Fungal Grapevine Trunk Diseases in Romanian Vineyards in the Context of the International Situation
Abstract
1. Introduction
2. State of the Art in GTDs Understanding at Global Level
2.1. Petri Disease
2.1.1. Fungi Involved
2.1.2. Symptoms
2.1.3. Management
2.2. Blackfoot Disease
2.2.1. Symptoms
2.2.2. Risk Factors
2.2.3. Fungi Involved
2.2.4. Management
2.3. Botryosphaeria Dieback
2.3.1. Fungi Involved
2.3.2. Symptoms
2.3.3. Management
2.4. Phomopsis Dieback
2.4.1. Symptoms
2.4.2. Fungi Involved
2.4.3. Management
2.5. Eutypa Dieback
2.5.1. Fungi Involved
2.5.2. Symptoms
2.5.3. Management
2.6. Esca Disease
2.6.1. Fungi Involved
2.6.2. Symptoms
2.6.3. Management
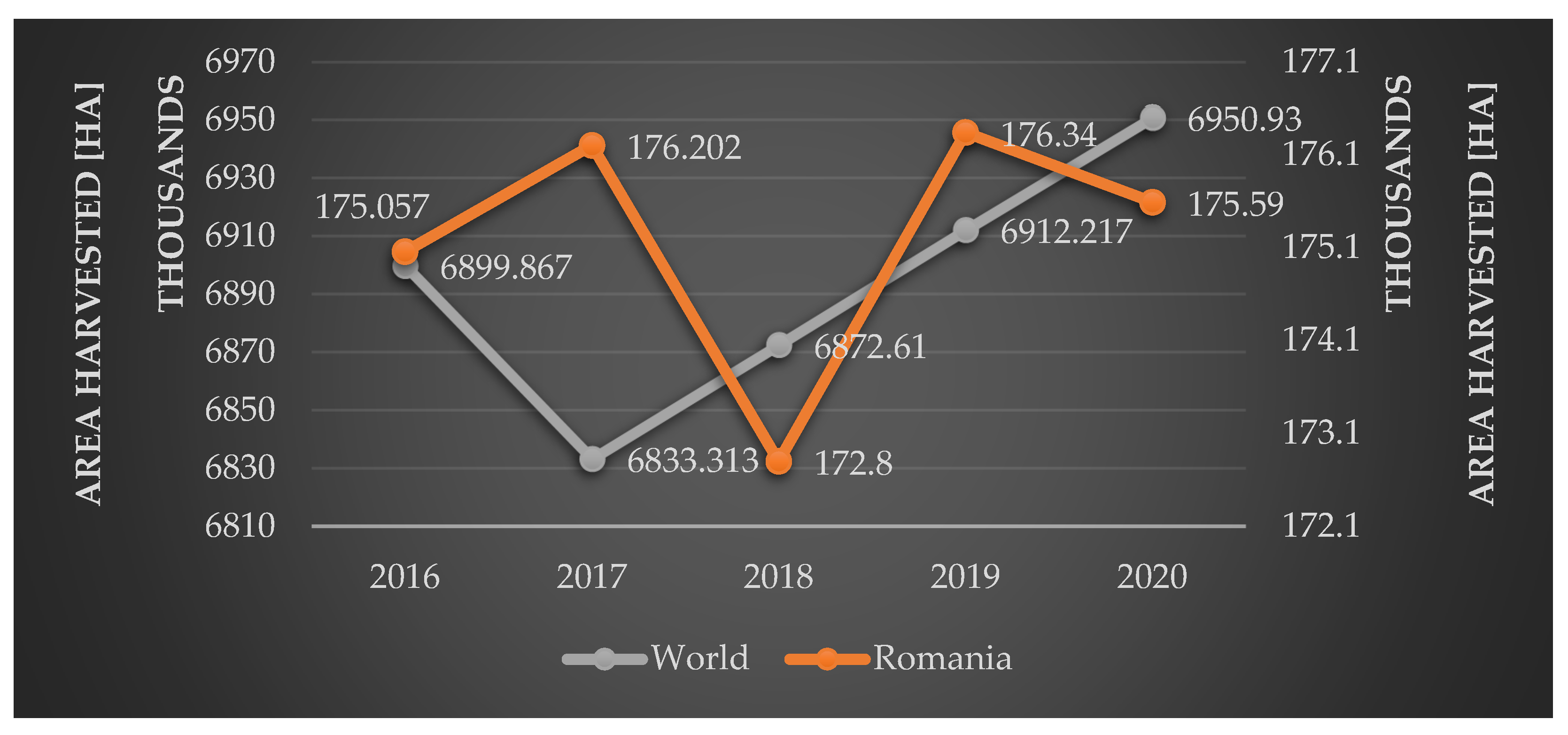
3. Viticulture in Romania
4. Grapevine Trunk Diseases in Romania
4.1. Eutypa Dieback in Romania
4.1.1. Risk Factors
4.1.2. Symptoms
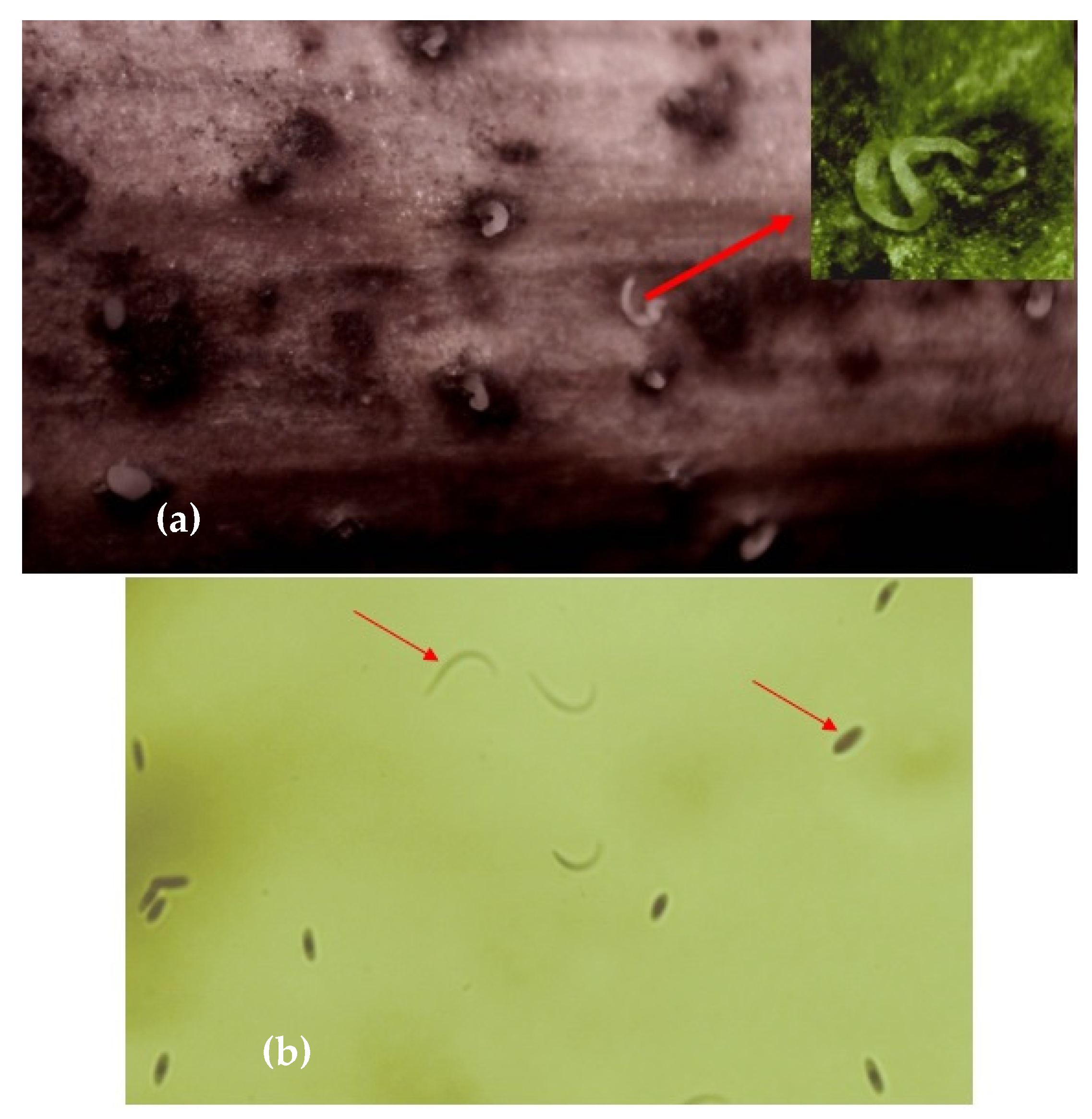
4.1.3. Fungi Involved
Temperature Influence on the In Vitro Development of Eutypa lata
Relative Atmospheric Humidity (RH) Influence on the In Vitro Development of Eutypa lata
Influence of pH Values on the In Vitro Development of Eutypa lata
The Energetic Resources of Eutypa lata Growing In Vitro
- (a)
- Carbon source influence
- (b)
- Light influence
4.2. Phomopsis Dieback in Romania
4.2.1. Symptoms
4.2.2. Fungi Involved
Life Cycle of Phomopsis viticola
Biology of Phomopsis viticola
4.3. Esca in Romania
4.3.1. Symptoms
4.3.2. Fungi Involved
5. Other GTDs Pathogens Identified in Romanian Vineyards
6. GTD Management in Romania
6.1. Management of Eutypa Dieback
- Prevention of stress conditions in the vineyard;
- Prevention of the infection and protection of the cutting wounds by wound sealing (painting or using an elastic sealing device);
- Avoid cutting in rainy weather when the release of fungal spores is at high levels;
- Renewal of infected vineyards (during the season, infected vines can be marked, and during winter they can be cut 20 cm above the grafting point; an offshoot that develops in the next growing season can be used to renew the vine; the wound in which the vine was cut must be covered with a sealing device);
- Removal and burning of the infected wood (dead wood must be cut during cutting season and then burned to reduce inoculation in the vineyard).
6.2. Management of Phomopsis Dieback
- Setting up plantations and filling gaps with certified material, free of phytopathogens;
- Correct application of grapevine maintenance work (pruning, tying, hoeing, and weed control);
- Reduce the source of infection in the plantation (cutting and removal of the attacked vines and burning them);
- Ensuring an optimal plant density to allow good air circulation.
