Prevalence of Swine Gastrointestinal Parasites in Two Free-Range Farms from Nord-West Region of Romania
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Traits | ATOL *, AHOL ** and OPL *** References |
|---|---|
| Parasite load traits | |
| Parasite Oocysts (OPG) | Oocyst Stage |
| Parasite Eggs (EPG) | Egg Stage |
| Parasites detected | |
| Eimeria spp. | AHOL_0004070 |
| Balantidium coli | AHOL_0004016 |
| Ascaris suum | AHOL_0004179 |
| Trichuris suis | AHOL_0004186 |
| Oesophagostomum spp. | AHOL_0004181 |
| Strongyloides ransomi | AHOL_0004178 |
| Cryptosporidium spp. | AHOL_0004175 |
| Diseases description | |
| Ascaridiosis | AHOL_0005382 |
| Coccidiosis | AHOL_0005374 |
| Cryptosporidiosis | AHOL_0005377 |
References
- Thomas, L.F.; de Glanville, W.A.; Cook, E.A.; Fèvre, E.M. The spatial ecology of free-ranging domestic pigs (Sus scrofa) in western Kenya. BMC Vet. Res. 2013, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, M.; Knecht, D.; Boruta, O.; Kot, M. Effect of breeding conditions, phenology, and age on the occurrence of helminths in pigs. A preliminary study. Bull. Vet. Inst. Pulawy 2009, 53, 213–220. [Google Scholar]
- Kochanowski, M.; Karamon, J.; Dąbrowska, J.; Dors, A.; Czyżewska-Dors, E.; Cencek, T. Occurrence of intestinal parasites in pigs in Poland-the influence of factors related to the production system. J. Vet. Res. 2017, 61, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Zhou, R.Q.; Huang, H.C.; Hu, S.J. Prevalence and risk factors associated with intestinal parasites in pigs in Chongqing, China. Res. Vet. Sci. 2011, 91, 121–124. [Google Scholar] [CrossRef]
- Joachim, A.; Dülmer, N.; Daugschies, A.; Roepstorff, A. Occurrence of helminthes in pig fattening units with different management systems in Northern Germany. Vet. Parasitol. 2001, 96, 135–146. [Google Scholar] [CrossRef]
- Ichim, O. An overview of organic pig farming in Romania. Porc. Res. 2012, 2, 50–65. [Google Scholar]
- Şuteu, M. Porcine milk protein polymorphisms. Porc. Res. 2011, 1, 1–112. [Google Scholar]
- Constantin, F. Economic Performance of Organic Farming in Romania and European Union. Econ. Ser. Manag. 2012, 15, 108–119. [Google Scholar]
- Nansen, P.; Roepstorff, A. Parasitic helminths of the pig: Factors influencing transmission and infection levels. Int. J. Parasitol. 1999, 29, 877–891. [Google Scholar] [CrossRef]
- Kagira, J.M.; Githigia, S.M.; Nganga, J.C.; Kanyari, P.W.N.; Maingi, N.; Gachohi, J.M. Prevalence of gastrointestinal protozoa and association with risk factors in free-range pigs in Kenya. J. Protozool. Res. 2010, 20, 1–9. [Google Scholar]
