Hepatitis E Virus (HEV) Infection among Hemodialysis Patients from Southern Bulgaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
- - Patients transferred to another dialysis center during the study period;
- - Transient dialysis patients (temporarily residing in the district due to travel, family visits, etc.);
- - Patients with an acute medical indication requiring hemodialysis treatment;
- - Patients undergoing peritoneal dialysis at home (they are not exposed to the same procedures and conditions as in the hemodialysis center).
2.2. Algorithm for Examining Patients
- Familiarizing the patients with the purpose of the study and filling out an informed consent form. The collection of blood samples (5 mL of venous blood each) was conducted after the submission of informed consent.
- Blood samples were immediately centrifuged (at 2000 rpm), and the serum was separated into two aliquots (one for anti-HEV antibodies detection and the second for HEV RNA detection) and stored at −80 °C until use.
- Initially, all serum samples were tested for the presence of specific HEV antibodies (anti-HEV IgM and anti-HEV IgG).
- Secondly, regardless of antibody results, all patients were tested for the presence of HEV RNA. According to the recommendations of the EASL (European Association for the Study of the Liver) [12], for the diagnosis of HEV infection (acute and chronic) in immunocompromised persons, it is recommended that serology and Nucleic Acid Amplification Testing (NAT) be used in combination, as a negative NAT does not exclude acute infection and serology is sometimes negative in immunosuppressed patients with chronic infection.
- All specimens were negative for HEV RNA. Therefore, for the purpose of analysis, the samples were divided into two groups based on the presence of HEV antibodies: (1) HEV IgG seroprevalence (anti-HEV IgG alone); (2) and overall HEV seroprevalence, based on all positive samples regardless of the type of HEV antibody marker (anti-HEV IgM and/or anti-HEV IgG).
- An additional examination for the presence of HBV (HBsAg) and HCV (anti-HCV) coinfection was performed.
2.2.1. Serological Testing
2.2.2. Quantification of the HEV RNA
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, G.W.; Dalton, H.R. Hepatitis E: An underestimated emerging threat. Ther. Adv. Infect. Dis. 2019, 6, 2049936119837162. [Google Scholar] [CrossRef] [PubMed]
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Moghaddam, S.M.; Zarei, A.; Alavian, S.M.; Mansouri, M. Hepatitis E virus infection: A general review with a focus on hemodialysis and kidney transplant patients. Am. J. Nephrol. 2010, 31, 398–407. [Google Scholar] [CrossRef]
- Tavakoli, A.; Alavian, S.M.; Moghoofei, M.; Mostafaei, S.; Abbasi, S.; Farahmand, M. Seroepidemiology of hepatitis E virus infection in patients undergoing maintenance hemodialysis: Systematic review and meta-analysis. Ther. Apher. Dial. 2021, 25, 4–15. [Google Scholar] [CrossRef]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Knotek, M.; Kudumija, B.; Ilic, M.; Gulin, M.; Zibar, L.; Hrstic, I.; Jurekovic, Z.; Kolaric, B.; et al. Seroepidemiology of hepatitis E in patients on haemodialysis in Croatia. Int. Urol. Nephrol. 2020, 52, 371–378. [Google Scholar] [CrossRef]
- Kogias, D.; Skeva, A.; Smyrlis, A.; Mourvati, E.; Kantartzi, K.; Romanidou, G.; Kalientzidou, M.; Rekari, V.; Konstantinidou, E.; Kiorteve, P.; et al. Hepatitis E Virus (HEV) Infection in Hemodialysis Patients: A Multicenter Epidemiological Cohort Study in North-Eastern Greece. Pathogens 2023, 12, 667. [Google Scholar] [CrossRef] [PubMed]
- Dalekos, G.N.; Zervou, E.; Elisaf, M.; Germanos, N.; Galanakis, E.; Bourantas, K.; Siamopoulos, K.C.; Tsianos, E.V. Antibodies to hepatitis E virus among several populations in Greece: Increased prevalence in an hemodialysis unit. Transfusion 1998, 38, 589–595. [Google Scholar] [CrossRef]
- Elimination of Viral Hepatitis in the Balkan Countries. Lessons Learnt and the Way Forward. Background Document. In Proceedings of the VHPB Multi-Country Meeting, Skopje, North Macedonia, 27–28 October 2022; Available online: https://www.vhpb.org/files/html/Meetings_and_publications/Pre-meeting_documents/Background_Document_BALKAN.pdf (accessed on 1 August 2023).