6.3. Management of Esca Disease
- The use of healthy viticultural material (free of wood fungi);
- Limitation of the source of inoculum by removing and burning affected vines in windless periods in order to reduce the spread of fungal spores;
- Avoidance of excess nitrogen and lack of water;
- Spring pruning during periods without rainfall;
- Avoid strong wounds at spring pruning and treatment if they occur;
- Treatment of the vine after dry pruning to avoid infection with GTD pathogens;
- Reformation of the vine with the help of a healthy offshoot (only if the necrosis has not reached the rootstock). The success of the vine reformation is 75% for vines up to 25 years old.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases with Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Irimia, L.M.; Patriche, C.V.; Roșca, B. Climate Change Impact on Climate Suitability for Wine Production in Romania. Theor. Appl. Climatol. 2018, 133, 1–14. [Google Scholar] [CrossRef]
- Chiurciu, I.-A.; Zaharia, I.; Soare, E. Production of Wine Grapes and Cultural Traditions Related to Vine in Romania. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2020, 20, 133–143. [Google Scholar]
- ANF-Autoritatea Naționlă Fitosanitară (Romanian national phytosanitary authority). Ghid Pentru Recunoașterea Și Combaterea Bolilor Și Dăunătorilor La Vița de Vie [Guide to Recognizing and Fighting Diseases and Pests of Grapevine RO]; Autoritatea Naţională Fitosanitară: Voluntari, Romania, 2016. [Google Scholar]
- FAOSTAT (Food and Agriculture Organization of the United Nation). Crops and Livestock Products; Ministry of Agriculture: Prague, Czech Republic, 2013. [Google Scholar]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on Fungal Phytotoxins and Their Role in Grapevine Trunk Diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.J.; Gelabert, M.; Moreira, V.; Mondino, P.; Alaniz, S. Grapevine nursery propagation material as source of fungal trunk disease pathogens in Uruguay. Front. Fungal Biol. 2022, 3, 958466. [Google Scholar] [CrossRef]
- Siebert, J.B. Eutypa: The Economic Toll on Vineyards. Wines Vines 2001, 82, 50–56. [Google Scholar]
- Surico, G.; Mugnai, L.; Marchi, G. The Esca Disease Complex. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Springer: Dordrecht, The Netherlands, 2008; pp. 119–136. [Google Scholar]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine Trunk Diseases: Complex and Still Poorly Understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Kaplan, J.; Travadon, R.; Cooper, M.; Hillis, V.; Lubell, M.; Baumgartner, K. Identifying Economic Hurdles to Early Adoption of Preventative Practices: The Case of Trunk Diseases in California Winegrape Vineyards. Wine Econ. Policy 2016, 5, 127–141. [Google Scholar] [CrossRef]
- Travadon, R.; Rolshausen, P.E.; Gubler, W.D.; Cadle-Davidson, L.; Baumgartner, K. Susceptibility of Cultivated and Wild Vitis Spp. to Wood Infection by Fungal Trunk Pathogens. Plant Dis. 2013, 97, 1529–1536. [Google Scholar] [CrossRef]
- Kovács, C.; Balling, P.; Bihari, Z.; Nagy, A.; Sándor, E. Incidence of Grapevine Trunk Diseases Is Influenced by Soil, Topology and Vineyard Age, but Not by Diplodia seriata Infection Rate in the Tokaj Wine Region, Hungary. Phytoparasitica 2017, 45, 21–32. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Nita, M.; Baumgartner, K. TrunkDiseaseID.Org: A Molecular Database for Fast and Accurate Identification of Fungi Commonly Isolated from Grapevine Wood. Crop Prot. 2017, 102, 110–117. [Google Scholar] [CrossRef]
- Raimondo, M.L.; Carlucci, A.; Ciccarone, C.; Sadallah, A.; Lops, F. Identification and Pathogenicity of Lignicolous Fungi Associated with Grapevine Trunk Diseases in Southern Italy. Phytopathol. Mediterr. 2019, 58, 639–662. [Google Scholar] [CrossRef]
- Gispert, C.; Kaplan, J.D.; Deyett, E.; Rolshausen, P.E. Long-Term Benefits of Protecting Table Grape Vineyards against Trunk Diseases in the California Desert. Agronomy 2020, 10, 1895. [Google Scholar] [CrossRef]
- Csótó, A.; Balling, P.; Nagy, A.; Sándor, E. The Role of Cultivar Susceptibility and Vineyard Age in GTD: Examples from the Carpathian Basin. Acta Agrar. Debr. 2020, 2, 57–63. [Google Scholar] [CrossRef]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine Trunk Disease Fungi: Their Roles as Latent Pathogens and Stress Factors That Favour Disease Develop-Ment and Symptom Expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar] [CrossRef]
- Urbez-Torres, J.R.; Peduto, F.; Striegler, R.K.; Urrea-Romero, K.E.; Rupe, J.C.; Cartwright, R.D.; Gubler, W.D. Characterization of Fungal Pathogens Associated with Grapevine Trunk Diseases in Arkansas and Missouri. Fungal Divers. 2012, 52, 169–189. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Guerin-Dubrana, L.; Fontaine, F.; Mugnai, L. Grapevine Trunk Disease in European and Mediterranean Vineyards: Occurrence, Distribution and Associated Disease-Affecting Cultural Factors. Phytopathol. Mediterr. 2019, 58, 49–71. [Google Scholar] [CrossRef]
- Lade, S.B.; Štraus, D.; Oliva, J. Variation in Fungal Community in Grapevine (Vitis vinifera) Nursery Stock Depends on Nursery, Variety and Rootstock. J. Fungi 2022, 8, 47. [Google Scholar] [CrossRef]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine Trunk Diseases under Thermal and Water Stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Úrbez-Torres, J.R.; Gubler, W.D. Susceptibility of Grapevine Pruning Wounds to Infection by Lasiodiplodia theobromae and Neofusicoccum parvum. Plant Pathol. 2011, 60, 261–270. [Google Scholar] [CrossRef]
- Reis, P.; Pierron, R.; Larignon, P.; Lecomte, P.; Abou-Mansour, E.; Farine, S.; Bertsch, C.; Jacques, A.; Trotel-Aziz, P.; Rego, C.; et al. Vitis Methods to Understand and Develop Strategies for Diagnosis and Sustainable Control of Grapevine Trunk Diseases. Phytopathology 2019, 109, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black Measles) and Brown Wood-Streaking: Two Old and Elusive Diseases of Grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Bénard-Gellon, M.; Farine, S.; Goddard, M.L.; Schmitt, M.; Stempien, E.; Pensec, F.; Laloue, H.; Mazet-Kieffer, F.; Fontaine, F.; Larignon, P.; et al. Toxicity of Extracellular Proteins from Diplodia seriata and Neofusicoccum parvum Involved in Grapevine Botryosphaeria Dieback. Protoplasma 2015, 252, 679–687. [Google Scholar] [CrossRef]
- Schilling, M.; Farine, S.; Péros, J.-P.; Bertsch, C.; Gelhaye, E. Wood Degradation in Grapevine Diseases. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 99, pp. 175–207. [Google Scholar]
- Bruno, G.; Sparapano, L. Effects of Three Esca-Associated Fungi on Vitis vinifera L.: Characterization of Secondary Metabolites in Culture Media and Host Responses to the Pathogens in Calli. Physiol. Mol. Plant Pathol. 2006, 69, 209–223. [Google Scholar] [CrossRef]
- Abou-Mansour, A.; Couché, E.; Tabacchi, R. Do Fungal Naphthalenones Have a Role in the Development of Esca Symptoms? Phytopathol. Mediterr. 2004, 43, 75–82. [Google Scholar]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F.; de la Fuente, M. Grapevine Trunk Diseases. A Review; OIV Publications: Paris, France, 2016; ISBN 979-10-91799-60-7. [Google Scholar]
- Rubio, J.; Garzón, E. Las Enfermedades de Madera de Vid Como Amenaza Del Sector Vitícola. Rev. Winetech 2011, 2, 18–21. [Google Scholar]
- Grosman, J.; Doublet, B. Maladies Du Bois de La Vigne: Synthèse Des Dispositifs d’observation Au Vignoble, de l’observatoire 2003–2008 Au Réseau d’épidémiosurveillance Actuel. Phytoma-La Défense des Végétaux 2012, 651, 31–35. [Google Scholar]
- Romanazzi, G.; Murolo, S.; Pizzichini, L.; Nardi, S. Esca in Young and Mature Vineyards, and Molecular Diagnosis of the Associated Fungi. Eur. J. Plant Pathol. 2009, 125, 277–290. [Google Scholar] [CrossRef]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wery, J. Current Knowledge on Grapevine Trunk Diseases with Complex Etiology: A Systemic Approach. Phytopathol. Mediterr. 2020, 1, 29–53. [Google Scholar] [CrossRef]
- Urbez-Torres, J.R.; Battany, M.; Bettiga, L.J.; Gispert, C.; McGourty, G.; Roncoroni, J.; Smith, R.J.; Verdegaal, P.; Gubler, W.D. Botryosphaeriaceae Species Spore-Trapping Studies in California Vineyards. Plant Dis. 2010, 94, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, P.E.; Rooney-Latham, S.; Eskalen, A. Evaluation of Pruning Wound Susceptibility and Protection against Fungi Associated with Grapevine Trunk Diseases. Am. Soc. Enol. Vitic. 2010, 61, 113–119. [Google Scholar]
- Petit, E.; Barriault, E.; Baumgartner, K.; Wilcox, W.F. Cylindrocarpon Species Associated with Black-Foot of Grapevine in Northeastern United States and Southeastern Canada. Am. J. Enol. Vitic 2011, 62, 177–183. [Google Scholar] [CrossRef]
- Gramaje, D.; Armengol, J. Fungal Trunk Pathogens in the Grapevine Propagation Process: Potential Inoculum Sources, Detection, Identification, and Management Strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Larignon, P.; Gramaje, D. Life Cycle of Pathogens Associated with Grapevine Trunk Diseases. In Abstracts of Oral and Poster, Proceedings of the 1rst COST Action FA 1303 Workshop on Grapevine Trunk Diseases, Cognac, France, 23–24 June 2015; Firenze University Press: Firenze, Italy, 2015; pp. 420–436. [Google Scholar]
- Petzoldt, C.; Sall, M.; Moller, W.J. Factors Determining the Relative Number of Ascospores Released by Eutypa armeniacae in California. Plant Dis. 1983, 67, 857–860. [Google Scholar]
- Rooney-Latham, S.; Eskalen, A.; Gubler, W.D. Occurrence of Togninia minima Perithecia in Esca-Affected Vineyards in California. Plant Dis. 2005, 89, 867–871. [Google Scholar] [CrossRef]
- Eskalen, A.; Feliciano, A.; Gubler, W.D. Susceptibility of Grapevine Pruning Wounds and Symptom Development in Response to Infection by Phaeoacremonium aleophilum and Phaeomoniella chlamydospora. Plant Dis. 2007, 91, 1100–1104. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Gubler, W.D. Double Pruning, a Potential Method to Control Bot Canker Disease of Grapes, and Susceptibility of Grapevine Pruning Wounds to Infection by Botryosphaeriaceae. Abstr. Phytopathol. Mediterr. 2008, 48, 185. [Google Scholar]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can Vessel Dimension Explain Tolerance toward Fungal Vascular Wilt Diseases in Woody Plants? Lessons from Dutch Elm Disease and Esca Disease in Grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef]