- Greve, J.H. Internal parasites: Helminths. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; John Wiley & Sons: Chichester, UK, 2012; pp. 912–913. [Google Scholar]
- Weng, Y.B.; Hu, Y.J.; Li, Y.; Li, B.S.; Lin, R.Q.; Xie, D.H.; Gasser, R.B.; Zhu, X.Q. Survey of intestinal parasites in pigs from intensive farms in Guangdong Province, People’s Republic of China. Vet. Parasitol. 2005, 127, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Balicka-Ramisz, A.; Wiśniewski, J.; Stadnytska, O. Extensity and intensity of intestinal parasite infections in pigs in different types of farm organization. Acta Sci. Pol. Zootech. 2020, 18, 47–50. [Google Scholar] [CrossRef]
- Trușcă, V.; Alecu, M. Romania General Facts. In Romania’s Third National Communication on Climate Change under the United Nations Framework Convention on Climate Change; Grue & Hornstrup: Holstebro, Denmark, 2005; pp. 21–67. [Google Scholar]
- Symeonidou, I.; Tassis, P.; Gelasakis, A.Ι.; Tzika, E.D.; Papadopoulos, E. Prevalence and Risk Factors of Intestinal Parasite Infections in Greek Swine Farrow-To-Finish Farms. Pathogens 2020, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Wieler, L.H.; Ilieff, A.; Herbst, W.; Bauer, C.; Vieler, E.; Bauerfeind, R.; Failing, K.; Klös, H.; Wengert, D.; Baljer, G.; et al. Prevalence of enteropathogens in suckling and weaned piglets with diarrhoea in southern Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 2001, 48, 151–159. [Google Scholar] [CrossRef]
- Eijck, I.A.J.M.; Borgsteede, F.H.M. A survey of gastrointestinal pig parasites on free-range, organic and conventional pig farms in The Netherlands. Vet. Res. Commun. 2005, 29, 407–414. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Roberts, H.; Fourie, P. Prevalence of gastrointestinal helminths and parasites in smallholder pigs reared in the central Free State Province. Onderstepoort J. Vet. Res. 2019, 86, a1687. [Google Scholar] [CrossRef]
- Dey, T.R.; Dey, A.R.; Begum, N.; Akther, S.; Barmon, B.C. Prevalence of endo parasites of pig at Mymensingh, Bangladesh. J. Agric. Vet. Sci. 2014, 7, 31–38. [Google Scholar]
- Tan, T.; Low, V.; Lee, S.; Chandrawathani, P.; Premaalatha, B.; Yvonne, A. Gastro-intestinal parasitism among two swine populations in Malaysia: Highlighting the zoonotic transmissible protozoan Balantidium coli infections. Malays. J. Vet. Res. 2014, 5, 63–68. [Google Scholar]
