Linear Peptide Epitopes Derived from ErpP, p35, and FlaB in the Serodiagnosis of Lyme Disease
Abstract
1. Background
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Epitope Mapping and Peptide Sequences
4.3. ELISA
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steere, A.C. Lyme disease. N. Engl. J. Med. 2001, 345, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Sikand, V.K. The presenting manifestations of Lyme disease and the outcomes of treatment. N. Engl. J. Med. 2003, 348, 2472–2474. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, A.F.; Connally, N.P.; Meek, J.I.; Johnson, B.J.; Kemperman, M.M.; Feldman, K.A.; White, J.L.; Mead, P.S. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis. 2014, 59, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef]
- AnonymousCase definitions for infectious conditions under public health surveillance. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 1997, 46, 1–55.
- Berger, B.W. Erythema migrans. Clin. Dermatol. 1993, 11, 359–362. [Google Scholar] [CrossRef]
- Nowakowski, J.; Schwartz, I.; Liveris, D.; Wang, G.; Aguero-Rosenfeld, M.E.; Girao, G.; McKenna, D.; Nadelman, R.B.; Cavaliere, L.F.; Wormser, G.P. Lyme Disease Study Group Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: A comparison of different techniques. Clin. Infect. Dis. 2001, 33, 2023–2027. [Google Scholar] [CrossRef]
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 2005, 18, 484–509. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 1995, 44, 590–591. [Google Scholar]
- Coulter, P.; Lema, C.; Flayhart, D.; Linhardt, A.S.; Aucott, J.N.; Auwaerter, P.G.; Dumler, J.S. Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme disease. J. Clin. Microbiol. 2005, 43, 5080–5084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wormser, G.P.; Aguero-Rosenfeld, M.E.; Nadelman, R.B. Lyme disease serology: Problems and opportunities. JAMA 1999, 282, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.; Petersen, J.; Hinkley, A. Updated CDC Recommendation for Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 703. [Google Scholar] [CrossRef]
- Waddell, L.A.; Greig, J.; Mascarenhas, M.; Harding, S.; Lindsay, R.; Ogden, N. The Accuracy of Diagnostic Tests for Lyme Disease in Humans, A Systematic Review and Meta-Analysis of North American Research. PLoS ONE 2016, 11, e0168613. [Google Scholar] [CrossRef] [PubMed]