- Munkvold, G.; Marois, J.J. Factors Associated with Variation in Susceptibility of Grapevine Pruning Wounds to Infection by Eutypa lata. Phytopathology 1995, 85, 249–256. [Google Scholar]
- Chacón-Vozmediano, J.L.; Gramaje, D.; León, M.; Armengol, J.; Moral, J.; Izquierdo-Cañas, P.M.; Martínez-Gascueña, J. Cultivar Susceptibility to Natural Infections Caused by Fungal Grapevine Trunk Pathogens in La Mancha Designation of Origin (Spain). Plants 2021, 10, 1171. [Google Scholar] [CrossRef]
- Lecomte, P.; Darrieutort, G.; Liminana, J.-M.; Comont, G.; Muruamendiaraz, A.; Legorburu, F.-J.; Choueiri, E.; Jreijiri, F.; el Amil, R.; Fermaud, M. New Insights into Esca of Grapevine: The Development of Foliar Symptoms and Their Association with Xylem Discoloration. Plant Dis. 2012, 96, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Larignon, R.; Dubos, B. Fungi Associated with Esca Disease in Grapevine. Eur. J. Plant Pathol. 1997, 103, 147–157. [Google Scholar] [CrossRef]
- Sparapano, L.; Graniti, A.; Bruno, G. Three-Year Observation of Grapevines Cross-Inoculated with Esca-Associated Fungi. Phytopathol. Mediterr. 2001, 40, 376–386. [Google Scholar]
- Valtaud, C.; Larignon, P.; Roblin, G.; Fleurat-Lessard, P. Developmental and Ultrastructural Features of Phaeomoniella chlamydospora and Phaeoacremonium aleophilum in Relation to Xylem Degradation in Esca Disease of the Grapevine. J. Plant Pathol. 2009, 91, 37–51. [Google Scholar]
- Surico, G. Towards a Redefinition of the Diseases within the Esca Complex of Grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar]
- Xie, Y.; Zhang, W.; Wang, Y.; Yan, J.-Y.; Liu, J.-K.; Hyde, K.D.; Seem, R.C.; Zhang, G.-Z.; Wang, Z.-Y.; Yao, S.-W.; et al. Species of Botryosphaeriaceae Involved in Grapevine Dieback in China. Fungal Divers. 2013, 61, 221–236. [Google Scholar] [CrossRef]
- Calzarano, F.; Osti, F.; Baranek, M.; di Marco, S. Rainfall and Temperature Influence Expression of Foliar Symptoms of Grapevine Leaf Stripe Disease (Esca Complex) in Vineyards. Phytopathol. Mediterr. 2018, 57, 488–505. [Google Scholar] [CrossRef]
- Halleen, F.; Crous, P.W.; Petrini, O. Fungi Associated with Healthy Grapevine Cuttings in Nurseries, with Special Reference to Pathogens Involved in the Decline of Young Vines. Australas. Plant Pathol. 2003, 32, 47–52. [Google Scholar] [CrossRef]
- Halleen, F.; Schroers, H.-J.; Groenewald, J.Z.; Crous, P.W.; Si, H.S. Novel Species of Cylindrocarpon (Neonectria) and Campylocarpon Gen. Nov. Associated with Black Foot Disease of Grapevines (Vitis Spp.). Stud. Mycol. 2004, 50, 431–455. [Google Scholar]
- Mostert, L.; Crous, P.W.; Fourie, P.; Halleen, F. A Review of Phaeoacremonium Species Involved in Petri Disease and Esca of Grapevines. Phytopathol. Mediterr. 2006, 45, S12–S29. [Google Scholar] [CrossRef]
- Giménez-Jaime, A.; Aroca, A.; Raposo, R.; García-Jiménez, J.; Armengol, J. Occurrence of Fungal Pathogens Associated with Grapevine Nurseries and the Decline of Young Vines in Spain. J. Phytopathol. 2006, 154, 598–602. [Google Scholar] [CrossRef]
- Whiteman, S.; Stewart, A.; Ridgway, H.J.; Jaspers, M. Infection of Rootstock Mother-Vines by Phaeomoniella chlamydospora Results in Infected Young Grapevines. Australas. Plant Pathol. 2007, 36, 198–203. [Google Scholar] [CrossRef]
- Gramaje, D.; García-Jiménez, J.; Armengol, J. Sensitivity of Petri Disease Pathogens to Hot-Water Treatments in Vitro. Ann. Appl. Biol. 2008, 153, 95–103. [Google Scholar] [CrossRef]
- Gramaje, D.; Aroca, Á.; Raposo, R.; García-Jiménez, J.; Armengol, J. Evaluation of Fungicides to Control Petri Disease Pathogens in the Grapevine Propagation Process. Crop Prot. 2009, 28, 1091–1097. [Google Scholar] [CrossRef]
- Rego, C.; Nascimento, T.; Cabral, A.; Silva, M.J.; Oliveira, H. Control of Grapevine Wood Fungi in Commercial Nurseries. Phytopathol. Mediterr. 2009, 48, 128–135. [Google Scholar]
- Agustí-Brisach, C.; Gramaje, D.; León, M.; García-Jiménez, J.; Armengol, J. Evaluation of Vineyard Weeds as Potential Hosts of Black-Foot and Petri Disease Pathogens. Plant Dis. 2011, 95, 803–810. [Google Scholar] [CrossRef]
- Pintos, C.; Redondo, V.; Costas, D.; Aguin, O. Fungi Associated with Grapevine Trunk Diseases in Nursery-Produced Vitis vinifera Plants. Phytopathol. Mediterr. 2018, 57, 407–424. [Google Scholar] [CrossRef]
- Fourie, P.; Halleen, F. Occurrence of Grapevine Trunk Disease Pathogens in Rootstock Mother Plants in South Africa. Australas. Plant Pathol. 2004, 33, 313–315. [Google Scholar] [CrossRef]
- Gramaje, D.; Armengol, J.; Salazar, D.; Lopez-Cortes, I. Effect of Hot-Water Treatments above 50 °C on Grapevine Viability and Survival of Petri Disease Pathogens. Crop Prot. 2009, 28, 280–285. [Google Scholar] [CrossRef]
- Fourie, P.H.; Halleen, F. Proactive Control of Petri Disease of Grapevine through Treatment of Propagation Material. Plant Dis. 2004, 88, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Pascoe, I.G. Pycnidial State of Phaeomoniella chlamydospora Found on Pinot Noir Grapevines in the Field. Australas. Plant Pathol. 2001, 30, 67. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B. Preliminary Studies on the Biology of “Phaeoacremonium”. Phytopathol. Mediterr. 2000, 39, 184–189. [Google Scholar]
- Pascoe, I.; Cottral, E. Developments in Grapevine Trunk Diseases Research in Australia. Phytopathol. Mediterr. 2000, 39, 68–75. [Google Scholar]
- Eskalen, A.; Gubler, W.D. Association of Spores of Phaeomoniella chlamydospora, Phaeoacremonium inflatipes, and Pm. Aleophilum with Grapevine Cordons in California. Phytopathol. Mediterr. 2001, 40, S429–S432. [Google Scholar]
- Feliciano, A.J.; Gubler, W.D. Histological Investigations on Infection of Grape Roots and Shoots by Phaeoacremonium spp. Phytopathol. Mediterr. 2001, 40, S387–S393. [Google Scholar]
- Fourie, P.H.; Halleen, F. Investigation on the Occurrence of Phaeomoniella chlamydospora in Canes of Rootstock Mother Vines. Australas. Plant Pathol. 2002, 31, 425–426. [Google Scholar] [CrossRef]
- Whiteman, S.; Jaspers, M.; Stewart, A.; Ridgway, H. Identification of Potential Sources of Phaeomoniella chlamydospora in the Grapevine Propagation Process. In Proceedings of the International Workshop on Grapevine Trunk Diseases, Lincoln, New Zealand, 17–18 November 2003; p. 26. [Google Scholar]
- Whiteman, S.; Jaspers, M.; Stewart, A.; Ridgway, H. Identification of Potential Sources of Phaeomoniella chlamydospora in the Grapevine Propagation Process. Phytopathol. Mediterr. 2004, 43, 152–153. [Google Scholar]
- Damm, U.; Fourie, P. A Cost-Effective Protocol for Molecular Detection of Fungal Pathogens in Soil. S. Afr. J. Sci. 2005, 101, 135–139. [Google Scholar] [CrossRef]
- Retief, E.; Damm, U.; van Niekerk, J.M.; Mcleod, A.; Fourie, P.H. A Protocol for Molecular Detection of Phaeomoniella chlamydospora in Grapevine Wood. S. Afr. J. Sci. 2005, 101, 139–142. [Google Scholar] [CrossRef]
- Retief, E.; McLeod, A.; Fourie, P.H. Potential Inoculum Sources of Phaeomoniella chlamydospora in South African Grapevine Nurseries. Eur. J. Plant Pathol. 2006, 115, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Constable, F.; Wiechel, T.; Salib, S. Comparison of the Molecular Tests-Single PCR, Nested PCR and Quantitative PCR (SYBR Green and TaqMan)—for Detection of Phaeomoniella chlamydospora during Grapevine Nursery Propagation. Phytopathol. Mediterr. 2007, 46, 58–72. [Google Scholar]
- Whiteman, S.; Jaspers, M.; Stewart, A.; Ridgway, H. Soil as a Source of Phaeomoniella chlamydospora (Causal Agent of Petri Disease). In Proceedings of the 4th International Workshop on Grapevine Trunk Diseases, Stellenbosch, South Africa, 20–21 January 2005; University of Stellenbosch: Stellenbosch, South Africa, 2005; p. 78. [Google Scholar]
- Rooney, S.; Eskalen, A.; Gubler, W.D. Recovery of “Phaemoniella chlamydospora” and “Phaeoacremonium inflatipes” from Soil and Grapevine Tissues. Phytopathol. Mediterr. 2001, 40, S351–S356. [Google Scholar]
- Wallace, J.; Edwards, J.; Pascoe, I.G.; May, P. Phaeomoniella chlamydospora Inhibits Callus Formation by Grapevine Rootstock and Scion Cultivars. Phytopathol. Mediterr. 2004, 43, 151–152. [Google Scholar]
- Ferreira, J.H.; van Wyk, P.S.; Calitz, F.J. Slow Dieback of Grapevine in South Africa: Stress-Related Predisposition of Young Vines for Infection by Phaeoacremonium chlamydosporum. S. Afr. J. Enol. Vitic. 1999, 20, 43–46. [Google Scholar]
- Sparapano, L.; Graniti, A.; Bruno, G. Effects on Plants of Metabolites Produced in Culture by “Phaeoacremonium chlamydosporum”, “P. aleophilum” and “Fomitiporia punctata”. Phytopathol. Mediterr. 2000, 39, 169–177. [Google Scholar]
- Waite, H.; Morton, L. Hot Water Treatment, Trunk Diseases and Other Critical Factors in the Production of High-Quality Grapevine Planting Material. Phytopathol. Mediterr. 2007, 46, 5–17. [Google Scholar]
- Abeywickrama, P.Z.W.; Li, X.; Jayawardena, R.; Hyde, K.; Yan, J. Campylocarpon fasciculare (Nectriaceae, Sordariomycetes); Novel Emergence of Black-Foot Causing Pathogen on Young Grapevines in China. Pathogens 2021, 10, 1555. [Google Scholar] [CrossRef]
- Edwards, J.; Pascoe, I.G. Occurrence of Phaeomoniella chlamydospora and Phaeoacremonium aleophilum Associated with Petri Disease and Esca in Australian Grapevines. Australas. Plant Pathol. 2004, 33, 273–279. [Google Scholar] [CrossRef]
- Whitelaw-Weckert, M.A.; Nair, N.G.; Lamont, R.; Alonso, M.; Priest, M.; Huang, R. Root Infection of Vitis vinifera by Cylindrocarpon liriodendri in Australia. Australas. Plant Pathol. 2007, 36, 403–406. [Google Scholar] [CrossRef]
- Scheck, H.J.; Vasquez, S.J.; Gubler, W.D.; Fogle, D. First Report of Black-Foot Disease, Caused by Cylindrocarpon obtusisporum, of Grapevine in California. Plant Dis. 1998, 82, 448. [Google Scholar] [CrossRef]
- Petit, E.; Gubler, W.D. Characterization of Cylindrocarpon Species, the Cause of Black Foot Disease of Grapevine in California. Plant Dis. 2005, 89, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Petit, E.; Gubler, W.D. First Report of Cylindrocarpon liriodendri Causing Black Foot Disease of Grapevine in California. Plant Dis. 2007, 91, 1060. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Droguett, A.; Esterio, M. The Red Globe Decline. In Proceedings of the 1st International Workshop on Grapevine Trunk Diseases: Esca and Grapevine Declines, Siena, Italy, 1 October 1999. [Google Scholar]