- Murthy, K.; Ananda, K.J.; Adeppa, J.; Satheesha, M.G. Studies on gastrointestinal parasites of pigs in Shimoga region of Karnataka. J. Parasit. Dis 2016, 40, 885–889. [Google Scholar] [CrossRef][Green Version]
- Li, Y.H.; Yao, Q.; Dong, H.P.; Wang, S.S.; Chen, R.R.; Song, J.K.; Yan, W.C.; Zhao, G.H. Molecular characterization of Balantioides coli in pigs from Shaanxi province, northwestern China. Parasitol. Res. 2020, 119, 3075–3081. [Google Scholar] [CrossRef]
- Vohra, H.K.S. Prevalence of gastrointestinal parasite in pigs of Hisar city, Haryana. Pharma Innov. J. 2021, 10, 476–478. [Google Scholar]
- Abdu, S.; Gashaw, A. Production system dynamism and parasitic interaction of swine in and around Holetta, Ethiopia. Ethiop. Vet. J. 2010, 14, 71–82. [Google Scholar]
- Tumusiime, M.; Ntampaka, P.; Niragire, F.; Sindikubwabo, T.; Habineza, F. Prevalence of Swine Gastrointestinal Parasites in Nyagatare District, Rwanda. J. Parasitol. Res. 2020, 2020, 8814136. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, X.Y.; Cong, M.M.; Ren, W.X.; Hu, B.; Cheng, W.Y.; Li, H.M.; Yu, S.K.; Zhao, G.H. Epidemiological investigation on swine intestinal parasites in Shaanxi province, China. Afr. J. Microbiol. Res. 2013, 7, 4251–4256. [Google Scholar]
- Robertson, L.J.; Björkman, C.; Axén, C.; Fayer, R. Cryptosporidiosis in farmed animals. In Cryptosporidium: Parasite and Disease; Cacciò, S.M., Widmer, G., Eds.; Springer: Vienna, Austria, 2014; pp. 149–235. [Google Scholar]
- Zintl, A.; Neville, D.; Maguire, D.; Fanning, S.; Mulcahy, G.; Smith, H.V.; De Waal, T. Prevalence of Cryptosporidium species in intensively farmed pigs in Ireland. Parasitology 2007, 134, 1575–1582. [Google Scholar] [CrossRef]
- Yin, J.; Shen, Y.; Yuan, Z.; Lu, W.; Xu, Y.; Cao, J. Prevalence of the Cryptosporidium pig genotype II in pigs from the Yangtze River Delta, China. PLoS ONE 2011, 6, e20738. [Google Scholar] [CrossRef]
- Budu-Amoako, E.; Greenwood, S.J.; Dixon, B.R.; Barkema, H.W.; Hurnik, D.; Estey, C.; McClure, J.T. Occurrence of Giardia and Cryptosporidium in pigs on Prince Edward Island, Canada. Vet. Parasitol. 2012, 184, 18–24. [Google Scholar] [CrossRef]
- Němejc, K.; Sak, B.; Květoňová, D.; Kernerová, N.; Rost, M.; Cama, V.A.; Kváč, M. Occurrence of Cryptosporidium suis and Cryptosporidium scrofarum on commercial swine farms in the Czech Republic and its associations with age and husbandry practices. Parasitol. Res. 2013, 112, 1143–1154. [Google Scholar] [CrossRef]