- Bacon, R.M.; Biggerstaff, B.J.; Schriefer, M.E.; Gilmore, R.D.; Philipp, M.T.; Steere, A.C.; Wormser, G.P.; Marques, A.R.; Johnson, B.J.B. Serodiagnosis of Lyme Disease by Kinetic Enzyme-Linked Immunosorbent Assay Using Recombinant VlsE1 or Peptide Antigens of Borrelia burgdorferi Compared with 2-Tiered Testing Using Whole-Cell Lysates. J. Infect. Dis. 2003, 187, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.T.; Steere, A.C.; Marques, A.R.; Johnson, B.J.; Miller, J.N.; Philipp, M.T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J. Clin. Microbiol. 1999, 37, 3990–3996. [Google Scholar] [CrossRef]
- Gomes-Solecki, M.J.; Meirelles, L.; Glass, J.; Dattwyler, R.J. Epitope length, genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin. Vaccine Immunol. 2007, 14, 875–879. [Google Scholar] [CrossRef]
- Heikkila, T.; Huppertz, H.I.; Seppala, I.; Sillanpaa, H.; Saxen, H.; Lahdenne, P. Recombinant or peptide antigens in the serology of Lyme arthritis in children. J. Infect. Dis. 2003, 187, 1888–1894. [Google Scholar] [CrossRef]
- Arnaboldi, P.M.; Seedarnee, R.; Sambir, M.; Callister, S.M.; Imparato, J.A.; Dattwyler, R.J. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early lyme disease. Clin. Vaccine Immunol. 2013, 20, 474–481. [Google Scholar] [CrossRef]
- Signorino, G.; Arnaboldi, P.M.; Petzke, M.M.; Dattwyler, R.J. Identification of OppA2 Linear Epitopes as Serodiagnostic Markers for Lyme Disease. Clin. Vaccine Immunol. 2014, 21, 704–711. [Google Scholar] [CrossRef][Green Version]
- Embers, M.E.; Jacobs, M.B.; Johnson, B.J.; Philipp, M.T. Dominant epitopes of the C6 diagnostic peptide of Borrelia burgdorferi are largely inaccessible to antibody on the parent VlsE molecule. Clin. Vaccine Immunol. 2007, 14, 931–936. [Google Scholar] [CrossRef][Green Version]
- Sillanpaa, H.; Lahdenne, P.; Sarvas, H.; Arnez, M.; Steere, A.; Peltomaa, M.; Seppala, I. Immune responses to borrelial VlsE IR6 peptide variants. Int. J. Med. Microbiol. 2007, 297, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Tjernberg, I.; Sillanpaa, H.; Seppala, I.; Eliasson, I.; Forsberg, P.; Lahdenne, P. Antibody responses to borrelia IR(6) peptide variants and the C6 peptide in Swedish patients with erythema migrans. Int. J. Med. Microbiol. 