- Pishmisheva, M.; Shikov, P.; Golkocheva-Markova, E.; Kotsev, S.; Naseva, E.; Vatev, N.; Argirova, R. Spread of Hepatitis E Viral Infection among Hemodialysis Patients in Pazardzhik District, Bulgaria. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1086–1092. [Google Scholar] [CrossRef]
- National Statistical Institute, Bulgaria, Healthcare. 2018. (In Bulgarian). Available online: https://www.nsi.bg/sites/default/files/files/publications/Zdraveopazvane_2018.pdf (accessed on 1 August 2023).
- Vazelov, E.S.; Bogov, B.I.; Gaydarova, M.S.; Georgiev, M.I.; Roussinov, D.L. Nephrology in Bulgaria. In Nephrology Worldwide; Moura-Neto, J.A., Divino-Filho, J.C., Ronco, C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Dalton, H.R.; Kamar, N.; Baylis, S.A.; Moradpour, D.; Wedemeyer, H.; Negro, F.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J. Hepatol. 2018, 68, 1256–1271. [Google Scholar] [CrossRef]
- Abravanel, F.; Parraud, D.; Chapuy-Regaud, S.; Miedogue, M.; Bonnin, E.; Larrieu, M.; Aversenq, A.; Lhomme, S.; Izopet, J. Diagnostic Performance of an Automated System for Assaying Anti-Hepatitis E Virus Immunoglobulins M and G Compared with a Conventional Microplate Assay. Viruses 2022, 14, 1065. [Google Scholar] [CrossRef]
- Norder, H.; Karlsson, M.; Mellgren, Å.; Konar, J.; Sandberg, E.; Lasson, A.; Castedal, M.; Magnius, L.; Lagging, M. Diagnostic Performance of Five Assays for Anti-Hepatitis E Virus IgG and IgM in a Large Cohort Study. J. Clin. Microbiol. 2016, 54, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.B.; Al Kassaa, I.; El Safadi, D.; Al Omari, S.; Mallat, H.; Dabboussi, F.; Hamze, M. Prevalence of anti-hepatitis E virus IgG antibodies in sera from hemodialysis patients in Tripoli, Lebanon. PLoS ONE 2020, 15, e0233256. [Google Scholar] [CrossRef] [PubMed]
- Jelicic, P.; Ferenc, T.; Mrzljak, A.; Jemersic, L.; Janev-Holcer, N.; Milosevic, M.; Bogdanic, M.; Barbic, L.; Kolaric, B.; Stevanovic, V.; et al. Insights into hepatitis E virus epidemiology in Croatia. World J. Gastroenterol. 2022, 28, 5494–5505. [Google Scholar] [CrossRef]
- Sezgin, O.; Yaraş, S.; Tezcan Ülger, S.; Aslan, G.; Naci Tiftik, E. The prevalence of hepatitis E virus infection in the adult turkish population: A systematic review of the literature and prevalence study in blood donors in Mersin province. Turk. J. Gastroenterol. 2021, 32, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Ordinance No. 41 of 24 September 2009 for the Approval of the Medical Standard “Dialysis Treatment”, Issued by the Minister of Health (Amended State Gazette No. 63 of July 30, 2021). (In Bulgarian). Available online: https://ekspertis.bg/document/view/law/129370 (accessed on 5 August 2023).
- Lapa, D.; Capobianchi, M.R.; Garbuglia, A.R. Epidemiology of Hepatitis E Virus in European Countries. Int. J. Mol. Sci. 2015, 16, 25711–25743. [Google Scholar] [CrossRef]
- Hartl, J.; Otto, B.; Madden, R.G.; Webb, G.; Woolson, K.L.; Kriston, L.; Vettorazzi, E.; Lohse, A.W.; Dalton, H.R.; Pischke, S. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses 2016, 8, 211. [Google Scholar] [CrossRef]
- The Global Prevalence of Hepatitis E Virus Infection and Susceptibility: A Systematic Review. WHO/IVB/10.14. Immunization, Vaccines and Biologicals. Available online: https://apps.who.int/iris/bitstream/handle/10665/70513/WHO_IVB_10.14_eng.pdf?sequence=1&isAllowed=y (accessed on 8 August 2023).
- Stefanidis, I.; Zervou, E.K.; Rizos, C.; Syrganis, C.; Patsidis, E.; Kyriakopoulos, G.; Sdrakas, L.; Tsianas, N.; Rigopoulou, E.I.; Liakopoulos, V.; et al. Hepatitis E virus antibodies in hemodialysis patients: An epidemiological survey in central Greece. Int. J. Artif. Organs 2004, 27, 842–847. [Google Scholar] [CrossRef]
- Harrison, A.; Scobie, L.; Crossan, C.; Parry, R.; Johnston, P.; Stratton, J.; Dickinson, S.; Ellis, V.; Hunter, J.G.; Prescott, O.R.; et al. Hepatitis E seroprevalence in recipients of renal transplants or haemodialysis in southwest England: A case-control study. J. Med. Virol. 2013, 85, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Jemersic, L.; Prpic, J.; Barbic, L.; Savic, V.; Stevanovic, V.; Vilibic-Cavlek, T. Epidemiology of hepatitis E in South-East Europe in the “One Health” concept. World J. Gastroenterol. 2019, 25, 3168–3182. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Hepatitis E in the EU/EEA, 2005–2015; ECDC: Stockholm, Sweden, 2017; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/HEV_Surveillance-report-2005-2015.pdf (accessed on 8 August 2023).