- Auger, J.; Esterio, M.; Pérez, I. First Report of Black Foot Disease of Grapevine Caused by Cylindrocarpon macrodidymum in Chile. Plant Dis. 2007, 91, 470. [Google Scholar] [CrossRef]
- Grasso, S.; Lio, G.M.D.S. Infezioni Di Cylindrocarpon obtusisporum Su Piante Di Vite in Sicilia [Infections of Cylindrocarpon obtusisporium on Grapevines in Sicily] [Italian]. Vitis 1975, 14, 36–39. [Google Scholar]
- Choueiri, E.; Jreijiri, F.; el Amil, R.; Chlela, P.; Bugaret, Y.; Liminana, J.M.; Mayet, V.; Lecomte, P. First Report of Black Foot Disease Associated with Cylindrocarpon Sp. in Lebanon. J. Plant Pathol. 2009, 91, 237. [Google Scholar]
- Rego, M.C. Nova e Grave Micose Da Videira Em Portugal. Agente Responsável: Cylindrocarpon destructans (Zins.) Scholten. Publicação Laboratório Patologia Vegetal Veríssimo Almeid 1994, 67, 1–4. [Google Scholar]
- Rego, C.; Phillips, A.; Carvalho, A.; Oliveira, H. Involvement of “Phaeoacremonium” Spp. and “Cylindrocarpon destructans” with Grapevine Decline in Portugal. Phytopathol. Mediterr. 2000, 39, 76–79. [Google Scholar]
- Rego, C.; Nascimento, T.; Oliveira, H. Characterisation of “Cylindrocarpon destructans” Isolates from Grapevines in Portugal. Phytopathol. Mediterr. 2001, 40, S343–S350. [Google Scholar]
- Armengol, J.; Vincent, A.; Torne, L.; Garcia-Figuerez, F.; Garcia-Jimenez, J. Fungi Associated with Esca and Grapevine Declines in Spain: A Three-Year Survey. Phytopathol. Mediterr. 2001, 40, 325–329. [Google Scholar]
- Alaniz, S.; Armengol, J.; León, M.; García-Jiménez, J.; Abad-Campos, P. Analysis of Genetic and Virulence Diversity of Cylindrocarpon liriodendri and C. macrodidymum Associated with Black Foot Disease of Grapevine. Mycol. Res. 2009, 113, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Agustí-Brisach, C.; Gramaje, D.; García-Jiménez, J.; Armengol, J. Detection of Black-Foot Disease Pathogens in the Grapevine Nursery Propagation Process in Spain. Eur. J. Plant Pathol. 2013, 137, 103–112. [Google Scholar] [CrossRef]
- Berlanas, C.; Ojeda, S.; López-Manzanares, B.; Andrés-Sodupe, M.; Bujanda, R.; del Pilar Martínez-Diz, M.; Díaz-Losada, E.; Gramaje, D. Occurrence and Diversity of Black-Foot Disease Fungi in Symptomless Grapevine Nursery Stock in Spain. Plant Dis. 2020, 104, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Diz, P.M.; Díaz-Losada, E.; Andrés-Sodupe, M.; Bujanda, R.; Maldonado-González, M.M.; Ojeda, S.; Yacoub, A.; Rey, P.; Gramaje, D. Field Evaluation of Biocontrol Agents against Black-foot and Petri Diseases of Grapevine. Pest Manag. Sci. 2021, 77, 697–708. [Google Scholar] [CrossRef]
- Abreo, E.; Martinez, S.; Bettucci, L.; Lupo, S. Morphological and Molecular Characterisation of Campylocarpon and Cylindrocarpon Spp. Associated with Black Foot Disease of Grapevines in Uruguay. Australas. Plant Pathol. 2010, 39, 446–452. [Google Scholar] [CrossRef]
- Sweetingham, M. Studies on the Nature of the Pathogenicity of Soil-Borne Cylindrocarpon Species; University of Tasmania: Hobart, Australia, 1983. [Google Scholar]
- Grasso, S. Infezioni Di Fusarium oxysporum e Di Cylindrocarpon destructans Associate a Una Moria Di Giovani Piante Di Vite in Sicilia [Infection of Fusarium oxysporum and Cylindrocarpon destructans, Associated with a Grapevine Decline in a Vineyard of Eastern Sicily]. [Italian]. Inf. Fitopatol. 1984, 1, 59–63. [Google Scholar]
- Gugino, B.K.; Travis, J.W. Suppression of Cylindrocarpon destructans Utilizing Composted Soil Amendments. Phytopathology 2003, 93, S31. [Google Scholar]
- Rumbou, A.; Rumbos, I. Fungi Associated with Esca and Young Grapevine Decline in Greece. Phytopathol. Mediterr. 2001, 40, S330–S335. [Google Scholar] [CrossRef]
- Fourie, P.H.; Halleen, F.; Volkmann, A.S. Fungi Associated with Grape Wood, Root and Trunk Diseases: A Summary of the 1999–2000 Results from the Diagnostic Service at Nietvoorbij. In Proceedings of the 2nd International Viticulture and Enology Congress, Cape Town, South Africa, 8 November 2000. [Google Scholar]
- Fourie, P.H.; Halleen, F. Diagnosis of Fungal Diseases and Their Involvement in Dieback Disease of Young Vines. Wynboer 2001, 149, 19–23. [Google Scholar]
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.; O’Gorman, D.T. Grapevine Trunk Diseases in British Columbia: Incidence and Characterization of the Fungal Pathogens Associated with Esca and Petri Diseases of Grapevine. Plant Dis. 2014, 98, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Akgül, D.; Savaş, N.G.; Keykubat, B.; Mayorquin, J.S.; Eskalen, A. Fungal Trunk Pathogens of Sultana Seedless Vineyards in Aegean Region of Turkey. Phytopathol. Mediterr. 2015, 54, 380–393. [Google Scholar] [CrossRef]
- Akgül, D.; Ahioğlu, M. Fungal Pathogens Associated with Young Grapevine Decline in the Southern Turkey Vineyards. BIO Web Conf. EDP Sci. 2019, 15, 10–27. [Google Scholar] [CrossRef]
- Mohammadi, H.; Alaniz, S.; Banihashemi, Z.; Armengol, J. Characterization of Cylindrocarpon liriodendri Associated with Black Foot Disease of Grapevine in Iran. J. Phytopathol. 2009, 157, 642–645. [Google Scholar] [CrossRef]
- Halleen, F.; Schroers, H.J.; Groenewald, J.Z.; Rego, C.; Oliveira, H.; Crous, P.W. Neonectria liriodendri Sp. Nov., the Main Causal Agent of Black Foot Disease of Grapevines. Stud. Mycol. 2006, 55, 227–234. [Google Scholar] [CrossRef]
- Alaniz, S.; León, M.; Vicent, A.; García-Jiménez, J.; Abad-Campos, P.; Armengol, J. Characterization of Cylindrocarpon Species Associated with Black Foot Disease of Grapevine in Spain. Plant Dis. 2007, 91, 1187–1193. [Google Scholar] [CrossRef]
- Brisach, C.A.; Fortí, J.A. Black-Foot Disease of Grapevine: An Update on Taxonomy, Epidemiology and Management Strategies. Phytopathol. Mediterr. 2013, 52, 380–387. [Google Scholar] [CrossRef]
- Scheck, H.; Vasquez, S.; Fogle, D.; Gubler, D. Grape Growers Report Losses to Black-Foot and Grapevine Decline. Calif. Agric. 1998, 52, 19–23. [Google Scholar] [CrossRef][Green Version]
- Larignon, P. Black Foot Disease in France. In Black Goo Occurrence and Symptoms of Grapevine Declines-IAS/ICGTD Proceedings 1998; International Ampelography Society: Fort Valley, VA, USA, 1999; pp. 89–90. [Google Scholar]
- Gubler, W.D.; Baumgartner, K.; Browne, G.T.; Eskalen, A.; Latham, S.R.; Petit, E.; Bayramian, L.A. Root Diseases of Grapevines in California and Their Control. Plant Pathol. 2004, 33, 157–165. [Google Scholar] [CrossRef]
- Probst, C.; Jones, E.E.; Ridgway, H.J.; Jaspers, M.V. Cylindrocarpon Black Foot in Nurseries-Two Factors That Can Increase Infection. Australas. Plant Pathol. 2012, 41, 157–163. [Google Scholar] [CrossRef]
- Brown, D.; Jones, E.; Ridgway, H.J. Effect of Partial Defoliation on Cylindrocarpon destructans Infection of Grapevines. New Zealand Plant Prot. 2012, 8, 256–261. [Google Scholar] [CrossRef]
- Lombard, L.; van der Merwe, A.; Groenewald, J.Z.; Crous, P.W. Lineages in Nectriaceae: Re-Evaluating the Generic Status of Ilyonectria and Allied Genera. Phytopathol. Mediterr. 2014, 53, 515–532. [Google Scholar] [CrossRef]
- Carlucci, A.; Lops, F.; Mostert, L.; Halleen, F.; Raimondo, M.L. Occurrence Fungi Causing Black Foot on Young Grapevines and Nursery Rootstock Plants in Italy. Phytopathol. Mediterr. 2017, 56, 10–39. [Google Scholar] [CrossRef]
- Cabral, A.; Groenewald, J.Z.; Rego, C.; Oliveira, H.; Crous, P.W. Cylindrocarpon Root Rot: Multi-Gene Analysis Reveals Novel Species within the Ilyonectria radicicola Species Complex. Mycol. Prog. 2012, 11, 655–688. [Google Scholar] [CrossRef]
- Cabral, A.; Rego, C.; Nascimento, T.; Oliveira, H.; Groenewald, J.Z.; Crous, P.W. Multi-Gene Analysis and Morphology Reveal Novel Ilyonectria Species Associated with Black Foot Disease of Grapevines. Fungal Biol. 2012, 116, 62–80. [Google Scholar] [CrossRef]
- Bleach, C.M. Management of Cylindrocarpon Black Foot Disease in New Zealand Nurseries and Vineyards; Lincoln University: Philadelphia, PA, USA, 2012. [Google Scholar]
- Langenhoven, S.D.; Halleen, F.; Spies, C.F.; Stempien, E.; Mostert, L. Detection and Quantification of Black Foot and Crown and Root Rot Pathogens in Grapevine Nursery Soils in the Western Cape of South Africa. Phytopathol. Mediterr. 2018, 57, 519–537. [Google Scholar] [CrossRef]
- Probst, C.M.; Jaspers, M.V.; Jones, E.E.; Ridgway, H.J. A Quantitative PCR Method for Detecting Two Cylindrocarpon Species in Soil. Phytopathol. Mediterr. 2010, 49, 115. [Google Scholar]
- Cardoso, M.; Diniz, I.; Cabral, A.; Rego, C.; Oliveira, H. Unveiling Inoculum Sources of Black Foot Pathogens in a Commercial Grapevine Nursery. Phytopathol. Mediterr. 2013, 52, 298–312. [Google Scholar]
- Agustí-Brisach, C.; Mostert, L.; Armengol, J. Detection and Quantification of Ilyonectria Spp. Associated with Black-Foot Disease of Grapevine in Nursery Soils Using Multiplex Nested PCR and Quantitative PCR. Plant Pathol. 2014, 63, 316–322. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.; Haag, P.; Bowen, P.; Lowery, T.; O’Gorman, D.T. Development of a DNA Macroarray for the Detection and Identification of Fungal Pathogens Causing Decline of Young Grapevines. Phytopathology 2015, 105, 1373–1388. [Google Scholar] [CrossRef]
- Berlanas, C.; López-Manzanares, B.; Gramaje, D. Estimation of Viable Propagules of Black-Foot Disease Pathogens in Grapevine Cultivated Soils and Their Relation to Production Systems and Soil Properties. Plant Soil 2017, 417, 467–479. [Google Scholar] [CrossRef]
- Oliveira, H.; Rego, M.; Nascimento, T. Decline of Young Grapevines Caused by Fungi. Acta Hortic. 2003, 652, 295–304. [Google Scholar] [CrossRef]
- Halleen, F.; Fourie, P.; Crous, P.W. Control of Black Foot Disease in Grapevine Nurseries. Plant Pathol. 2007, 56, 637–645. [Google Scholar] [CrossRef]
- Nascimento, T.; Rego, C.; Oliveira, H. Potential Use of Chitosan in the Control of Grapevine Trunk Diseases. Phytopathol. Mediterr. 2007, 46, 218–224. [Google Scholar]
- Petit, E.; Gubler, W.D. Influence of Glomus intraradices on Black Foot Disease Caused by Cylindrocarpon macrodidymum on Vitis rupestris under Controlled Conditions. Plant Dis. 2006, 90, 1481–1484. [Google Scholar] [CrossRef]