2009, 299, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, P.M.; Sambir, M.; Dattwyler, R.J. Decorin binding proteins A and B in the serodiagnosis of Lyme disease in North America. Clin. Vaccine Immunol. 2014, 21, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Lahey, L.J.; Panas, M.W.; Mao, R.; Delanoy, M.; Flanagan, J.; Binder, S.R.; Rebman, A.W.; Montoya, J.G.; Soloski, M.J.; Steere, A.C.; et al. Development of a multi-antigen panel for improved detection of Borrelia burgdorferi infection in early Lyme disease. J. Clin. Microbiol. 2015, 53, 3834–3841. [Google Scholar] [CrossRef]
- Arnaboldi, P.M.; Dattwyler, R.J. Cross-Reactive Epitopes in Borrelia burgdorferi p66. Clin. Vaccine Immunol. 2015, 22, 840–843. [Google Scholar] [CrossRef][Green Version]
- Baarsma, M.E.; Schellekens, J.; Meijer, B.C.; Brandenburg, A.H.; Souilljee, T.; Hofhuis, A.; Hovius, J.W.; van Dam, A.P. Diagnostic parameters of modified two-tier testing in European patients with early Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2143–2152. [Google Scholar] [CrossRef]
- Liang, F.T.; Yan, J.; Mbow, M.L.; Sviat, S.L.; Gilmore, R.D.; Mamula, M.; Fikrig, E. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 2004, 72, 5759–5767. [Google Scholar] [CrossRef]
- Gomes-Solecki, M.J.; Wormser, G.P.; Schriefer, M.; Neuman, G.; Hannafey, L.; Glass, J.D.; Dattwyler, R.J. Recombinant assay for serodiagnosis of Lyme disease regardless of OspA vaccination status. J. Clin. Microbiol. 2002, 40, 193–197. [Google Scholar] [CrossRef][Green Version]
- Gomes-Solecki, M.J.; Wormser, G.P.; Persing, D.H.; Berger, B.W.; Glass, J.D.; Yang, X.; Dattwyler, R.J. A first-tier rapid assay for the serodiagnosis of Borrelia burgdorferi infection. Arch. Intern. Med. 2001, 161, 2015–2020. [Google Scholar] [CrossRef][Green Version]
- Robertson, J.; Guy, E.; Andrews, N.; Wilske, B.; Anda, P.; Granstrom, M.; Hauser, U.; Moosmann, Y.; Sambri, V.; Schellekens, J.; et al. A European multicenter study of immunoblotting in serodiagnosis of lyme borreliosis. J. Clin. Microbiol. 