- De Felice, L.A.; Moré, G.; Cappuccio, J.; Venturini, M.C.; Unzaga, J.M. Molecular characterization of Cryptosporidium spp. from domestic pigs in Argentina. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100473. [Google Scholar] [CrossRef]
- Wang, W.; Gong, Q.L.; Zeng, A.; Li, M.H.; Zhao, Q.; Ni, H.B. Prevalence of Cryptosporidium in pigs in China: A systematic review and meta-analysis. Transbound. Emerg. Dis. 2021, 68, 1400–1413. [Google Scholar] [CrossRef]
- Nissen, S.; Poulsen, I.H.; Nejsum, P.; Olsen, A.; Roepstorff, A.; Rubaire-Akiiki, C.; Thamsborg, S.M. Prevalence of gastrointestinal nematodes in growing pigs in Kabale District in Uganda. Trop. Anim. Health Prod. 2011, 43, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Sowemimo, O.A.; Asaolu, S.O.; Adegoke, F.O.; Ayanniyi, O.O. Epidemiological survey of gastrointestinal parasites of pigs in Ibadan, Southwest Nigeria. J. Public Health Epidemiol. 2012, 4, 294–298. [Google Scholar]
- Aiyedun, J.O.; Oludairo, O.O. Prevalence of intestinal parasitism of swine in a North Central State of Nigeria. J. Adv. Vet. Anim. Res. 2016, 3, 278–281. [Google Scholar] [CrossRef]
- Roepstorff, A. Natural Ascaris suum infections in swine diagnosed by coprological and serological (ELISA) methods. Parasitol. Res. 1998, 84, 537–543. [Google Scholar] [CrossRef]
- Boes, J.; Kanora, A.; Havn, K.T.; Christiansen, S.; Vestergaard-Nielsen, K.; Jacobs, J.; Alban, L. Effect of Ascaris suum infection on performance of fattening pigs. Vet. Parasitol. 2010, 172, 269–276. [Google Scholar] [CrossRef]
- Katakam, K.K.; Thamsborg, S.M.; Dalsgaard, A.; Kyvsgaard, N.C.; Mejer, H. Environmental contamination and transmission of Ascaris suum in Danish organic pig farms. Parasites Vectors 2016, 9, 80. [Google Scholar] [CrossRef]
- Pietrosemoli, S.; Tang, C. Animal Welfare and Production Challenges Associated with Pasture Pig Systems: A Review. Agriculture 2020, 10, 223. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, M.J.; Smith, R.P.; Kang, S.; Lewis, F.; Nielen, M.; Gunn, G.J.; Edwards, S.A. Identification of factors influencing the occurrence of milk spot livers in slaughtered pigs: A novel approach to understanding Ascaris suum epidemiology in British farmed pigs. Vet. Parasitol. 2010, 173, 271–279. [Google Scholar] [CrossRef]
- Çani, Y.M.; Bizhga, B. Ascaris suum Infection Estimate. Anglisticum 2016, 5, 8–13. [Google Scholar]
- Marufu, M.C.; Chanayiwa, P.; Chimonyo, M.; Bhebhe, E. Prevalence of gastrointestinal nematodes in Mukota pigs in a communal area of Zimbabwe. Afr. J. Agric. Res. 2008, 3, 91–95. [Google Scholar]