- Alaniz, S.; Abad-Campos, P.; García-Jiménez, J.; Armengol, J. Evaluation of Fungicides to Control Cylindrocarpon liriodendri and Cylindrocarpon macrodidymum in Vitro, and Their Effect during the Rooting Phase in the Grapevine Propagation Process. Crop Prot. 2011, 30, 489–494. [Google Scholar] [CrossRef]
- Stephens, P.M.; Davoren, C.W.; Wicks, T. Effect of Methyl Bromide, Metham Sodium and the Biofumigants Indian Mustard and Canola on the Incidence of Soilborne Fungal Pathogens and Growth of Grapevine Nursery Stock. Australas. Plant Pathol. 1999, 28, 187–196. [Google Scholar] [CrossRef]
- Bleach, C.M.; Jones, E.E.; Jaspers, M.V. Biofumigation Using Brassicaceous Plant Products to Control Cylindrocarpon Black Foot Disease in New Zealand Soils. Phytopathol. Mediterr. 2010, 49, 10–1007. [Google Scholar]
- Pitt, W.M.; Sosnowski, M.R.; Qiu, Y.; Steel, C.C.; Savocchia, S. Evaluation of Fungicides for the Management of Botryosphaeria Canker of Grapevines. Plant Dis. 2012, 96, 1303–1308. [Google Scholar] [CrossRef]
- Spagnolo, A.; Mondello, V.; Larignon, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Fontaine, F. Defense Responses in Grapevine (Cv. Mourvèdre) after Inoculation with the Botryosphaeria Dieback Pathogens Neofusicoccum parvum and Diplodia seriata and Their Relationship with Flowering. Int. J. Mol. Sci. 2017, 18, 393. [Google Scholar] [CrossRef]
- Martínez-Diz, M.D.P.; Díaz-Losada, E.; Díaz-Fernández, Á.; Bouzas-Cid, Y.; Gramaje, D. Protection of Grapevine Pruning Wounds against Phaeomoniella chlamydospora and Diplodia seriata by Commercial Biological and Chemical Methods. Crop Prot. 2021, 143, 105465. [Google Scholar] [CrossRef]
- Larach, A.; Torres, C.; Riquelme, N.; Valenzuela, M.; Salgado, E.; Seeger, M.; Besoain, X. Yield Loss Estimation and Pathogen Identification from Botryosphaeria Dieback in Vineyards of Central Chile over Two Growing Seasons. Phytopathol. Mediterr. 2020, 59, 537–548. [Google Scholar] [CrossRef]
- Urbez-Torres, J.R.; Phillips, A.; Gubler, W. Botryosphaeria Dieback. In Compendium of Grape Diseases, Disorders, and Pests; American Phytopathological Society: Saint Paul, MN, USA, 2015; pp. 33–39. [Google Scholar]
- Lehoczky, J. Black Dead-Arm Disease of Grapevine Caused by Botryosphaeria stevensii Infection. Acta Phytopathol. Acad. Sci. Hung. 1974, 9, 319–327. [Google Scholar]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.O.; Phillips, A.J.L.; Alves, A.; Burgess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic Lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Urbez-Torres, J.R. The Status of Botryosphaeriaceae Species Infecting Grapevines. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B.; Cere, L.; Fulchic, R. Observation on Black Dead Arm in French Vineyards. Phytopathol. Mediterr. 2001, 40, S336–S342. [Google Scholar] [CrossRef]
- van Niekerk, J.M.; Fourie, P.H.; Halleen, F.; Crous, P.W. “Botryosphaeria” Spp. as Grapevine Trunk Disease Pathogens. Phytopathol. Mediterr. 2006, 45, S43–S54. [Google Scholar]
- Pitt, W.M.; Úrbez-Torres, J.R.; Trouillas, F.P. Dothiorella vidmadera, a Novel Species from Grapevines in Australia and Notes on Spencermartinsia. Fungal Divers. 2013, 61, 209–219. [Google Scholar] [CrossRef]
- Pitt, W.M.; Trouillas, F.P.; Gubler, W.D.; Savocchia, S.; Sosnowski, M.R. Pathogenicity of Diatrypaceous Fungi on Grapevines in Australia. Plant Dis. 2013, 97, 749–756. [Google Scholar] [CrossRef]
- Pitt, W.M.; Úrbez-Torres, J.R.; Trouillas, F.P. Dothiorella and Spencermartinsia, New Species and Records from Grapevines in Australia. Australas. Plant Pathol. 2015, 44, 43–56. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Akgül, D.S.; Perez, R.; Eskalen, A.; Gispert, C. First Report of Wood Canker Caused by Neoscytalidium dimidiatum on Grapevine in California. Plant Dis. 2013, 97, 1511. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Groenewald, J.Z.; Cheewangkoon, R.; Jami, F.; Abdollahzadeh, J.; Lombard, L.; Crous, P.W. Families, Genera, and Species of Botryosphaeriales. Fungal Biol. 2017, 121, 322–346. [Google Scholar] [CrossRef] [PubMed]
- Savocchia, S.; Steel, C.; Stodart, B.; Somers, A. Pathogenicity of Botryosphaeria Species Isolated from Declining Grapevines in Sub Tropical Regions of Eastern Australia. Vitis-Geilweilerhof 2007, 46, 27–32. [Google Scholar]
- Auger, J.; Esterio, M.; Ricke, G.; Pérez, I. Black Dead Arm and Basal Canker of Vitis vinifera Cv. Red Globe Caused by Botryosphaeria obtusa in Chile. Plant Dis. 2004, 88, 1286. [Google Scholar] [CrossRef] [PubMed]
- Kaliternam, J.; Milicevic, T.; Bencic, D.; Duralija, B. First Report of Neofusicoccum parvum Associated with Grapevine Trunk Diseases in Croatia. Plant Dis. 2013, 97, 1656. [Google Scholar] [CrossRef] [PubMed]
- Larignon, P.; Dubos, B. Le Black Dead Arm. Maladie Nouvelle à Ne Pas Confondre Avec l’esca. Phytoma 2001, 538, 26–29. [Google Scholar]
- Larignon, P.; Fontaine, F.; Farine, S.; Clément, C. Esca et Black Dead Arm: Deux Acteurs Majeurs des Maladies Du Bois Chez La Vigne. C R Biol. 2009, 332, 765–783. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Guerrero, J.C.; Guevara, J.; Gubler, W.D. Identification and Pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the Causal Agents of Bot Canker Disease of Grapevines in Mexico. Plant Dis. 2008, 92, 519–529. [Google Scholar] [CrossRef]
- Phillips, A.J.L. Botryosphaeria Species Associated with Diseases of Grapevines in Portugal. Phytopathol. Mediterr. 2002, 41, 3–18. [Google Scholar]
- van Niekerk, J.M.; Crous, P.W.; Groenewald, J.Z.; Fourie, P.H.; Halleen, F. DNA Phylogeny, Morphology and Pathogenicity of Botryosphaeria Species on Grapevines. Mycologia 2004, 96, 781–798. [Google Scholar] [CrossRef]
- Luque, J.; Elena, G.; Garcia-Figueres, F.; Reyes, J.; Barrios, G.; Legorburu, F.J. Natural Infections of Pruning Wounds by Fungal Trunk Pathogens in Mature Grapevines in Catalonia (Northeast Spain). Aust. J. Grape Wine Res. 2014, 20, 134–143. [Google Scholar] [CrossRef]
- Chacón-Vozmediano, J.L.; Gramaje, D.; Izquierdo, P.M.; Martínez, J.; Mena, A. Evaluation of Six Red Grapevine Cultivars Inoculated with Neofusicoccum parvum. Eur. J. Plant Pathol. 2020, 158, 811–815. [Google Scholar] [CrossRef]
- Masi, M.; Reveglia, P.; Femina, G.; Baaijens-Billones, R.; Savocchia, S.; Evidente, A. Luteoethanones A and B, Two Phytotoxic 1-Substituted Ethanones Produced by Neofusicoccum luteum, a Causal Agent of Botryosphaeria Dieback on Grapevine. Nat. Prod. Res. 2021, 35, 4542–4549. [Google Scholar] [CrossRef] [PubMed]
- Luque, J.; Martos, S.; Aroca, A.; Raposo, R.; Garcia-Figueres, F. Symptoms and Fungi Associated with Declining Mature Grapevine Plants in Northeast Spain. J. Plant Pathol. 2009, 91, 381–390. [Google Scholar]
- Amponsah, N.; Jones, E.; Ridgway, H.J.; Jaspers, M.V. Identification, Potential Inoculum Sources and Pathogenicity of Botryosphaeriaceous Species Associated with Grapevine Dieback Disease in New Zealand. Eur. J. Plant Pathol. 2011, 131, 467–482. [Google Scholar] [CrossRef]
- Abreo, E.; Martinez, S.; Bettucci, L.; Lupo, S. Characterization of Botryosphaeriaceae Species Associated with Grapevines in Uruguay. Australas. Plant Pathol. 2013, 42, 241–249. [Google Scholar] [CrossRef]
- Chebil, S.; Fersi, R.; Yakoub, A.; Chenenaoui, S.; Chattaoui, M.; Melki, I.; Zemni, H.; Rhouma, A.; Durante, G.; Zacchi, E.; et al. First Report of Botryosphaeria dothidea, Diplodia seriata, and Neofusicoccum luteum Associated with Canker and Dieback of Grapevines in Tunisia. Plant Dis. 2014, 98, 420. [Google Scholar] [CrossRef]
- Leavitt, G. The Occurrence, Distribution, Effects and Control of Botryodiplodia theobromae on Vitis vinifera in California, Arizona and Northern Mexico; University of California: Riverside, CA, USA, 1991. [Google Scholar]
- Úrbez-Torres, J.; Leavitt, G.; Voegel, T.M.; Gubler, W.D. Identification and Distribution of Botryosphaeria Spp. Associated with Grapevine Cankers in California. Plant Dis. 2006, 90, 1490–1503. [Google Scholar] [CrossRef]
- Stempien, E.; Goddard, M.L.; Leva, Y.; Bénard-Gellon, M.; Laloue, H.; Farine, S.; Kieffer-Mazet, F.; Tarnus, C.; Bertsch, C.; Chong, J. Secreted Proteins Produced by Fungi Associated with Botryosphaeria Dieback Trigger Distinct Defense Responses in Vitis vinifera and Vitis rupestris Cells. Protoplasma 2018, 255, 613–628. [Google Scholar] [CrossRef]
- Zhao, L.; You, S.; Zou, H.; Guan, X. Transcriptome Analysis and Cell Morphology of Vitis rupestris Cells to Botryosphaeria Dieback Pathogen Diplodia Seriata. Genes 2021, 12, 179. [Google Scholar] [CrossRef]
- Billones-Baaijens, R.; Jones, E.; Ridgway, H.; Jaspers, M. Susceptiblity of Common Rootstock and Scion Varieties of Grapevines to Botryosphaeriaceae Species. Australas. Plant Pathol. 2014, 43, 25–31. [Google Scholar] [CrossRef]
- Guan, X.; Essakhi, S.; Laloue, H.; Nick, P.; Bertsch, C.; Chong, J. Mining New Resources for Grape Resistance against Botryosphaeriaceae: A Focus on Vitis vinifera Subsp. Sylvestris. Plant Pathol. 2016, 65, 273–284. [Google Scholar] [CrossRef]
- Ulea, E. Declinul Plantatiilor Viticole. [The Decline of Grapevine Plantations-RO]; Ion Ionescu de la Brad: Iași, Romania, 2003; ISBN 973-7921-05-4. [Google Scholar]
- Úrbez-Torres, J.R.; Peduto, F.; Smith, R.J.; Gubler, W.D. Phomopsis Dieback: A Grapevine Trunk Disease Caused by Phomopsis viticola in California. Plant Dis. 2013, 97, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.L. Excoriose, Cane Blight and Related Diseases of Grapevines: A Taxonomic Review of the Pathogens. Phytopathol. Mediterr. 2000, 39, 341–356. [Google Scholar]
- Ephytia (INRAE) Grapevine-Phomopsis-Viticola-Excoriose. Available online: http://ephytia.inra.fr/en/C/6093/Grapevine-Phomopsis-viticola-Excoriose (accessed on 28 April 2022).
- Fabre, E.; Dunal, M. Observations Sur Les Maladies Régnantes de La Vigne; Typographie de Pierre Grollier: Montpellier, France, 1853. [Google Scholar]
- Ravaz, L.; Verge, G. Sur Une Maladie de La Vigne, l’Excoriose. Comptes Rendus Séances l’Académie Sci. 1925, 180, 313–315. [Google Scholar]
- Tomás, I.L.; Rego, M.C. Vida Rural; Abilways: Lisboa, Portugal, 1990; pp. 12–20. [Google Scholar]
- Bugaret, Y. Comment Lutter Efficacement Contre l’excoriose de La Vigne [Phomopsis viticola]. Progrès Agricole et Viticole 1987, 104, 121–126. [Google Scholar]