- Ordinance No. 21 of 15 July 2005 on the Procedure for Registration, Reporting and Reporting of Communicable Diseases. Issued by the Minister of Health (Suppl. SG No. 55 of 15 July 2022). (In Bulgarian). Available online: https://lex.bg/laws/ldoc/2135508238 (accessed on 12 August 2023).
- Teoharov, P.; Tiholova, M.; Draganov, P.; Lilyanova, V.; Ivanova, R.; Varleva, T.; Dimitrova, T. First cases of hepatitis E virus infection in Bulgaria. Infectology 1995, 32, 17–18. (In Bulgarian) [Google Scholar]
- Pismisheva, M.; Baymakova, M.; Golkocheva-Markova, E.; Kundurzhiev, T.; Pepovich, R.; Popov, G.T.; Tsachev, I. First serological study of hepatitis E virus infection in pigs in Bulgaria. Comptes Rendus L’academie Bulg. Sci. 2018, 71, 1001–1008. [Google Scholar] [CrossRef]
- Tsachev, I.; Baymakova, M.; Pepovich, R.; Palova, N.; Marutsov, P.; Gospodinova, K.; Kundurzhiev, T.; Ciccozzi, M. High seroprevalence of hepatitis E virus infection among East Balkan Swine (Sus scrofa) in Bulgaria: Preliminary results. Pathogens 2020, 9, 911. [Google Scholar] [CrossRef]
- Takova, K.; Koynarski, T.; Minkov, I.; Ivanova, Z.; Toneva, V.; Zahmanova, G. Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Tsachev, I.; Gospodinova, K.; Pepovich, R.; Takova, K.; Kundurzhiev, T.; Zahmanova, G.; Kaneva, K.; Baymakova, M. First Insight into the Seroepidemiology of Hepatitis E Virus (HEV) in Dogs, Cats, Horses, Cattle, Sheep, and Goats from Bulgaria. Viruses 2023, 15, 1594. [Google Scholar] [CrossRef]
- Teoharov, P.; Kevorkyan, A.; Raycheva, R.; Golkocheva-Markova, E.; Trandeva-Bankova, D.; Andonov, A. Data on the prevalence of hepatitis E virus in Bulgaria. Comptes Rendus L’academie Bulg. Sci. 2014, 67, 1427–1432. [Google Scholar]
- Bruni, R.; Villano, U.; Equestre, M.; Chionne, P.; Madonna, E.; Trandeva-Bankova, D.; Peleva-Pishmisheva, M.; Tenev, T.; Cella, E.; Ciccozzi, M.; et al. Hepatitis E virus genotypes and subgenotypes causing acute hepatitis, Bulgaria, 2013–2015. PLoS ONE 2018, 13, e0198045. [Google Scholar] [CrossRef]
- Cella, E.; Golkocheva-Markova, E.; Sagnelli, C.; Scolamacchia, V.; Bruni, R.; Villano, U.; Ciccaglione, A.R.; Equestre, M.; Sagnelli, E.; Angeletti, S.; et al. Human hepatitis E virus circulation in Bulgaria: Deep Bayesian phylogenetic analysis for viral spread control in the country. J. Med. Virol. 2019, 91, 132–138. [Google Scholar] [CrossRef]
- Golkocheva-Markova, E.; Kevorkyan, A.; Raycheva, R.; Ismailova, C.; Yoncheva, V.; Tenev, T.; Emilova, R.; Grigorova, L.; Baltadzhiev, I.; Komitova, R. Assessment of hepatitis E seropositivity among HIV-infected patients in Bulgaria. Braz. J. Infect. Dis. 2022, 26, 102329. [Google Scholar] [CrossRef] [PubMed]