2000, 38, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Dattwyler, R.J.; Arnaboldi, P.M. Comparison of Lyme Disease Serologic Assays and Lyme Specialty Laboratories. Clin. Infect. Dis. 2014, 59, 1711–1713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brissette, C.A.; Haupt, K.; Barthel, D.; Cooley, A.E.; Bowman, A.; Skerka, C.; Wallich, R.; Zipfel, P.F.; Kraiczy, P.; Stevenson, B. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect. Immun. 2009, 77, 300–306. [Google Scholar] [CrossRef]
- Bykowski, T.; Woodman, M.E.; Cooley, A.E.; Brissette, C.A.; Wallich, R.; Brade, V.; Kraiczy, P.; Stevenson, B. Borrelia burgdorferi complement regulator-acquiring surface proteins (BbCRASPs): Expression patterns during the mammal-tick infection cycle. Int. J. Med. Microbiol. 2008, 298 (Suppl. 1), 249–256. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, A.; Meri, T.; Lankinen, H.; Seppala, I.; Lahdenne, P.; Hefty, P.S.; Akins, D.; Meri, S. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 2002, 169, 3847–3853. [Google Scholar] [CrossRef]
- Nowalk, A.J.; Gilmore, R.D., Jr.; Carroll, J.A. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 2006, 74, 3864–3873. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.T.; Nelson, F.K.; Fikrig, E. DNA microarray assessment of putative Borrelia burgdorferi lipoprotein genes. Infect. Immun. 2002, 70, 3300–3303. [Google Scholar] [CrossRef]
- Dowdell, A.S.; Murphy, M.D.; Azodi, C.; Swanson, S.K.; Florens, L.; Chen, S.; Zückert, W.R. Comprehensive Spatial Analysis of the Borrelia burgdorferi Lipoproteome Reveals a Compartmentalization Bias toward the Bacterial Surface. J. Bacteriol. 2017, 199, e00658-16. [Google Scholar] [CrossRef]
- Jiang, W.; Luft, B.J.; Schubach, W.; Dattwyler, R.J.; Gorevic, P.D. Mapping the major antigenic domains of the native flagellar antigen of Borrelia burgdorferi. J. Clin. Microbiol. 1992, 30, 1535–1540. [Google Scholar] [CrossRef]
- Robinson, J.M.; Pilot-Matias, T.J.; Pratt, S.D.; Patel, C.B.; Bevirt, T.S.; Hunt, J.C. Analysis of the humoral response to the flagellin protein of Borrelia burgdorferi: Cloning of regions capable of differentiating Lyme disease from syphilis. J. Clin. Microbiol. 1993, 31, 629–635. [Google Scholar] [CrossRef]