- Knecht, D.; Popiołek, M.; Zaleśny, G. Does meatiness of pigs depend on the level of gastro-intestinal parasites infection? Prev. Vet. Med. 2011, 99, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Kagira, J.M.; Kanyari, P.N.; Githigia, S.M.; Maingi, N.; Nganga, J.C.; Gachohi, J.M. Risk factors associated with occurrence of nematodes in free range pigs in Busia District, Kenya. Trop. Anim. Health Prod. 2012, 44, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kouam, M.K.; Ngueguim, F.D. Prevalence, Intensity, and Risk Factors for Helminth Infections in Pigs in Menoua, Western Highlands of Cameroon, with Some Data on Protozoa. J. Parasitol. Res. 2022, 2022, 9151294. [Google Scholar] [CrossRef] [PubMed]
- Thamsborg, S.M.; Ketzis, J.; Horii, Y.; Matthews, J.B. Strongyloides spp. infections of veterinary importance. Parasitology 2017, 144, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Török, I.; Croitoru, A.E.; Man, T.C. A new approach to assess the impact of extreme temperature conditions on social vulnerability. Nat. Hazards Earth Syst. Sci. Discuss. 2021, 45, 1–26. [Google Scholar]
- Manser, M.M.; Saez, A.C.; Chiodini, P.L. Faecal Parasitology: Concentration Methodology Needs to be Better Standardised. PLoS Negl. Trop. Dis. 2016, 10, e0004579. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, S.A.; Pohlenz, J.F. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet. Scand. 1981, 22, 594–596. [Google Scholar] [CrossRef]
- Mircean, V.; Cozma, V.; Gyorke, A. Diagnostic Coproscopic in Bolile Parazitare la Animale, (Coproparasitological Diagnostic in Parasitic Diseases in Animals); Risoprint: Cluj-Napoca, Romania, 2011; pp. 23–35. [Google Scholar]
- Lukyanova, G.A.; Yagenich, L.V.; Pasechnik, A.A. Morphometric parameters of egg-shells and thirdstage juveniles as diagnostic features of Oesophagostomum dentatum and O. quadrispinulatum (Strongyloidea: Chabertiidae). Russ. J. Nematol. 2020, 28, 41–44. [Google Scholar]
- Honer, M.R. The Routine Differentiation of the Ova and Larvae of Two Parasites of Swine, Hyostrongylus rubidus (Hassall et Stiles, 1892) and Oesophagostomum dentatum (Rud., 1803). Z. Für Parasitenkd. 1967, 29, 40–45. [Google Scholar] [CrossRef]

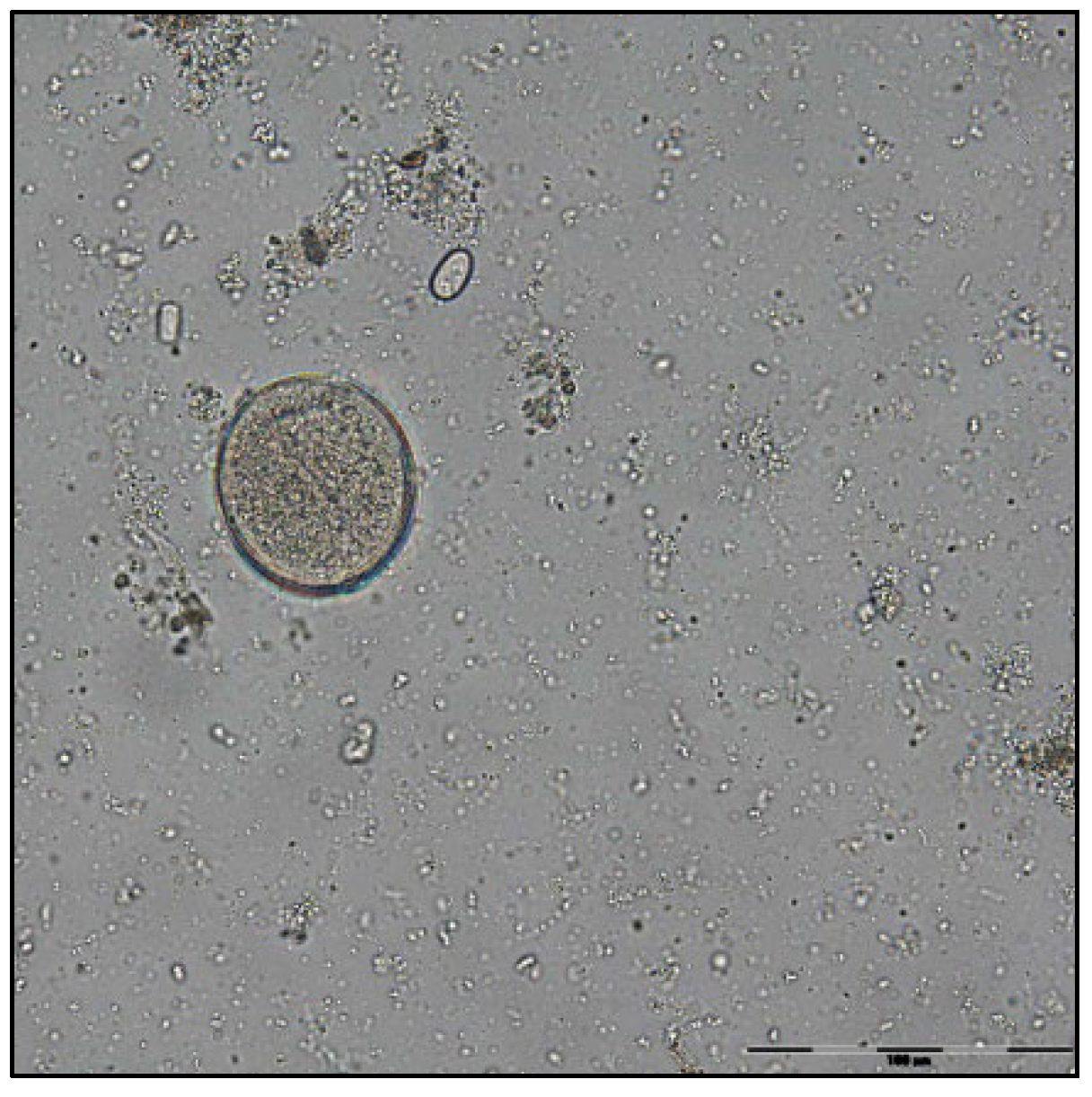
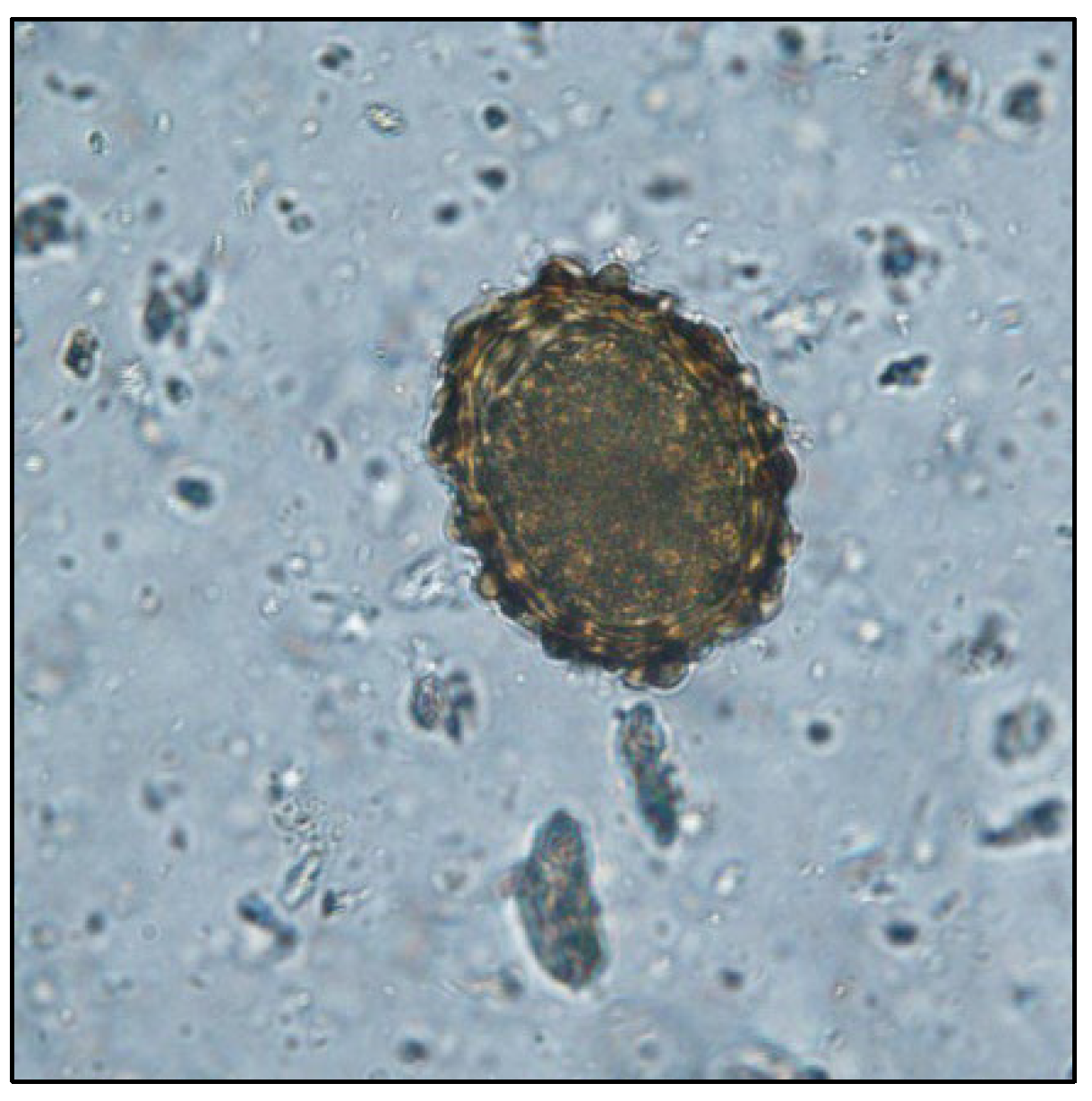
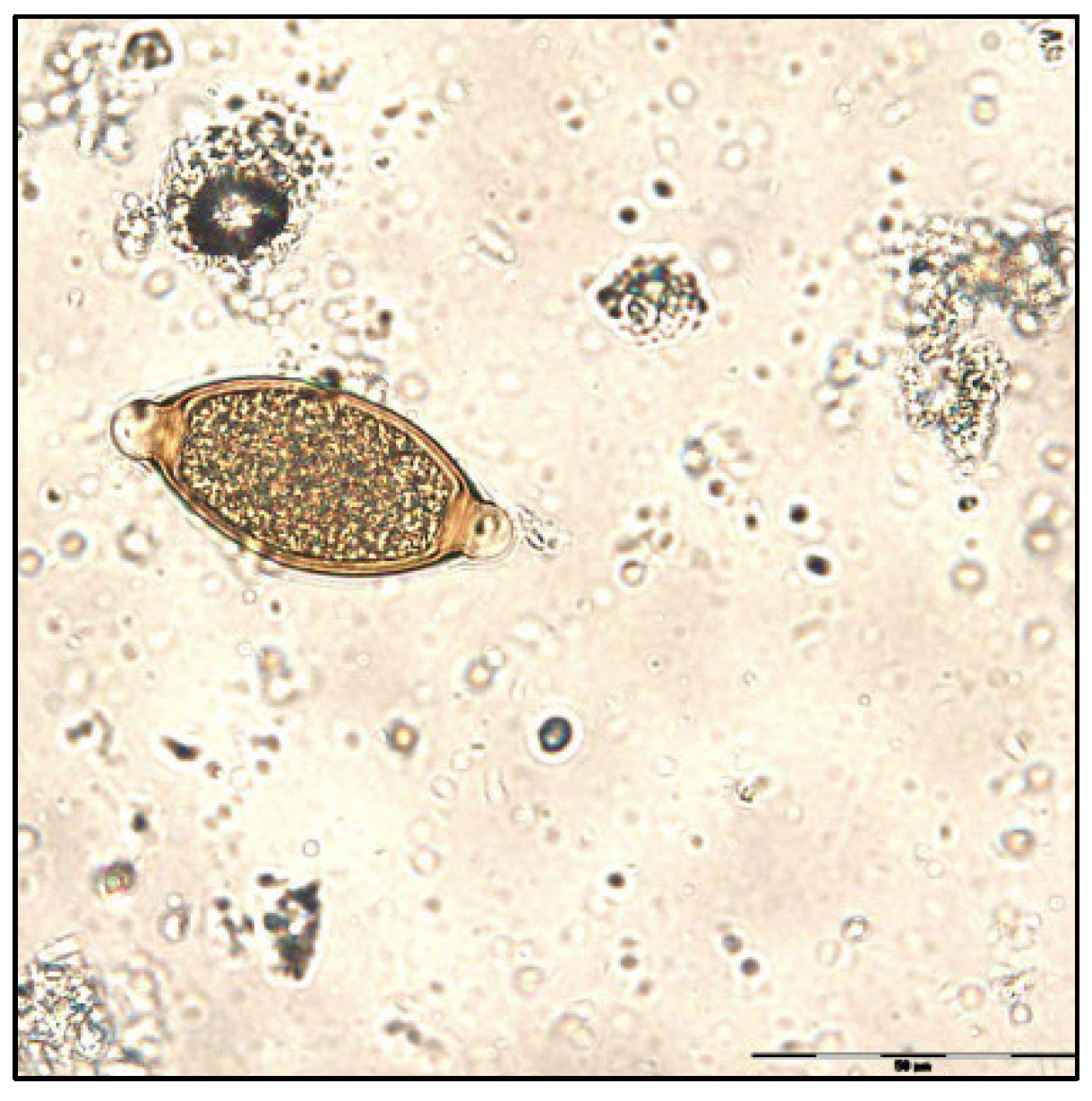



| F1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring (n = 40) | Summer (n = 40) | Autumn (n = 40) | Winter (n = 40) | |||||||||