- Rouseva, A.; Nikolovska, D. Two cases of hepatitis E in Bulgaria. Pediatr. Infect Dis. 2010, 2, 26–28. (In Bulgarian) [Google Scholar]
- Baymakova, M.; Sakem, B.; Plochev, K.; Popov, G.T.; Mihaylova-Garnizova, R.; Kovaleva, V.; Kundurdjiev, T. Epidemiological characteristics and clinical manifestations of hepatitis E virus infection in Bulgaria: A report on 20 patients. Srp. Arh. Celok. Lek. 2016, 144, 63–68. [Google Scholar] [CrossRef]
- Stoykova, Z.; Ivanova, L.; Tsaneva-Damyanova, D.; Kostadinova, T. Hepatitis E virus infection in Northeastern Bulgaria. Med. Rev. 2017, 53, 30–34. [Google Scholar]
- Baymakova, M.; Kunchev, M.; Mihaylova-Garnizova, R.; Zasheva, A.; Plochev, K.; Kundurzhiev, T.; Tsachev, I. Comparative Analysis on Clinical Characteristics Among Patients with Acute Hepatitis A Virus (HAV) and Patients with Acute Hepatitis E Virus (HEV): A Single-Center Retrospective Study from Bulgaria. Infect. Drug Resist. 2023, 16, 3349–3366. [Google Scholar] [CrossRef]
- Golkocheva-Markova, E.; Peleva-Pishmisheva, M.; Bruni, R.; Villano, U.; Pisani, G.; Equestre, M.; Kevorkyan, A.; Ciccozzi, M.; Ciccaglione, A.R. Following a patient with prolonged response against hepatitis E virus. Panminerva Med. 2018, 60, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Parana, R.; Cotrim, H.P.; Cortey-Boennec, M.L.; Trepo, C.; Lyra, L. Prevalence of hepatitis E virus IgG antibodies in patients from a referral unit of liver diseases in Salvador, Bahia, Brazil. Am. J. Trop. Med. Hyg. 1997, 57, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Ouji, M.; Taherkhani, R.; Farshadpour, F. High prevalence of hepatitis E among regular hemodialysis patients in South of Iran. Int. J. Artif. Organs 2021, 44, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.B.; Balderramo, D.; Wassaf, M.M.; Lotto, M.; Carlino, Y.; Ré, V.E.; Debes, J.D. Hepatitis E virus infection in patients on dialysis and solid organ transplant recipients in Argentina: Exploring associated risk factors. Arch. Virol. 2017, 162, 787–792. [Google Scholar] [CrossRef]
- Wenzel, J.J.; Preiss, J.; Schemmerer, M.; Huber, B.; Jilg, W. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J. Infect. Dis. 2013, 207, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Huang, Y.; Wang, P.; Li, Q.; Li, Z.; Jiang, J.; Guo, Q.; Gui, H.; Xie, Q. Dynamics of Hepatitis E Virus (HEV) Antibodies and Development of a Multifactorial Model to Improve the Diagnosis of HEV Infection in Resource-Limited Settings. J. Clin. Microbiol. 2021, 59, e02321-20. [Google Scholar] [CrossRef]
- Alavian, S.M.; Ataei, B.; Ebrahimi, A.; Pirhaji, O.; Azad, R.; Olya, B.; Ataei, A.M. Anti-Hepatitis E Antibody in Hemodialysis Patients in Isfahan, Iran: Prevalence and Risk Factors. Hepat. Mon. 2015, 15, e23633. [Google Scholar] [CrossRef] [PubMed]