- Toumanios, C.; Prisco, L.; Dattwyler, R.J.; Arnaboldi, P.M. Linear B Cell Epitopes Derived from the Multifunctional Surface Lipoprotein BBK32 as Targets for the Serodiagnosis of Lyme Disease. mSphere 2019, 4, e00111-19. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Carter, J.M.; Sigal, L.H.; Stein, S. Multi-well ELISA based on independent peptide antigens for antibody capture. Application to Lyme disease serodiagnosis. J. Immunol. Methods 1996, 198, 25–33. [Google Scholar] [CrossRef]
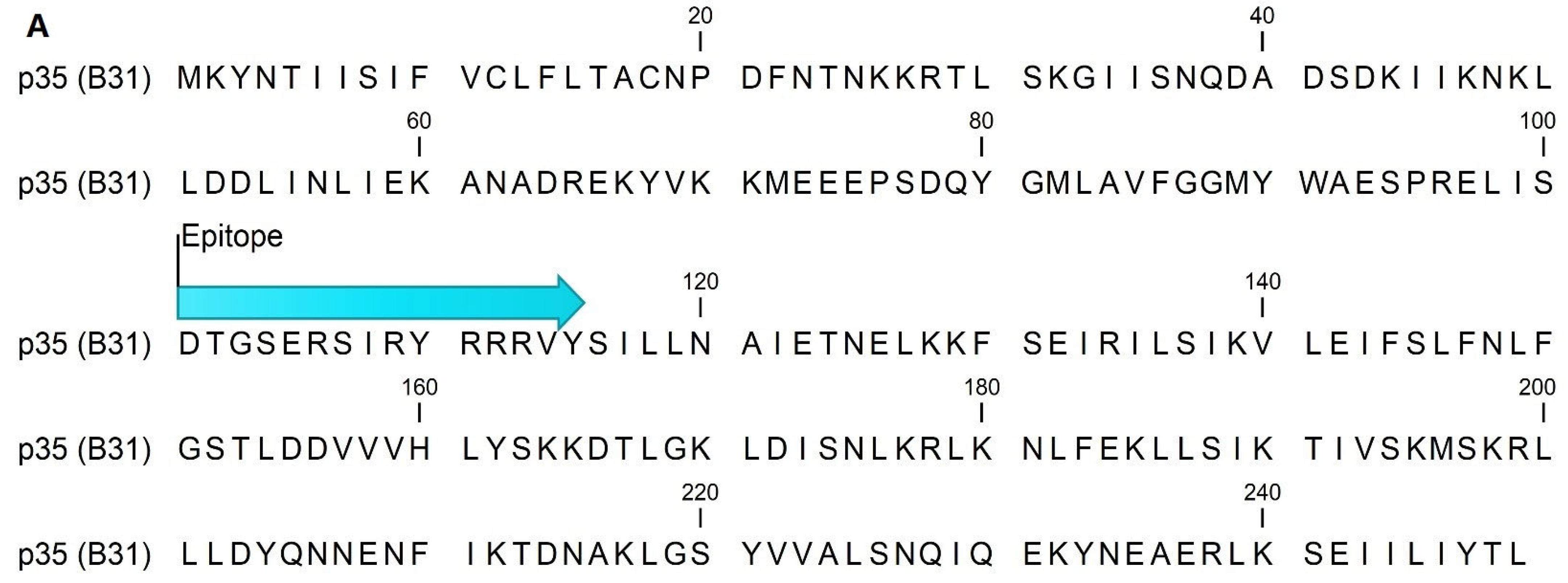
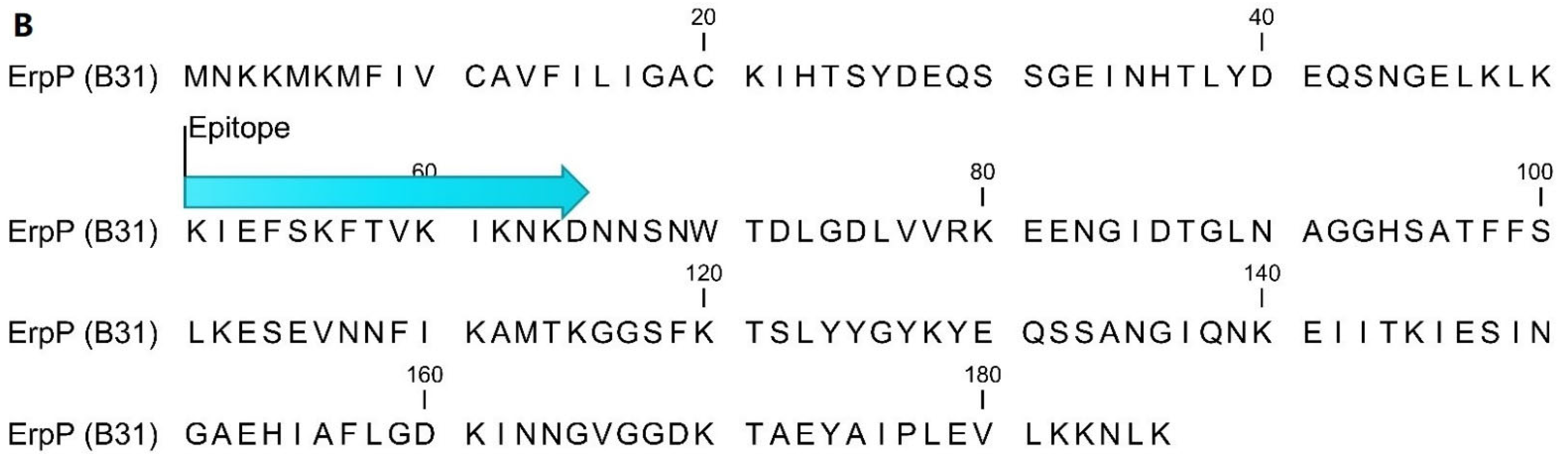
| Early Lyme (EM+) 1 (n = as Indicated) | Late Lyme (LA) 2 (n = as Indicated) | ||||||
|---|---|---|---|---|---|---|---|
| IgM | IgG | IgM + IgG 3 | IgM | IgG | IgM + IgG | ||
| p35(101–115) | Positive 4 | 19/145 13.1% | 9/144 6.3% | 24/145 16.6% | 0/20 0.0% | 3/20 15.0% | 3/20 15.0% |
| Equivocal 5 | 13/145 9.0% | 20/144 13.9% | 25/145 17.2% | 1/20 5.0% | 7/20 35.0% | 7/20 35.0% | |
| Negative 6 | 113/145 77.9% | 115/144 79.9% | 96/145 66.2% | 19/20 95.0% | 10/20 50.0% | 10/20 50.0% | |
| pp35-mV | Positive | 36/139 25.9% | 75/139 54.0% | 78/139 56.9% | 2/20 10.0% | 19/20 95.0% | 19/20 95.0% |
| Equivocal | 15/139 10.8% | 15/139 10.8% | 14/139 10.2% | 0/20 0.0% | 1/20 5.0% | 1/20 5.0% | |
| Negative | 88/139 63.3% | 49/139 35.3% | 45/139 32.8% | 18/20 90.0% | 0/20 0.0% | 0/20 0.0% | |
| ErpP(51–65) | Positive | 34/145 23.4% | 17/144 11.8% | 45/145 31.0% | 3/20 15.0% | 9/20 45.0% | 10/20 50.0% |
| Equivocal | 13/145 9.0% | 20/144 13.9% | 22/145 15.2% | 2/20 10.0% | 2/20 10.0% | 3/20 15.0% | |
| Negative | 98/145 67.6% | 107/144 74.3% | 78/145 53.8% | 15/20 75.0% | 9/20 45.0% | 7/20 35.0% | |
| pErpP-mV | Positive | 60/139 43.2% | 71/139 51.1% | 82/139 59.0% | 1/20 5.0% | 17/20 85.0% | 17/20 85.0% |
| Equivocal | 12/139 8.6% | 15/139 10.8% | 17/139 12.2% | 1/20 5.0% | 1/20 5.0% | 1/20 5.0% | |
| Negative | 67/139 48.2% | 53/139 38.1% | 40/139 28.8% | 18/20 90.0% | 2/20 10.0% | 2/20 10.0% | |
| FlaB(211–223) | Positive | 50/133 37.6% | 23/133 17.3% | 60/133 45.1% | 2/20 10.0% | 15/20 75.0% | 15/20 75.0% |
| Equivocal | 4/133 3.0% | 16/133 12.0% | 10/133 7.5% | 0/20 0.0% | 1/20 5.0% | 1/20 5.0% | |
| Negative | 79/133 59.4% | 94/133 70.7% | 63/133 47.4% | 18/20 90.0% | 4/20 20.0% | 4/20 20.0% | |
| pFlaB-mV | Positive | 76/137 55.5% | 75/137 54.7% | 86/137 62.8% | 7/20 35.0% | 17/20 85.0% | 17/20 85.0% |