- Pearson, R.C.; Goheen, A. Phomopsis Cane and Leaf Spot. In Compendium of Grape Diseases; American Phytopathological Society: Saint Paul, MN, USA, 1994; pp. 17–18. [Google Scholar]
- Gregory, C.T. A Rot of Grapes Caused by Cryptosporella viticola. Phytopathology 1913, 3, 20–23. [Google Scholar]
- Bugaret, Y. L’excoriose de La Vigne: Recherches Sur Le Phomopsis viticola Sacc. Nouvelles Possibilites de Lutte. In Comptes Rendus de l’Académie d’Agriculture de France; L’Académie: Constanta, Romania, 1984. [Google Scholar]
- Phillips, A. Botryosphaeria dothidea and Other Fungi Associated with Excoriose and Dieback of Grapevines in Portugal. J. Phytopathol. 1998, 146, 327–332. [Google Scholar] [CrossRef]
- Arya, A.; Lal, B. Biochemical Changes in Grapes Infected with Phomopsis viticola. J. Plant Sci. 1986, 2, 53–59. [Google Scholar]
- Erincik, O.; Madden, L.V.; Ferree, D.C.; Ellis, M.A. Effect of Growth Stage on Susceptibility of Grape Berry and Rachis Tissues to Infection by Phomopsis viticola. Plant Dis. 2001, 85, 517–520. [Google Scholar] [CrossRef]
- Nita, M.; Ellis, M.A.; Madden, L.V. Variation in Disease Incidence of Phomopsis Cane and Leaf Spot of Grape in Commercial Vineyards in Ohio. Plant Dis. 2008, 92, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Reddick, D. Necrosis of the Grape Vine. Exp. Stn. Geneva 1909, 263, 323–343. [Google Scholar]
- Willison, R.S.; Chamberlain, G.C.; Townshend, J.L.; Ronde, J.H. de Epidemiology and Control of Dead-Arm of Grapes. Can. J. Bot. 1965, 43, 901–914. [Google Scholar] [CrossRef]
- Erincik, O.; Madden, L.V.; Ferree, D.C.; Ellis, M.A. Temperature and Wetness-Duration Requirements for Grape Leaf and Cane Infection by Phomopsis viticola. Plant Dis. 2003, 87, 832–840. [Google Scholar] [CrossRef]
- Anco, D.J.; Erincik, O.; Ellis, M.A. Phomopsis Cane and Leaf Spot of Grape. Available online: https://ohioline.osu.edu/factsheet/plpath-fru-47 (accessed on 2 June 2022).
- Rawnsley, B. Wine Australia for Australian Wine; Australian Government: Adelaide, Australia, 2012; pp. 1–5. [Google Scholar]
- Pearson, R.C.; Goheen, A.C. Compendium of Grape Diseases; American Phytopathological Society: Saint Paul, MN, USA, 1988. [Google Scholar]
- Ellis, M.; Welty, C.; Funt, R.C.; Doohan, D.; Wiliams, R.N.; Brown, M.; Bordelon, B. Midwest Small Fruit Pest Management Handbook; Ohio State University Extension: Columbus, OH, USA, 2004. [Google Scholar]
- Pscheidt, J.; Pearson, R.C. Time of Infection and Control of Phomopsis Fruit Rot of Grape. Plant Dis. 1989, 73, 829–833. [Google Scholar] [CrossRef]
- Carter, M.V. Eutypa Dieback. In Compendium of Grape Diseases; Pearson, R.C., Goheen, A.C., Eds.; APS Press: StPaul, MN, USA, 1988; pp. 32–34. [Google Scholar]
- Munkvold, G.; Duthie, J.A.; Marois, J.J. Reductions in Yield and Vegetative Growth of Grapevines Due to Eutypa Dieback. Phytopathology 1994, 84, 186–192. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Rolshausen, P.E.; Cantu, D. Draft Genome Sequence of the Grapevine Dieback Fungus Eutypa lata UCR-EL1. Genome Announc. 2013, 1, e00228-13. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Baumgartner, K.; Travadon, R.; Fujiyoshi, P.; Pouzoulet, J.; Wilcox, W.F. Identification of Eutypa spp. Causing Eutypa Dieback of Grapevine in Eastern North America. Plant Dis. 2014, 98, 483–491. [Google Scholar] [CrossRef]
- Carter, M.V. Further Studies on Eutypa armeniacae Hansf. & Carter. Aust. J. Agric. Res 1960, 11, 498–504. [Google Scholar]
- Moller, W.J.; Kasimatis, A.N. Dieback of Grapevines Caused by Eutypa armeniacae. Plant Dis. Rep. 1978, 62, 254–258. [Google Scholar]
- Pascoe, I. Grapevine Trunk Diseases-Black Goo Decline, Esca, Eutypa Dieback and Others. Aust. Grapegrow. Winemak. 1999, 429, 27–28. [Google Scholar]
- Sosnowski, M.R.; Creaser, M.L.; Wicks, T.J.; Lardner, R.; Scott, E.S. Protection of Grapevine Pruning Wounds from Infection by Eutypa lata. Aust. J. Grape Wine Res. 2008, 14, 134–142. [Google Scholar] [CrossRef]
- Rolshausen, P.; Mahoney, N.; Molyneux, R.J.; Gubler, W.D. A Reassessment of the Species Concept in Eutypa lata, the Causal Agent of Eutypa Dieback of Grapevine. Phytopathology 2006, 96, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Moyo, P.; Mostert, L.; Spies, C.F.J.; Damm, U.; Halleen, F. Diversity of Diatrypaceae Species Associated with Dieback of Grapevines in South Africa, with the Description of Eutypa cremea sp. Nov. Plant Dis. 2018, 102, 220–230. [Google Scholar] [CrossRef]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; el Ghadraoui, L.; Belabess, Z.; Fontaine, F.; el Hamss, H.; Amiri, S.; Lahlali, R.; et al. A Panoramic View on Grapevine Trunk Diseases Threats: Case of Eutypa Dieback, Botryosphaeria Dieback, and Esca Disease. Fungi 2022, 8, 595. [Google Scholar] [CrossRef]
- Ricard, J.L.; Grosclaude, C.; Ale-Agha, N. Antagonism between Eutypa armeniacae and Gliocladium roseum. Plant Dis. Rep. 1974, 58, 983–984. [Google Scholar]
- Péros, J.; Jamaux-Despréaux, I.; Berger, G. The Potential Importance of Diversity in Eutypa lata and Co-Colonising Fungi in Explaining Variation in Development of Grapevine Dieback. Mycol. Res. 1999, 103, 1385–1390. [Google Scholar] [CrossRef]
- Wagschal, I.; Abou-Mansour, E.; Petit, A.N.; Clément, C.; Fontaine, F. Wood Diseases of Grapevine: A Review on Eutypa Dieback and Esca. In Plant—Microbe Interactions; Barka, E.A., Clément, C., Eds.; Research Signpost: Kerala, India, 2008; pp. 367–391. ISBN 9788178953144. [Google Scholar]
- Carter, M.V. The Status of Eutypa lata as a Pathogen; Surrey: Caterham, UK, 1991. [Google Scholar]
- Travadon, R.; Rolshausen, P.E.; Gubler, W.; Sosnowski, M.R. Genetic Structure of the Fungal Grapevine Pathogen Eutypa lata from Four Continents. Plant Pathol. 2012, 61, 85–95. [Google Scholar] [CrossRef]
- Trouillas, F.; Gubler, W.D. Host Range, Biological Variation, and Phylogenetic Diversity of Eutypa lata in California. Am. Phytopath Soc. 2010, 100, 1048–1056. [Google Scholar] [CrossRef]
- Travadon, R.; Baumgartner, K. Molecular Polymorphism and Phenotypic Diversity in the Eutypa Dieback Pathogen Eutypa lata. Phytopathology 2015, 105, 255–264. [Google Scholar] [CrossRef]
- Pearson, R.C. Discharge of Ascospores of Eutypa armeniacae in New York. Plant Dis. 1980, 64, 171–174. [Google Scholar] [CrossRef]
- Trese, A.; Burton, C.; Ramsdell, D.C. Eutypa armeniacae in Michigan Vineyards: Ascospore Production and Survival, Host Infection, and Fungal Growth at Low Temperatures. Phytopathology 1980, 70, 788–793. [Google Scholar] [CrossRef]
- Moller, W.; Carter, M.V. Production and Dispersal of Ascospores in Eutypa armeniacae. Aust. J. Biol. Sci. 1965, 18, 67–80. [Google Scholar] [CrossRef]
- Mahoney, N.; Molyneux, R.; Smith, L.R. Dying-Arm Disease in Grapevines: Diagnosis of Infection with Eutypa lata by Metabolite Analysis. J. Agric. Food Chem. 2005, 53, 8148–8155. [Google Scholar] [CrossRef] [PubMed]
- Emmett, R.W.; Magarey, P.A. Eutypa Dieback. In Diseases and Pests; Royal Horticulture Society: London, UK, 2003; pp. 28–30. [Google Scholar]
- Rolshausen, P.E.; Mahoney, N.; Molyneux, R.J.; Gubler, W. Pathogenesis of Eutypa lata in Grapevine: Identification of Virulence Factors and Biochemical Characterization of Cordon Dieback. Phytopathology 2008, 98, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lardner, R.; Mahoney, N.; Zanker, T.P.; Molyneux, R.J.; Scott, E.S. Secondary Metabolite Production by the Fungal Pathogen Eutypa lata: Analysis of Extracts from Grapevine Cultures and Detection of Those Metabolites in Planta. Aust. J. Grape Wine Res. 2006, 12, 107–114. [Google Scholar] [CrossRef]
- Mahoney, N.; Lardner, R.; Molyneux, R.J.; Scott, E.S.; Smith, L.R.; Schoch, T.K. Phenolic and Heterocyclic Metabolite Profiles of the Grapevine Pathogen Eutypa lata. Phytochemistry 2003, 64, 475–484. [Google Scholar] [CrossRef]
- Gendloff, E.H.; Ramsdell, D.C.; Burton, C.L. Fungicidal Control of Eutypa armeniacae Infecting Concord Grapevine in Michigan. Plant Dis. 1983, 67, 754–756. [Google Scholar] [CrossRef]
- Moller, W.; Kasimatis, A.N. Protection of Grapevine Pruning Wounds from Eutypa Dieback. Plant Dis. 1980, 64, 278–280. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Marois, J.J. The Effects of Fungicides on Eutypa lata Germination, Growth, and Infection of Grapevines. Plant Dis. 1993, 77, 50–55. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Gubler, W.D. Use of Boron for the Control of Eutypa Dieback of Grapevines. Plant Dis. 2005, 89, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Matthee, F.; Thomas, A.C. Biological Control of Eutypa lata on Grapevine by an Antagonistic Strain of Bacillus Subtilis. Phytopathology 1991, 81, 283–287. [Google Scholar]
- Munkvold, G.; Marois, J. Efficacy of Natural Epiphytes and Colonizers of Grapevine Pruning Wounds for Biological Control of Eutypa Dieback. Phytopathology 1993, 83, 624–629. [Google Scholar] [CrossRef]
- Irelan, N.; Gubler, W.D.; DeScenzo, R. Efficacy Testing of Eutypa Chemical and Biological Control Candidates with DNA-Based Diagnostics. Pract. Winery Vineyard 1999, 19, 47–56. [Google Scholar]
- Kovács, C.S.; Sándor, E. The Increasing Importance of Grapevine Trunk Diseases. Int. J. Hortic. Sci. 2016, 22, 21–30. [Google Scholar] [CrossRef]
- Bortolami, G.; Gambetta, G.A.; Delzon, S.; Lamarque, L.J.; Pouzoulet, J.; Badel, E.; Burlett, R.; Charrier, G.; Cochard, H.; Dayer, S.; et al. Exploring the Hydraulic Failure Hypothesis of Esca Leaf Symptom Formation. Plant Physiol. 2019, 181, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- di Marco, S.; Cesari, A.; Calzarano, F.; Mazzullo, A. The Control of Esca: Status and Perspectives. Phytopathol. Mediterr. 2000, 39, 232–240. [Google Scholar] [CrossRef]
- Choueiri, E.; Jreijiri, F.; Chlela, P.; Mayet, V.; Comont, G.; Liminana, J.-M.; Mostert, L.; Fischer, M.; Lecomte, P. Fungal Community Associated with Grapevine Wood Lesions in Lebanon. OENO One 2014, 48, 293–302. [Google Scholar] [CrossRef]
- Foglia, R.; Landi, L.; Romanazzi, G. Analyses of Xylem Vessel Size on Grapevine Cultivars and Relationship with Incidence of Esca Disease, a Threat to Grape Quality. Appl. Sci. 2022, 12, 1177. [Google Scholar] [CrossRef]