- Pirmoradi, R.; Makvandi, M.; Haghi Navand, A.; Shayanpour, S.; Shahbazian, H.; Makvandi, K.; Eynali Varnosfaderani, S.; Nasimzadeh, S. Status of anti-HEV IgG and IgM antibodies among the hemodialysis patients in southwest region of Iran. Iran. J. Microbiol. 2022, 14, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Yang, R.; Wu, C.; Xia, J. Hepatitis E virus and blood transfusion safety. Epidemiol. Infect. 2020, 148, E158. [Google Scholar] [CrossRef] [PubMed]
- Slot, E.; Hogema, B.M.; Riezebos-Brilman, A.; Kok, T.M.; Molier, M.; Zaaijer, H.L. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill 2013, 18, 20550. [Google Scholar] [CrossRef]
- Ijaz, S.; Vyse, A.J.; Morgan, D.; Pebody, R.G.; Tedder, R.S.; Brown, D. Indigenous hepatitis E virus infection in England: More common than it seems. J. Clin. Virol. 2009, 44, 272–276. [Google Scholar] [CrossRef]
- Kaufmann, A.; Kenfak-Foguena, A.; André, C.; Canellini, G.; Bürgisser, P.; Moradpour, D.; Darling, K.E.A.; Cavassini, M. Hepatitis E Virus Seroprevalence among Blood Donors in Southwest Switzerland. PLoS ONE 2011, 6, e21150. [Google Scholar] [CrossRef] [PubMed]
- Dharmesti, N.W.W.; Wibawa, I.D.N.; Kandarini, Y. Hepatitis C Seroconversion Remains High among Patients with Regular Hemodialysis: Study of Associated Risk Factors. Int. J. Hepatol. 2022, 2022, 8109977. [Google Scholar] [CrossRef] [PubMed]
- Crossan, C.; Scobie, L.; Godwin, J.; Hunter, J.G.; Hawkes, T.; Dalton, H.R. Hepatitis E virus and porcine-derived heparin. Emerg. Infect. Dis. 2013, 19, 686–688. [Google Scholar] [CrossRef]
- Psichogiou, M.; Vaindirli, E.; Tzala, E.; Voudiclari, S.; Boletis, J.; Vosnidis, G.; Moutafis, S.; Skoutelis, G.; Hadjiconstantinou, V.; Troonen, H.; et al. Hepatitis E virus (HEV) infection in haemodialysis patients. The Multicentre Haemodialysis Cohort Study on Viral Hepatitis. Nephrol. Dial. Transplant. 1996, 11, 1093–1095. [Google Scholar] [CrossRef] [PubMed]

| Variable | Hemodialysis Patients (n = 225) |
|---|---|
| Age (year) | |
| Median | 64 |
| 25th; 75th percentile | 52.5; 71.5 |
| Min–max | 24–95 |
| Age decades, n (%) | |
| 18–49 | 44 (19.6) |
| 50–59 | 43 (19.1) |
| 60–69 | 61 (27.1) |
| ≥70 | 77 (34.2) |
| Gender, n (%) | |
| Male | 126 (56.0) |
| Female | 99 (44.0) |
| M:F | 1.3:1 |
| Place of residence, n (%) | |
| Town | 153 (68.0) |
| Village | 72 (32.0) |
| Duration of hemodialysis (months) | |
| Median (25th percentile; 75th percentile) | 48 (24; 72) |
| Duration of dialysis, n (%) | |
| ≤1 year | 60 (26.7) |
| 2–5 years | 91 (40.4) |
| >5 years | 74 (32.9) |
| Vascular access, n (%) | |
| AV fistula | 121 (53.8) |
