabi, Muhammad A. Halwani, Amjad A. Yousuf, Amer Alshengeti, Amal H. Alfaraj, Faryal Khamis, Maha F. Al-Subaie, and et al. 2023. "Prevalence of Hepatocellular Carcinoma in Hepatitis B Population within Southeast Asia: A Systematic Review and Meta-Analysis of 39,050 Participants" Pathogens 12, no. 10: 1220. https://doi.org/10.3390/pathogens12101220
APA StyleRabaan, A. A., Bello, K. E., Irekeola, A. A., Kaabi, N. A. A., Halwani, M. A., Yousuf, A. A., Alshengeti, A., Alfaraj, A. H., Khamis, F., Al-Subaie, M. F., AlShehail, B. M., Almuthree, S. A., Ibraheem, N. Y., Khalifa, M. H., Alfaresi, M., Fares, M. A. A., Garout, M., Alsayyah, A., Alshehri, A. A., ... Alissa, M. (2023). Prevalence of Hepatocellular Carcinoma in Hepatitis B Population within Southeast Asia: A Systematic Review and Meta-Analysis of 39,050 Participants. Pathogens, 12(10), 1220. https://doi.org/10.3390/pathogens12101220