| Parasite | F | P% | MI (±SD) | F | P% | MI (±SD) | F | P% | MI (±SD) | F | P% | MI (±SD) |
| Eimeria spp. | 18 | 45.0 | 155 (±158) | 32 | 80.0 | 1016 (±730) | 10 | 25.0 | 300 (±351) | 31 | 77.5 | 762 (±439) |
| B. coli | 29 | 72.5 | 1367 (±692) | 30 | 75.0 | 427 (±371) | 26 | 65.0 | 723 (±638) | 38 | 95.0 | 1151 (±590) |
| Oesophagostomum spp. | 7 | 17.5 | 114 (±80) | 4 | 10.0 | 88 (±77) | 6 | 12.5 | 380 (±480) | 14 | 35.0 | 457 (±199) |
| S. ransomi | - | - | - | - | - | - | 12 | 30.0 | 817 (±501) | - | - | - |
| Cryptosporidium spp. | 9 | 22.5 | - | 10 | 25.0 | - | 4 | 10.0 | - | 8 | 20.0 | - |
| F2 | ||||||||||||
| Eimeria spp. | 22 | 55.0 | 1261 (±808) | 23 | 57.5 | 9883 (±12563) | 39 | 97.5 | 9974 (±43366) | 27 | 67.5 | 2252 (±2184) |
| B. coli | 32 | 80.0 | 1575 (±893) | 28 | 70.0 | 1114 (±624) | 23 | 57.5 | 1220 (±702) | 19 | 47.5 | 963 (±603) |
| Cryptosporidium spp. | 6 | 15.0 | - | 8 | 20.0 | - | 7 | 17.5 | - | 6 | 15.0 | - |
| F1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring (n = 40) | Summer (n = 40) | Autumn (n = 40) | Winter (n = 40) | |||||||||
| Parasite | F | P% | MI (±SD) | F | P% | MI (±SD) | F | P% | MI (±SD) | F | P% | MI (±SD) |
| Eimeria spp. | 15 | 37.5 | 137 (±126) | 16 | 40.0 | 356 (±298) | 8 | 20.0 | 156 (±192) | 14 | 35.0 | 354 (±251) |
| B. coli | 38 | 95.0 | 1126 (±576) | 18 | 45.0 | 261 (±215) | 30 | 77.5 | 848 (±629) | 38 | 95.0 | 1258 (±443) |
| A. suum | 13 | 32.5 | 881 (±423) | 12 | 30.0 | 458 (±372) | 16 | 40.0 | 2681 (±3963) | 34 | 85.0 | 1265 (±453) |
| T. suis | 11 | 27.5 | 209 (±111) | 16 | 40.0 | 181 (±129) | 10 | 25.0 | 130 (±118) | 21 | 52.5 | 379 (±245) |
| F2 | ||||||||||||
| Eimeria spp. | 24 | 60.0 | 679 (±524) | 16 | 40.0 | 278 (±255) | 40 | 100 | 4075 (±4079) | 30 | 75.0 | 2693 (±2220) |
| B. coli | 33 | 82.5 | 948 (±669) | 30 | 77.5 | 853 (±453) | 23 | 57.5 | 1008 (±758) | 20 | 50.0 | 850 (±676) |
| A. suum | 35 | 87.5 | 2890 (±2382) | 30 | 77.5 | 2494 (±1645) | 37 | 92.5 | 3973 (±2337) | 24 | 60.0 | 3475 (±1964) |
| T. suis | 18 | 45.0 | 847 (±580) | 6 | 15.0 | 208 (±111) | 24 | 60.0 | 788 (±596) | 19 | 47.5 | 574 (±447) |
| F1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring (n = 40) | Summer (n = 40) | Autumn (n = 40) | Winter (n = 40) | |||||||||
| F | P% | MI (±SD) | F | P% | MI (±SD) | F | P% | MI (±SD) | F | P% | MI (±SD) | |
| Eimeria spp. | 13 | 32.5 | 127 (±133) | 12 | 30.0 | 88 (±68) | 8 | 20.0 | 1050 (±1165) | 16 | 40.0 | 404 (±200) |