- Larignon, P. Maladies Cryptogamiques Du Bois de La Vigne: Symptomatologie et Agents Pathogens; Institut Français de la Vigne et du Vin: Grau du Roi dans le Gard, France, 2012; Volume 2. [Google Scholar]
- Ravaz, L. Sur Le Folletage. Rev. De Vitic. 1898, 10, 184–186. [Google Scholar]
- Viala, P. Recherches Sur Les Maladies de La Vigne. Esca. Ann. Des Epiphyt. Fasc. 1926, 1, 1–108. [Google Scholar]
- Bruez, E.; Vallance, J.; Gerbore, J.; Lecomte, P.; da Costa, J.-P.; Guerin-Dubrana, L.; Rey, P. Analyses of the Temporal Dynamics of Fungal Communities Colonizing the Healthy Wood Tissues of Esca Leaf-Symptomatic and Asymptomatic Vines. PLoS ONE 2014, 9, e95928. [Google Scholar] [CrossRef]
- Kubátová, A.; Kolařík, M.; Pažoutová, S. Phaeoacremonium rubrigenum—Hyphomycete Associated with Bark Beetles Found in Czechia. Folia Microbiol. 2004, 49, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Essakhi, S.; Mugnai, L.; Crous, P.W.; Groenewald, J.Z.; Surico, G. Molecular and Phenotypic Characterisation of Novel Phaeoacremonium Species Isolated from Esca Diseased Grapevines. Pers. Mol. Phylogeny Evol. Fungi 2008, 21, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Ashnaei, S.P. Grapevine, Esca Complex, and Environment: The Disease Triangle. Phytopathol. Mediterr. 2019, 58, 17–37. [Google Scholar] [CrossRef]
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What If Esca Disease of Grapevine Were Not a Fungal Disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef]
- Borie, B.; Jacquiot, L.; Jamaux-Despréaux, I.; Larignon, P.; Péros, J.P. Genetic Diversity in Populations of the Fungi Phaeomoniella Chlamydospora and Phaeoacremonium aleophilum on Grapevine in France. Plant Pathol. 2002, 51, 85–96. [Google Scholar] [CrossRef]
- Aroca, Á.; Gramaje, D.; Armengol, J.; García-Jiménez, J.; Raposo, R. Evaluation of the Grapevine Nursery Propagation Process as a Source of Phaeoacremonium spp. and Phaeomoniella chlamydospora and Occurrence of Trunk Disease Pathogens in Rootstock Mother Vines in Spain. Eur. J. Plant Pathol. 2010, 126, 165–174. [Google Scholar] [CrossRef]
- Latinovic, N.; Vucinic, Z.; Latinovic, J. Phaeoacremonium aleophilum One of the Causal Agents of Esca Disease of Grapevine in Montenegro. In Proceedings of the 7th Conference on Plant Protection, Soko Banja, Serbia, 15 November 2005; pp. 125–126. [Google Scholar]
- Gramaje, D.; Armengol, J.; Mohammadi, H.; Banihashemi, Z.; Mostert, L. Novel Phaeoacremonium Species Associated with Petri Disease and Esca of Grapevine in Iran and Spain. Mycologia 2009, 101, 920–929. [Google Scholar] [CrossRef]
- Gubler, W.; Eskalen, A.; Feliciano, A.; Eskalen, A. Pathogenicity of “Phaeoacremonium aleophilum” and “Phaeomoniella chlamydospora” on Grape Berries in California. Phytopathol. Mediterr. 2004, 43, 70–74. [Google Scholar]
- Ciccarone, C.; Marras, F.; Schiaffino, A.; Marras, F. Molecular Analysis of “Fomitiporia Mediterranea” Isolates from Esca-Affected Grapevines in Southern Italy. Phytopathol. Mediterr. 2004, 43, 268–272. [Google Scholar]
- Lehoczky, J.; Szabolcs, M. A Szolotokek Sztereumos Elhalasa. Kertgazdasag 1983, 15, 53–66. [Google Scholar]
- Nieder, G.; Reisenzein, H.; Berger, N. Esca in Austria. Phytopathol. Mediterr. 2000, 39, 26–34. [Google Scholar]
- Cloete, M.; Fischer, M.; Mostert, L.; Halleen, F. Hymenochaetales Associated with Esca-Related Wood Rots on Grapevine with a Special Emphasis on the Status of Esca in South African Vineyards. Phytopathol. Mediterr. 2015, 54, 299–312. [Google Scholar] [CrossRef]
- Surico, G.; Mugnai, L.; Marchi, G. Older and More Recent Observations on Esca: A Critical Overview. Phytopathol. Mediterr. 2006, 45, S68–S86. [Google Scholar]
- Bortolami, G.; Gambetta, G.A.; Cassan, C.; Dayer, S.; Farolfi, E.; Ferrer, N.; Gibon, Y.; Jolivet, J.; Lecomte, P.; Delmas, C.E.L. Grapevines under Drought Do Not Express Esca Leaf Symptoms. Proc. Natl. Acad. Sci. USA 2021, 118, e2112825118. [Google Scholar] [CrossRef]
- Graniti, A.; Surico, G.; Mugnai, L. Esca of Grapevine. A Disease Complex or a Complex of Diseases? Phytopathol. Mediterr. 2000, 39, 16–20. [Google Scholar]
- Maher, N.; Piot, J.; Bastien, S.; Vallance, J.; Rey, P.; Guérin-Dubrana, L. Wood Necrosis in Esca-Affected Vines: Types, Relationships and Possible Links with Foliar Symptom Expression. OENO One 2012, 46, 15–27. [Google Scholar]
- Chiarappa, L. Phellinus ignarius: The Cause of Spongy Wood Decay of Black Measles (“Esca”) Disease of Grapevines. Phytopathol. Mediterr. 1997, 36, 109–111. [Google Scholar]
- Fischer, M. A New Wood-Decaying Basidiomycete Species Associated with Esca of Grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol. Prog. 2002, 1, 315–324. [Google Scholar] [CrossRef]
- Fischer, M.; Kassemeyer, H.H. Fungi Associated with Esca Disease of Grapevine in Germany. Vitis-Geilweilerhof 2003, 42, 109–116. [Google Scholar]
- Baumgartner, K.; Hillis, V.; Lubell, M.; Norton, M.; Kaplan, J. Managing Grapevine Trunk Diseases in California’s Southern San Joaquin Valley. Am. J. Enol. Vitic 2019, 70, 267–276. [Google Scholar] [CrossRef]
- Mazzullo, A.; di Marco, S.; Cesari, A. Interazione in Vitro Di Fitoalessine Della Vite e Di Acido Fosforoso Con Funghi Associati al “Mal Dell’esca” Della Vite. In Proceedings of the Atti del Convegno “Aspetti molecolari e fisiologici dell’interazione ospite patogeno”, Ragusa, Italy, 31 May 1996. [Google Scholar]
- di Marco, S.; Mazzullo, A.; Calzarano, F.; Cesari, A. In Vitro Studies on the Phosphorous Acid—Vitis stilbenes Interaction, and in Vivo Phosetyl Al Activity towards Phaeoacremonium spp. Grapevine Wood Decay Agents. In Modern fungicides and antifungal compounds II, Proceedings of the 12th International Reinhardsbrunn Symposium, Friedrichroda, Thuringia, Germany, 24–29 May 1998; Intercept Limited: London, UK, 1999; pp. 171–177. [Google Scholar]
- Díaz, G.A.; Auger, J.; Besoain, X.; Bordeu, E.; Latorre, B.A. Prevalence and Pathogenicity of Fungi Associated with Grapevine Trunk Diseases in Chilean Vineyards. Cienc. Investig. Agrar. 2013, 40, 327–339. [Google Scholar] [CrossRef]
- di Marco, S.; Osti, F.; Calzarano, F.; Roberti, R.; Veronesi, A.; Amalfitano, C. Effects of Grapevine Applications of Fosetyl-Aluminium Formulations for Downy Mildew Control on “Esca” and Associated Fungi. Phytopathol. Mediterr. 2011, 50, S285–S299. [Google Scholar]
- Fourie, P.; Halleen, F. Chemical and Biological Protection of Grapevine Propagation Material from Trunk Disease Pathogens. Eur. J. Plant Pathol. 2006, 116, 255–265. [Google Scholar] [CrossRef]
- Calzarano, F.; di Marco, S.; D’agostino, V.; Schiff, S.; Mugnai, L. Grapevine Leaf Stripe Disease Symptoms (Esca Complex) Are Reduced by a Nutrients and Seaweed Mixture. Phytopathol. Mediterr. 2014, 53, 543–558. [Google Scholar] [CrossRef]
- Calzarano, F.; Osti, F.; D’Agostino, V.; Pepe, A.; di Marco, S. Mixture of Calcium, Magnesium and Seaweed Affects Leaf Phytoalexin Contents and Grape Ripening on Vines with Grapevine Leaf Stripe Disease. Phytopathol. Mediterr. 2017, 56, 445–457. [Google Scholar] [CrossRef]
- Calzarano, F.; di Marco, S. Further Evidence That Calcium, Magnesium and Seaweed Mixtures Reduce Grapevine Leaf Stripe Symptoms and Increase Grape Yields. Phytopathol. Mediterr. 2018, 57, 459–471. [Google Scholar] [CrossRef]
- Mazzullo, A.; Cesari, A.; Osti, F.; di Marco, S. Bioassays on the Activity of Resveratrol, Pterostilbene and Phosphorous Acid towards Fungi Associated with Esca of Grapevine. Phytopathol. Mediterr. 2000, 39, 357–365. [Google Scholar]
- Santos, C.; Fragoeiro, S.; Oliveira, H.; Phillips, A. Response of Vitis vinfifera L. Plants Inoculated with Phaeoacremonium angustius and Phaeomoniella chlamydospora to Thiabendazole, Resveratrol and Sodium Arsenite. Sci. Hortic. 2006, 107, 131–136. [Google Scholar] [CrossRef]
- Cobos, R.; Mateos, R.M.; Álvarez-Pérez, J.M.; Olego, M.A.; Sevillano, S.; González-García, S.; Garzón-Jimeno, E.; Coque, J.J.R. Effectiveness of Natural Antifungal Compounds in Controlling Infection by Grapevine Trunk Disease Pathogens through Pruning Wounds. Appl. Environ. Microbiol. 2015, 81, 6474–6483. [Google Scholar] [CrossRef] [PubMed]
- Mounier, E.; Boulisset, F.; Cortes, F.; Cadiou, M.; Dubournet, P.; Pajot, E. Esquive® WP Limits Development of Grapevine Trunk Diseases and Safeguards the Production Potential of Vineyards. In Biocontrol of Major Grapevine Diseases: Leading Research; CAB International: Wallingford, UK, 2016; pp. 160–170. [Google Scholar]
- Yacoub, A.; Gerbore, J.; Magnin, N.; Chambon, P.; Dufour, M.C.; Corio-Costet, M.F.; Rey, P. Ability of Pythium oligandrum Strains to Protect Vitis vinifera L., by Inducing Plant Resistance against Phaeomoniella chlamydospora, a Pathogen Involved in Esca, a Grapevine Trunk Disease. Biol. Control 2016, 92, 7–16. [Google Scholar] [CrossRef]
- Chedea, V.; Drăgulinescu, A.-M.; Tomoiagă, L.; Bălăceanu, C.; Iliescu, M. Climate Change and Internet of Things Technologies—Sustainable Premises of Extending the Culture of the Amurg Cultivar in Transylvania—A Use Case for Târnave Vineyard. Sustainability 2021, 13, 8170. [Google Scholar] [CrossRef]
- Administrația Națională de Meteorologie [National Meteorological Administration-RO] Clima Romaniei [Romania’s Climate-RO]. Available online: https://www.meteoromania.ro/clima/clima-romaniei/ (accessed on 6 June 2022).
- Bǎlteanu, D.; Chendeş, V.; Sima, M.; Enciu, P. A Country-Wide Spatial Assessment of Landslide Susceptibility in Romania. Geomorphology 2010, 124, 102–112. [Google Scholar] [CrossRef]
- Hitchins, K. Romania. Available online: https://www.britannica.com/place/Romania/Settlement-patterns (accessed on 15 March 2022).
- Oficiul National al Viei si Produselor Vitivinicole [National Office of Wine and Wine Products-RO] Harta Vinului Românesc [Romanian Wine Map-RO]. Available online: http://storage0.dms.mpinteractiv.ro/media/1/1481/21335/13386089/3/12-regiuni-harta.jpg (accessed on 10 March 2022).
- Crişan Aurelia Micromycete Noi Pentru Flora Micologică a României [New Micromycetes for the Mycological Flora of Romania-RO]. Contribuţii Botanice 1962, 1, 45–51.
- Mărmureanu, M.; Dumitru, C.; Oprea, M. Bioecologia, Transmiterea Şi Combaterea Ciupercii Phomopsis viticola Sacc. [Bioecology, Transmission and Control of the Fungus Phomopsis viticola Sacc—RO]. Horticultura 1990, 39, 26–30. [Google Scholar]