| Permanent catheter | 99 (44.0) |
| Vascular prosthesis | 5 (2.2) |
| Previous kidney transplantation, n (%) | |
| Yes | 9 (4.0) |
| No | 216 (96.0) |
| Blood transfusion, n (%) | |
| Yes | 132 (58.7) |
| No | 93 (41.3) |
| History of surgical intervention, n (%) | |
| Yes | 159 (70.7) |
| No | 66 (29.3) |
| Diseases that led to hemodialysis, n (%) | |
| Autosomal dominant polycystic kidney disease | 34 (15.2) |
| Chronic tubulointerstitial nephritis | 34 (15.2) |
| Chronic glomerulonephritis | 33 (14.7) |
| Chronic calculous pyelonephritis | 25 (11.1) |
| Diabetic nephropathy | 21 (9.3) |
| Hypertensive kidney disease | 18 (8.0) |
| Bilateral hydronephrosis | 10 (4.4) |
| Chronic nephritic syndrome | 10 (4.4) |
| Other disease | 40 (17.7) |
| Kidney disease in other family member, n (%) | |
| Yes | 50 (22.2) |
| No | 175 (77.8) |
| Hepatitis (jaundice) in the past *, n (%) | |
| Yes | 22 (9.8) |
| No | 203 (90.2) |
| Comorbidities **, n (%) | |
| Arterial hypertension | 151 (67.1) |
| Secondary anemia | 132 (58.7) |
| Ischemic heart disease | 43 (19.1) |
| Heart failure | 29 (12.9) |
| Diabetes | 27 (12.0) |
| Nephrectomy | 12 (5.3) |
| Oncological diseases | 10 (4.4) |
| Liver cirrhosis | 3 (1.3) |
| Traveled abroad in the last 3 months, n (%) | |
| Yes | 1 (0.4) |
| No | 224 (99.6) |
| Variable | Center 1 (n = 80), % | Center 2 (n = 112), % | Center 3 (n = 33), % | p-Value |
| Overall HEV seroprevalence *, (n = 24) | 7 (8.8%) | 14 (12.5%) | 3 (9.1%) | 0.645 |
| HEV IgG seroprevalence (n = 14) | 4 (5%) | 10 (8.9%) | 0 (0%) | 0.150 |
| HBsAg (+), n (%) | 5 (6.3%) | 4 (3.6%) | 4 (12.1%) | 0.195 |
| anti-HCV (+), n (%) | 5 (6.3%) | 8 (7.1%) | 2 (6.1%) | 0.937 |
| Parameters | HEV IgG Seroprevalence (n = 14) n (%) | HEV Markers (−) (n = 201) n (%) | p-Value | Overall HEV Seroprevalence (n = 24) n (%) | HEV Markers (−) (n = 201) n (%) | p-Value |
|---|---|---|---|---|---|---|
| Age decades | 0.473 | 0.462 | ||||
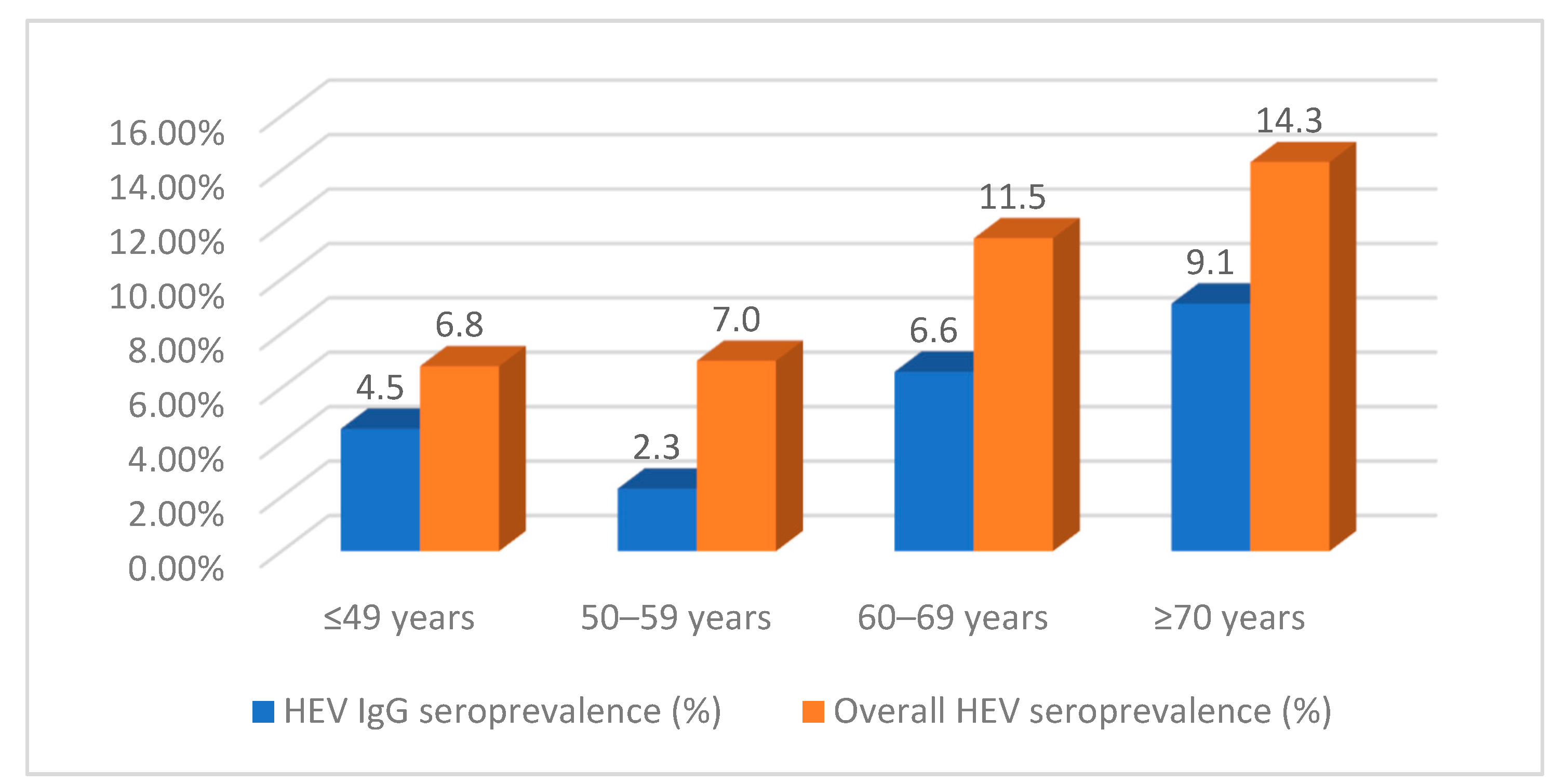
| 18–49 | 2 (14.3) | 41 (20.4) | 3 (12.5) | 41 (20.4) | ||
| 50–59 | 1 (7.1) | 40 (19.9) | 3 (12.5) | 40 (19.9) | ||
| 60–69 | 4 (28.6) | 54 (26.9) | 7 (29.2) | 54 (26.9) | ||
| ≥70 | 7 (50.0) | 66 (32.8) | 66 (32.8) | |||
| Gender | 0.918 | 0.808 | ||||
| Male | 8 (57.1) | 112 (55.7) | 14 (58.3) | 112 (55.7) | ||
| Female | 6 (42.9) | 89 (44.3) | 10 (41.7) | 89 (44.3) | ||
| Place of residence | 0.770 | 0.753 | ||||
| Town | 10 (71.4) | 136 (67.7) | 17 (70.8) | 136 (67.7) | ||
| Village | 4 (28.6) | 65 (32.3) | 7 (29.2) | 65 (32.3) | ||
| Hemodialysis duration (yrs.) | 0.522 | 0.306 | ||||
| ≤1 year | 2 (14.2) | 56 (27.9) | 4 (16.7) | 56 (27.9) | ||
| 2–5 years | 6 (42.9) | 78 (38.8) | 13 (54.2) | 78 (38.8) | ||