| B. coli | 24 | 60.0 | 1108 (±534) | 24 | 60.0 | 244 (±270) | 30 | 75.0 | 823 (±619) | 38 | 95.0 | 859 (±508) |
| A. suum | 4 | 10.0 | 88 (±75) | 12 | 30.0 | 100 (±74) | 12 | 30.0 | 683 (±744) | 16 | 40.0 | 472 (±310) |
| Oesophagostomum spp. | 4 | 10.0 | 125 (±87) | 11 | 27.5 | 182 (±129) | 12 | 30.0 | 171 (±329) | 15 | 37.5 | 187 (±109) |
| S. ransomi | - | - | - | 3 | 7.5 | 467 (±115) | 2 | 5.0 | 125 (±106) | - | - | - |
| Cryptosporidium spp | 4 | 10.0 | - | 6 | 15.0 | - | 2 | 5.0 | - | 6 | 15.0 | - |
| F2 | ||||||||||||
| Eimeria spp. | 26 | 65.0 | 5900 (±4287) | 15 | 37.5 | 282 (±305) | 19 | 47.5 | 560 (±365) | 16 | 40.0 | 516 (±381) |
| B. coli | 23 | 57.5 | 985 (±703) | 9 | 22.5 | 782 (±430) | 30 | 72.5 | 767 (±611) | 22 | 55.0 | 857 (±602) |
| A. suum | 16 | 40.0 | 1013 (±492) | 18 | 45.0 | 469 (±382) | 17 | 42.5 | 741 (±468) | 14 | 35.0 | 643 (±361) |
| Oesophagostomum spp. | 4 | 10.0 | 125 (±87) | 13 | 32.5 | 219 (±180) | 17 | 42.5 | 874 (±479) | 11 | 27.5 | 623 (±376) |
| Cryptosporidium spp. | 1 | 2.5 | - | 4 | 10.0 | - | 4 | 10.0 | - | 3 | 7.5 | - |
| Eimeriaspp. | ||||||
| F1 | F2 | |||||
| Seasons | Weaners | Fatteners | Sows | Weaners | Fatteners | Sows |
| Sp/Su | ssv | ssv | 0.54 | 0.42 | 0.06 | ssv |
| Sp/A | 0.21 | 0.98 | ssv | ssv | ssv | ssv |
| Sp/W | ssv | ssv | ssv | ssv | ssv | ssv |
| Su/A | ssv | ssv | ssv | 0.53 | ssv | ssv |
| Su/W | 0.40 | 0.84 | ssv | 0.71 | ssv | ssv |
| A/W | ssv | ssv | ssv | 0.33 | 0.28 | 0.69 |
| Sp/Sp | ssv | ssv | ssv | ssv | ssv | ssv |
| Su/Su | 0.22 | ssv | ssv | 0.22 | 0.27 | ssv |
| A/A | ssv | ssv | 0.21 | ssv | ssv | 0.21 |
| W/W | ssv | ssv | 0.71 | ssv | ssv | 0.71 |
| B. coli | ||||||
| F1 | F2 | |||||
| Seasons | Weaners | Fatteners | Sows | Weaners | Fatteners | Sows |
| Sp/Su | ssv | ssv | ssv | ssv | 0.94 | 0.50 |
| Sp/A | ssv | ssv | ssv | 0.09 | 0.70 | 0.17 |
| Sp/W | 0.53 | 0.09 | 0.06 | ssv | 0.34 | 0.63 |
| Su/A | 0.32 | ssv | ssv | 0.92 | 0.64 | 0.44 |
| Su/W | ssv | ssv | ssv | 0.36 | 0.28 | 0.85 |
| A/W | ssv | ssv | 0.48 | 0.53 | 0.65 | 0.36 |
| Sp/Sp | 0.29 | 0.08 | 0.20 | 0.29 | 0.08 | 0.20 |
| Su/Su | ssv | ssv | ssv | ssv | ssv | ssv |
| A/A | ssv | 0.64 | 0.91 | ssv | 0.64 | 0.91 |
| W/W | 0.30 | ssv | 0.87 | 0.30 | ssv | 0.87 |
| A. suum | T. suis | |||||
| F1 | F2 | F1 | F2 | |||
| Seasons | Fatteners | Sows | Fatteners | Sows | Fatteners | Fatteners |
| Sp/Su | ssv | 0.77 | 0.83 | ssv | 0.40 | ssv |
| Sp/A | 0.70 | 0.10 | ssv | 0.12 | 0.07 | 0.78 |
| Sp/W | ssv | ssv | ssv | ssv | 0.12 | 0.27 |