- Rafailă, C. Excorioza—O Boală Păgubitoare a Viţei de Vie. [Excoriosis—A Harmful Disease of Grapevine—RO]. Revista de Horticultura si Viticultura 1970, 19, 78–80. [Google Scholar]
- Oprea, M.; Dumitru, C. Cercetări Privind Ciupercile Patogene Implicate În Declinul Viţei de Vie. [Research on Pathogenic Fungi Involved in Grapevine Decline—RO]. An. ICPP Bucureşti 1989, 22, 11–24. [Google Scholar]
- Podosu, A.; Oprea, M. Phomopsis viticola Sacc, Ciupercă Implicată În Declinul Viţei de Vie.Combatere. [Phomopsis viticola Sacc, Fungus Involved in the Grapevine Decline-RO]. An. ASAS 1999, 2, 24. [Google Scholar]
- Podosu, A.; Oprea, M.; Tică, C.; Oancea, V. Studii Privind Rolul Ciupercilor Lignicole În Uscarea Prematură a Viţei de Vie. [Studies on the Role of Wood Fungi in the Premature Drying of Vines—RO]. Lucr. Științifice USAMV Iași 1998, 41, 320–327. [Google Scholar]
- Tică, C.; Şesan, T.; Oprea, M. Cercetări Privind Biologia Şi Combaterea Excoriozei Viţei de Vie (Phomopsis viticola Sacc.), În Codiţiile Podgoriilor Din Vrancea. [Research on the Biology and Control of Vine Excoriosis (Phomopsis viticola Sacc.), in the Vineyards of Vrancea—RO]; Institutul de Cercetare Dezvoltare Pentru Viticultura si Vinificatie: Călugărească, Romania, 1994; Volume 14. [Google Scholar]
- Ulea, V. Contribuţii La Studiul Ciupercilor Parazite Şi Saprofite Care Atacă Scoarţa Şi Lemnul Butucilor de Viţă de Vie Cu Privire Specială Asupra Excoriozei, Produsă de Phomopsis viticola Sacc. [Contributions to the Study of Parasitic and Saprophytic Fungi That Attack the Bark and Wood of Vines with a Special Focus on Excoriation, Produced by Phomopsis viticola Sacc—RO]; USAMV: Iaşi, Romania, 1997. [Google Scholar]
- Stoica, M.; Ulea, E. Cauzele Pieirii Premature a Butucilor În Plantaţiile Viticole Din Moldova. [Causes of Premature Loss of Vines in Moldovan Vineyards—RO]; An. ICDVV: Valea Calugareasca, Romania, 2004; Volume 17, pp. 213–219. [Google Scholar]
- Oprea, M.; Podosu, A. Grape Dieback in Romania Induced by Pathogenic Lignicoulus Fungi. Fruit Grow. Technol. 2008, 52, 128–133. [Google Scholar]
- Tomoiagă, L.; Oroian, I. Identifying and Setting the Parameters of the Risk Factors Involved in the Process of Biological Decline of the Vines in Romania. J. Environ. Prot. Ecol. 2012, 13, 1350–1356. [Google Scholar]
- Comșa, M.L.; Cudur, F.; Cudur, C.; Cristea, C. Research on Some Pathogenic Fungi Involved in the Biological Decline of the Grapevine at the Blaj Viticultural Centre. In Seria Horticultură; Universitatea de Științe Agricole și Medicină Veterinară “Ion Ionescu de la Brad”: Iași, Romania, 2012; pp. 503–508. [Google Scholar]
- Comșa, M.; Tomoiagă, L.; Popescu, D.; Cristea, C. Researches Regarding the Eutypa lata Lignicole Fungus Manifestation in Vineyards from Blaj Wine Centre. Bull. UASVM Hortic. 2014, 71, 2. [Google Scholar] [CrossRef][Green Version]
- Comșa, M.; Tomoiagă, L.; Botea, V.; Sîrbu, A.; Dobromir, D. Identification by Plate Culture Method of the Fungal Pathogens Causing the Grapevine Trunk Diseases in Romanian Vineyards. Rom. J. Hortic. 2021, 2, 137–142. [Google Scholar] [CrossRef]
- Savu, S.; Tomoiaga, L.L.; Chedea, V.S. Ecological Microclimate Influence on Grapevine Phomopsis viticola Attack Frequency in Aiud-Ciumbrud Vineyards. Bull. USAMV Cluj-Napoca. Hortic. 2020, 77, 1843–5394. [Google Scholar] [CrossRef]
- Botea, V.; Tomoiagă, L.L.; Vasiu, I.; Sîrbu, A.; Răcoare, H.S.; Chedea, V.S. Phomopsis viticola Management in Târnave Vineyards. Rom. J. Hortic. 2022; in press. [Google Scholar]
- Tică, C.; Şesan, T.; Oprea, M. Excorioza (Phomopsis viticola), Boală de Importanţă Majoră a Viţei de Vie. [Excoriosis (Phomopsis viticola), a Grapevine Disease of Major Importance—RO]. Probleme Protecţia Plantelor 1994, 22, 21–51. [Google Scholar]
- Tomoiaga, L.; Oprea, M.; Podosu, A.; Voiculescu, I.; Stoica, C.; Florian, V. Grape Dieback in Romania Induced by Pathogenic Lignicoulus Fungi. Rom. J. Plant Prot. 2008, 1, 1–15. [Google Scholar]
- Tomoiaga, L.; Chedea, V.S. Grapevine Trunk Diseases Management in Vineyards from Central Transylvania. Bull. USMV Cluj-Napoca. Hortic. 2020, 77, 117–121. [Google Scholar] [CrossRef]
- Iacob, V. Bolile Plantelor Cultivate—Prevenire Și Combatere. [Diseases of Cultivated Plants—Prevention and Control—RO]; Editura “Ion Ionescu de la Brad”: Iaşi, Romania, 2006; ISBN 632. [Google Scholar]
- Mirică, I.; Mirică, A. Protecția Viței-de-Vie Împotriva Bolilor Și Dăunătorilor [Grapevine Protection against Diseases and Pests—RO]; Editura CERES: București, Romania, 1986. [Google Scholar]
- Mirică, I.; Mărmureanu, M.; Oprea, M.; Dumitru, C.; Mirică, A.; Filip, I.; Julei, S. Researches on Bioecology, Transmission and Control of Eutypa lata Fungi. Prod. Veg. Hortic. 1989, 2, 35–39. [Google Scholar]
- Podosu, A.; Mihu, G.; Stoian, I. Research Concerning the Biology of the Grapevine Excoriosis (Phomopsis viticola SACC.) under the Conditions of the Vineyards in Vrancea. Lucr. Științifice USAMV Iași Ser. Hortic. 2008, 51, 1197–1204. [Google Scholar]
- Bulit, J.; Bugaret, Y.; Verdu, D. On the Possibilities on Conservation in Winter of Botrytis Cinerea Pers. and Phomopsis viticola Sacc. in the Buds of the Grapevine. Revue Zool. Agricole et Pathol. Vegetale 1973, 1, 1–12. [Google Scholar]
- Bugaret, Y. New Facts on Phomopsis Cane Blight and Leaf Spot Epidemiology and Their Consequences for the Control-French. Phytoma 1986, 375, 36–41. [Google Scholar]
- Matei, P.; Iacomi, B.; Dragan, G. Fungi Associated with Esca Decline and Their in Vitro Control by Chitosan. Sci. Pap. UASVM Buchar. 2010, 53, 448–453. [Google Scholar]
- di Marco, S.; Osti, F.; Cesari, A. Experiments on the Control of Esca by Trichoderma. Phytopathol. Mediterr. 2004, 43, 108–115. [Google Scholar]
- Smart, R. Trunk Diseases: Timely Trunk Renewal to Overcome Trunk Disease. Wine Vitic. J. 2015, 30, 46–47. [Google Scholar]



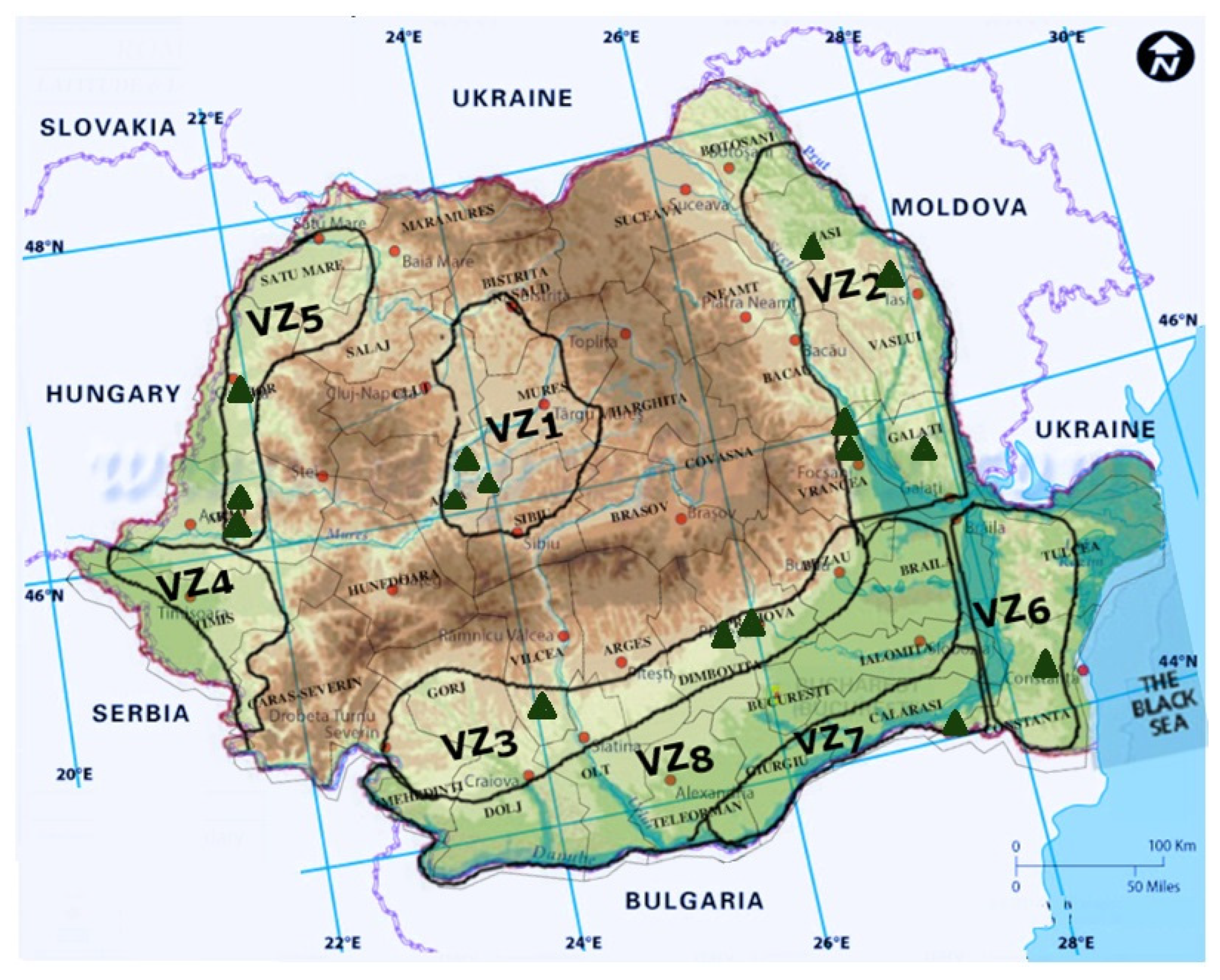

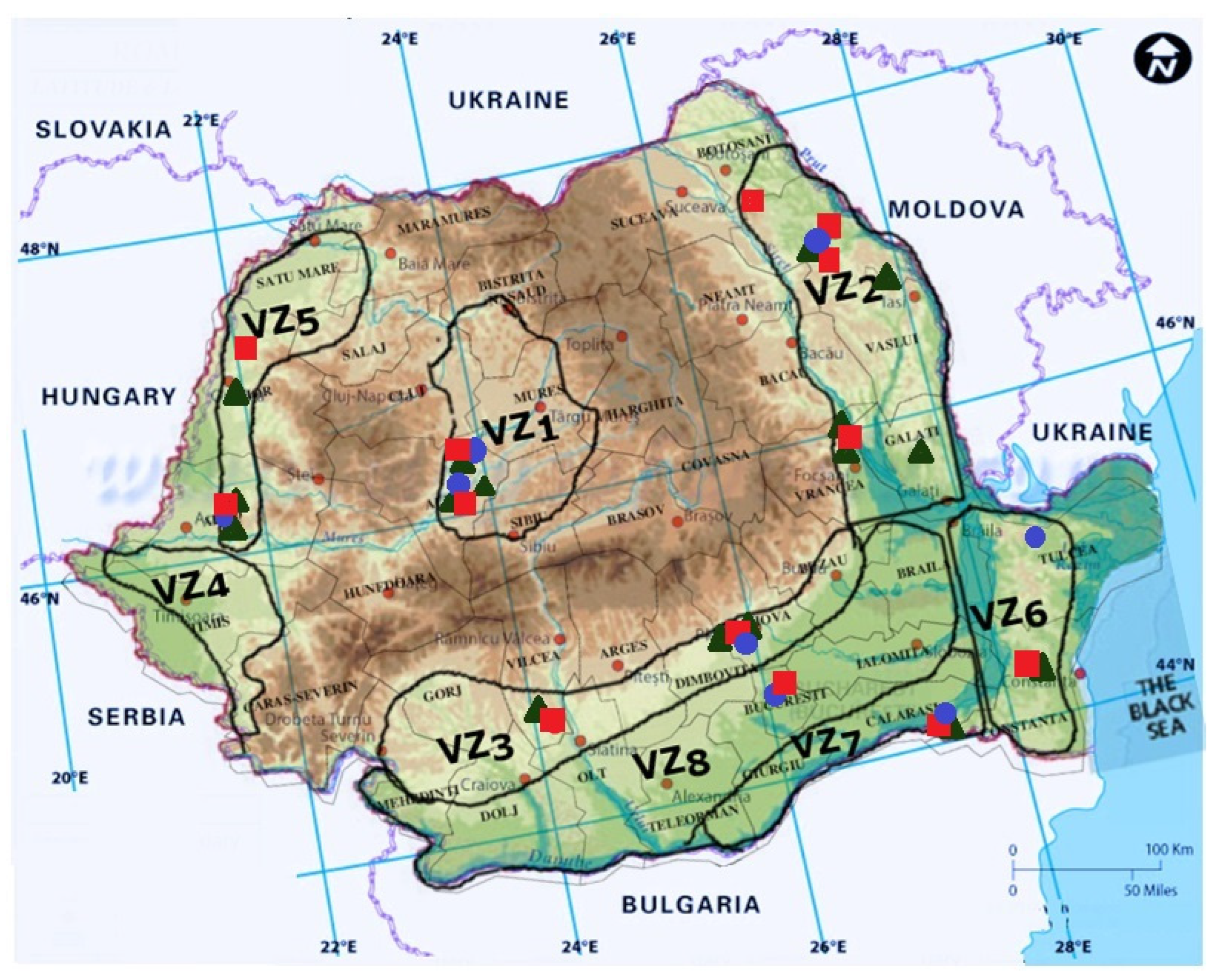
| Viticultural Zone | Harvested Area [ha] | Vineyards | Characteristics |
|---|---|---|---|
| Transylvanian Plateau (VZ1) | 6800 | Târnave |
|
| Alba | |||
| Sebeş-Apold | |||
| Ciumbrud-Aiud | |||
| Lechinţa | |||
| Moldavian Hills (VZ2) | 69,134 | Cotnari |
|
| Iaşi | |||
| Huşi | |||
| Colinele Tutovei | |||
| Dealul Bujorului | |||
| Nicoreşti | |||
| Iveşti | |||
| Covurlui | |||
| Zeletin | |||
| Panciu | |||
| Odobeşti | |||
| Coteşti | |||
| Muntenia and Oltenia Hills (VZ3) | 53,450 | Dealurile Buzăului |
|
| Dealu Mare | |||
| Ştefăneşti | |||
| Sâmbureşti | |||
| Drăgăşani | |||
| Dealurile Craiovei | |||
| Podgoria Severin | |||
| Plaiurile Drâncei | |||
| Banat Hills (VZ4) | 2930 | Dealurile Banatului |
|
| Crişana and Maramureş Hills (VZ5) | 9100 | Miniş-Măderat |
|
| Diosig | |||
| Valea lui Mihai | |||
| Podgoria Silvaniei | |||
| Dobrogea Hills (VZ6) | 17,564 | Sarica-Niculiţel |
|
| Istria-Babadag | |||
| Murfatlar | |||
| Danube Terraces (VZ7) | 11,234 | Ostrov |
|
| Greaca | |||
| Sands and other suitable terrains from the South (VZ8) | 12,960 | Podgoria Dacilor |
|
| Calafat | |||
| Sadova-Corabia |
| Crt. No. | Date | Commercial Name | Active Substance |
|---|---|---|---|
| 1 | 14 May 2020 | Fantic M 0.25% + Thiovit Jet 80 WG 0.3% | Benalaxyl-M 4% + Mancozeb 65% Wetable sulfur 80% |
| 2 | 22 May 2020 | Polyram DF 0.25% + Topas 100 ec 0.025% | Metiram 70% Penconazol 100 g/L |
| 3 | 28 May 2020 | Equation PRO 0.04% + Topas 100 ec 0.025% | Cimoxanil 30% + Fomaxadon 22.5% Penconazol 100 g/L |
| 4 | 11 June 2020 | Universalis 593.5 SC 0.2% | Azoxistrobin 93.5 g/L + Folpet 500 g/L |
| 5 | 19 June 2020 | Mikal Flasch 75 WG 0.3% + Flint Max 75 WG 0.016% | Trifloxistrobin 25% + Tebuconazol 50% Aluminum fosetil 50% + Folpet 25% |
| 6 | 29 June 2020 | Mikal Flasch 75 WG 0.3% | Aluminum fosetil% + Folpet 25% |
| 7 | 9 July 2020 | Valis M 0.2% | Mancozeb 60% + Valifenalat 6% |
| 8 | 19 July 2020 | Triumf 0.25% | Copper hydroxide 40% |
| Treatment No. | Active Substance |
|---|---|
| 13 March 2020-first Phomopsis viticola frequency evaluation | |
| 1 | Copper Sulphate Pentahidrate + Sulphur 80% |
| 2 | Copper hydroxide + 50% Metallic copper. Abamectin 18 g/L Sulphur 80% |
| 3 | 55% Metiram, 5% Pyraclostrobin Sulphur 80% |
| 4 | Fosetyl-aluminum 50% + Folpet 25% Sulphur 80% Cypermethrin (100 g/L) 240 g/L Myclobutanil Cyclohexanone |
| 5 | Trifloxystrobin 250 g/kg + Tebuconazole 500 g/kg Fosetyl-aluminum + Fluopicolide |
| 6 | Fosetyl-aluminum 50% + Folpet 25% 240 g/L Myclobutanil Cyclohexanone Cypermethrin (100 g/L) Boron 15%-ethanolamine |
| 27 June 2020-second Phomopsis viticola frequency evaluation | |
| 7 | 65% Mancozeb + 4% Benalaxil M + Metrafenone 500 g/L + Sulphur 80% |
| 8 | 5% Mandipropamid 40% Folpet Sulphur 80% 300 g/L Fluxapiroxad |
| 9 | 5% Mandipropamid 40% Folpet Sulphur 80% Fluopyram 75 g/L + Spiroxamina 200 g/L 500 g/L Clofentezine + Copper hydroxide + 50% Metallic Copper |
| 10 | Sulphur 80% + Copper sulphate pentahydrate |
| 16 August 2020-third Phomopsis viticola frequency evaluation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, M.-D.; Drăgulinescu, A.-M.; Tomoiagă, L.L.; Comșa, M.; Răcoare, H.-S.; Sîrbu, A.D.; Chedea, V.S. Fungal Grapevine Trunk Diseases in Romanian Vineyards in the Context of the International Situation. Pathogens 2022, 11, 1006. https://doi.org/10.3390/pathogens11091006
Muntean M-D, Drăgulinescu A-M, Tomoiagă LL, Comșa M, Răcoare H-S, Sîrbu AD, Chedea VS. Fungal Grapevine Trunk Diseases in Romanian Vineyards in the Context of the International Situation. Pathogens. 2022; 11(9):1006. https://doi.org/10.3390/pathogens11091006
Chicago/Turabian StyleMuntean, Maria-Doinița, Ana-Maria Drăgulinescu, Liliana Lucia Tomoiagă, Maria Comșa, Horia-Silviu Răcoare, Alexandra Doina Sîrbu, and Veronica Sanda Chedea. 2022. "Fungal Grapevine Trunk Diseases in Romanian Vineyards in the Context of the International Situation" Pathogens 11, no. 9: 1006. https://doi.org/10.3390/pathogens11091006
APA StyleMuntean, M.-D., Drăgulinescu, A.-M., Tomoiagă, L. L., Comșa, M., Răcoare, H.-S., Sîrbu, A. D., & Chedea, V. S. (2022). Fungal Grapevine Trunk Diseases in Romanian Vineyards in the Context of the International Situation. Pathogens, 11(9), 1006. https://doi.org/10.3390/pathogens11091006