| >5 years | 6 (42.9) | 67 (33.3) | 7 (29.2) | 67 (33.3) | ||
| Vascular access (Yes) | 0.007 | 0.275 | ||||
| AV fistula | 2 (14.3) | 111 (55.2) | 10 (41.7) | 111 (55.2) | ||
| Permanent catheter | 12 (85.7) | 85 (42.3) | 14 (58.3) | 85 (42.3) | ||
| Vascular prosthesis | 0 (0) | 5 (2.5) | 0 (0) | 5 (2.5) | ||
| Predictor variables | ||||||
| Blood transfusion | 0.938 | 0.687 | ||||
| Yes | 8 (57.1) | 117 (58.2) | 15 (62.5) | 117 (58.2) | ||
| No | 6 (42.9) | 84 (41.8) | 9 (37.5) | 84 (41.8) | ||
| Surgical intervention | 0.458 | 0.149 | ||||
| Yes | 11 (78.6) | 139 (69.2) | 20 (83.3) | 139 (69.2) | ||
| No | 3 (21.4) | 62 (30.8) | 4 (16.7) | 62 (30.8) | ||
| Hepatitis infection in the past | 0.203 | 0.327 | ||||
| Yes | 0 (0) | 21 (10.4) | 1 (4.2) | 21 (10.4) | ||
| No | 14 (100) | 180 (89.6) | 23 (95.8) | 180 (89.6) | ||
| Food consumption | ||||||
| Pork | 0.840 | 0.559 | ||||
| Yes | 12(85.7) | 176 (87.6) | 22 (91.7) | 176 (87.6) | ||
| No | 2 (14.3) | 25 (12.4) | 2 (8.3) | 25 (12.4) | ||
| Fish | 0.226 | 0.054 | ||||
| Yes | 13 (92.9) | 160 (79.6) | 23 (95.8) | 160 (79.6) | ||
| No | 1 (7.1) | 41 (20.4) | 1 (4.2) | 41 (20.4) | ||
| Vegetable leafy greens | 0.885 | 0.519 | ||||
| Yes | 12 (85.7) | 175 (87.1) | 22 (91.7) | 175 (87.1) | ||
| No | 2 (14.3) | 26 (12.9) | 2 (8.3) | 26 (12.9) | ||
| Water intake | ||||||
| Tap water | 0.548 | 0.687 | ||||
| Yes | 7 (50.0) | 117 (58.2) | 15 (62.5) | 117 (58.2) | ||
| No | 7 (50.0) | 84 (41.8) | 9 (37.5) | 84 (41.8) | ||
| Bottled water | 0.268 | 0.975 | ||||
| Yes | 8 (57.1) | 143 (71.1) | 17 (70.8) | 143 (71.1) | ||
| No | 6 (42.9) | 58 (28.9) | 7 (29.2) | 58 (28.9) | ||
| Private water source | 0.127 | 0.162 | ||||
| Yes | 0 (0) | 29 (14.4) | 1 (4.2) | 29 (14.4) | ||
| No | 14 (100) | 172 (85.6) | 23 (95.8) | 172 (85.6) | ||
| Contact with animals | 0.714 | 1.000 | ||||
| Yes | 4 (28.6) | 67 (33.3) | 8 (33.3) | 67 (33.3) | ||
| No | 10 (71.4) | 134 (66.7) | 16 (66.7) | 134 (66.7) | ||
| HBsAg | 0.792 | 0.570 | ||||
| Yes | 1 (7.1) | 11 (5.5) | 2 (8.3) | 11 (5.5) | ||
| No | 13 (92.9) | 190 (94.5) | 22 (91.7) | 190 (94.5) | ||
| anti-HCV | 0.980 | 0.603 | ||||
| Yes | 1 (7.1) | 14 (7.0) | 1 (4.2) | 14 (7.0) | ||
| No | 13 (92.9) | 187 (93) | 23 (95.8) | 187 (93.0) |
| Model | Unstandardized Coefficients | Wald | df | Sig. | Exp(B) | 95% Confidence Interval for B | ||
|---|---|---|---|---|---|---|---|---|
| B | Std. Error | Lower Bound | Upper Bound | |||||
| (Constant) | −4.16 | 1.97 | 4.42 | 1 | 0.036 | 0.02 | ||
| Age groups | ||||||||
| 18–49 yrs. (baseline) | ||||||||
| 50–59 yrs. | −1.92 | 1.36 | 1.98 | 1 | 0.159 | 0.15 | 0.01 | 2.12 |
| 60–69 yrs. | −0.28 | 1.01 | 0.08 | 1 | 0.785 | 0.76 | 0.11 | 5.48 |