| Su/A | ssv | ssv | ssv | ssv | 0.28 | ssv |
| Su/W | ssv | ssv | ssv | 0.07 | ssv | 0.08 |
| A/W | 0.36 | 0.75 | 0.44 | 0.66 | ssv | 0.37 |
| Sp/Sp | 0.07 | ssv | 0.07 | ssv | ssv | ssv |
| Su/Su | ssv | ssv | ssv | ssv | 0.48 | 0.48 |
| A/A | ssv | 0.09 | ssv | 0.09 | ssv | ssv |
| W/W | ssv | 0.14 | ssv | 0.14 | - | 0.27 |
| Oesophagostomumspp. | S. ransomi | |||||
| F1 | F2 | F1 | ||||
| Seasons | Weaners | Sows | Sows | Sows | ||
| Sp/Su | 0.60 | 0.18 | 0.46 | - | ||
| Sp/A | 0.43 | 0.79 | ssv | - | ||
| Sp/W | ssv | 0.08 | ssv | - | ||
| Su/A | 0.38 | 0.20 | ssv | 0.07 | ||
| Su/W | ssv | 0.75 | ssv | - | ||
| A/W | ssv | 0.08 | 0.31 | - | ||
| Sp/Sp | - | 0.54 | 0.54 | - | ||
| Su/Su | - | 0.81 | 0.81 | - | ||
| A/A | - | ssv | ssv | - | ||
| W/W | - | ssv | ssv | - | ||
| Parasite | Weaners (n = 320) | Fatteners (n = 320) | Sows (n = 320) | |||
|---|---|---|---|---|---|---|
| F | % | F | % | F | % | |
| Eimeria spp. | 203 | 63.4 | 163 | 50.9 | 125 | 39.0 |
| B. coli | 225 | 70.3 | 232 | 72.5 | 199 | 62.1 |
| A. suum | - | - | 202 | 63.1 | 109 | 34.0 |
| T. suis | - | - | 125 | 39.0 | - | - |
| Oesophagostomum spp. | 30 | 9.3 | - | - | 87 | 27.1 |
| S. ransomi | 12 | 3.7 | - | - | 5 | 1.5 |
| Cryptosporidium spp. | 58 | 18.1 | - | - | 30 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Băieş, M.-H.; Boros, Z.; Gherman, C.M.; Spînu, M.; Mathe, A.; Pataky, S.; Lefkaditis, M.; Cozma, V. Prevalence of Swine Gastrointestinal Parasites in Two Free-Range Farms from Nord-West Region of Romania. Pathogens 2022, 11, 954. https://doi.org/10.3390/pathogens11090954
Băieş M-H, Boros Z, Gherman CM, Spînu M, Mathe A, Pataky S, Lefkaditis M, Cozma V. Prevalence of Swine Gastrointestinal Parasites in Two Free-Range Farms from Nord-West Region of Romania. Pathogens. 2022; 11(9):954. https://doi.org/10.3390/pathogens11090954
Chicago/Turabian StyleBăieş, Mihai-Horia, Zsolt Boros, Călin Mircea Gherman, Marina Spînu, Attila Mathe, Stefan Pataky, Menelaos Lefkaditis, and Vasile Cozma. 2022. "Prevalence of Swine Gastrointestinal Parasites in Two Free-Range Farms from Nord-West Region of Romania" Pathogens 11, no. 9: 954. https://doi.org/10.3390/pathogens11090954
APA StyleBăieş, M.-H., Boros, Z., Gherman, C. M., Spînu, M., Mathe, A., Pataky, S., Lefkaditis, M., & Cozma, V. (2022). Prevalence of Swine Gastrointestinal Parasites in Two Free-Range Farms from Nord-West Region of Romania. Pathogens, 11(9), 954. https://doi.org/10.3390/pathogens11090954