| ≥70 yrs. | −0.18 | 0.95 | 0.04 | 1 | 0.852 | 0.84 | 0.13 | 5.41 |
| Hemodialysis duration | ||||||||
| ≤1 yr. (baseline) | ||||||||
| 2–5 yrs. | 0.92 | 0.94 | 0.97 | 1 | 0.325 | 2.52 | 0.40 | 15.91 |
| >5 yrs. | 1.90 | 0.97 | 3.84 | 1 | 0.049 | 6.67 | 1.01 | 44.37 |
| Vascular access | ||||||||
| AV fistula (baseline) | ||||||||
| Vein prosthesis | −16.35 | 16 574.32 | 0.00 | 1 | 0.999 | 0.00 | 0.00 | 0.00 |
| Permanent central vein catheter | 2.54 | 0.88 | 8.38 | 1 | 0.004 | 12.72 | 2.27 | 71.20 |
| Blood transfusion | ||||||||
| No (baseline) | ||||||||
| Yes | −0.69 | 0.68 | 1.02 | 1 | 0.31 | 0.50 | 0.13 | 1.92 |
| Surgical intervention | ||||||||
| No (baseline) | ||||||||
| Yes | 0.89 | 0.82 | 1.17 | 1 | 0.280 | 2.43 | 0.49 | 12.12 |
| Food habits—consumption of pork | ||||||||
| No (baseline) | ||||||||
| Yes | −0.49 | 0.95 | 0.26 | 1 | 0.608 | 0.61 | 0.10 | 3.96 |
| Food habits—consumption of fish | ||||||||
| No (baseline) | ||||||||
| Yes | 1.06 | 1.17 | 0.82 | 1 | 0.366 | 2.88 | 0.29 | 28.54 |
| Type of drinking water intake—tap water | ||||||||
| No (baseline) | ||||||||
| Yes | −1.25 | 0.85 | 2.16 | 1 | 0.142 | 0.29 | .05 | 1.52 |
| Type of drinking water intake—bottled water | ||||||||
| No (baseline) | ||||||||
| Yes | −1.69 | 0.85 | 3.91 | 1 | 0.048 | 0.19 | 0.04 | 0.99 |
| Animal contact | ||||||||
| No (baseline) | ||||||||
| Yes | −0.37 | 0.78 | 0.23 | 1 | 0.635 | 0.69 | 0.15 | 3.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kevorkyan, A.; Golkocheva-Markova, E.; Raycheva, R.; Rangelova, V.; Komitova, R.; Atanasova, M.; Tzekov, V.; Kostadinova, T.; Chardakova, T. Hepatitis E Virus (HEV) Infection among Hemodialysis Patients from Southern Bulgaria. Pathogens 2023, 12, 1208. https://doi.org/10.3390/pathogens12101208
Kevorkyan A, Golkocheva-Markova E, Raycheva R, Rangelova V, Komitova R, Atanasova M, Tzekov V, Kostadinova T, Chardakova T. Hepatitis E Virus (HEV) Infection among Hemodialysis Patients from Southern Bulgaria. Pathogens. 2023; 12(10):1208. https://doi.org/10.3390/pathogens12101208
Chicago/Turabian StyleKevorkyan, Ani, Elitsa Golkocheva-Markova, Ralitsa Raycheva, Vanya Rangelova, Radka Komitova, Mariya Atanasova, Valeri Tzekov, Tanya Kostadinova, and Tsvetelina Chardakova. 2023. "Hepatitis E Virus (HEV) Infection among Hemodialysis Patients from Southern Bulgaria" Pathogens 12, no. 10: 1208. https://doi.org/10.3390/pathogens12101208
APA StyleKevorkyan, A., Golkocheva-Markova, E., Raycheva, R., Rangelova, V., Komitova, R., Atanasova, M., Tzekov, V., Kostadinova, T., & Chardakova, T. (2023). Hepatitis E Virus (HEV) Infection among Hemodialysis Patients from Southern Bulgaria. Pathogens, 12(10), 1208. https://doi.org/10.3390/pathogens12101208