| Equivocal | 7/137 5.1% | 5/137 3.6% | 9/137 6.6% | 3/20 15.0% | 2/20 10.0% | 2/20 10.0% | |
| Negative | 54/137 39.4% | 57/137 41.6% | 42/137 30.7% | 10/20 50.0% | 1/20 5.0% | 1/20 5.0% | |
| mVlsE(275–291) (mV) | Positive | 10/83 12.0% | 22/83 26.5% | 23/83 27.7% | 1/19 5.3% | 13/19 68.4% | 13/19 68.4% |
| Equivocal | 2/83 2.4% | 3/83 3.6% | 3/83 3.6% | 0/19 0.0% | 1/19 5.3% | 1/19 5.3% | |
| Negative | 71/83 85.6% | 58/83 69.9% | 57/83 68.7% | 18/19 94.7% | 5/19 26.3% | 5/19 26.3% | |
| Healthy Controls 1 | RA 2 | Syphilis 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgM + IgG 4 | IgM | IgG | IgM + IgG | IgM | IgG | IgM + IgG | ||
| p35(101–115) | Positive 5 | 0/64 0.0% | 0/64 0.0% | 0/64 0.0% | 0/40 0.0% | 2/40 5.0% | 2/40 5.0% | 1/35 2.9% | 3/35 8.6% | 4/35 11.4% |
| Equivocal 6 | 2/64 3.1% | 2/64 3.1% | 4/64 6.3% | 0/40 0.0% | 3/40 7.5% | 3/40 7.5% | 1/35 2.9% | 6/35 17.1% | 7/35 20.0% | |
| Negative 7 | 62/64 96.9% | 62/64 96.9% | 60/64 93.8% | 40/40 100.0% | 35/40 87.5% | 35/40 87.5% | 33/35 94.3% | 26/35 74.3% | 24/35 68.6% | |
| pp35-mV | Positive | 0/64 0.0% | 0/64 0.0% | 0/64 0.0% | 1/38 2.6% | 2/38 5.3% | 3/38 7.9% | 0/34 0.0% | 4/34 11.8% | 4/34 11.8% |
| Equivocal | 3/64 4.7% | 4/64 6.3% | 6/64 9.4% | 2/38 5.3% | 6/38 15.8% | 8/38 21.1% | 0/34 0.0% | 8/34 23.5% | 8/34 23.5% | |
| Negative | 61/64 95.3% | 60/64 93.8% | 58/64 90.6% | 35/38 92.1% | 30/38 78.9% | 27/38 71.1% | 34/34 100.0% | 22/34 64.7% | 22/34 64.7% | |
| ErpP(51–65) | Positive | 0/64 0.0% | 0/64 0.0% | 0/64 0.0% | 0/40 0.0% | 0/40 0.0% | 0/40 0.0% | 2/35 5.7% | 1/35 2.9% | 3/35 8.6% |
| Equivocal | 3/64 4.7% | 1/64 1.6% | 4/64 6.3% | 4/40 10.0% | 1/40 2.6% | 5/40 12.8% | 0/35 0.0% | 3/35 8.6% | 3/35 8.6% | |
| Negative | 61/64 95.3% | 63/64 98.4% | 60/64 93.8% | 36/40 90.0% | 38/40 97.4% | 34/40 87.2% | 33/35 94.3% | 31/35 88.6% | 29/35 82.9% | |
| pErpP-mV | Positive | 1/64 1.6% | 1/64 1.6% | 1/64 1.6% | 5/38 13.2% | 0/38 0.0% | 5/38 13.2% | 1/34 2.9% | 1/34 2.9% | 2/34 5.9% |
| Equivocal | 4/64 6.3% | 2/64 3.1% | 6/64 9.4% | 2/38 5.3% | 2/38 5.3% | 4/38 10.5% | 3/34 8.8% | 0/34 0.0% | 3/34 8.8% | |
| Negative | 59/64 92.2% | 61/64 95.3% | 57/64 89.1% | 31/38 81.6% | 36/38 94.7% | 29/38 76.3% | 30/34 88.2% | 33/34 97.1% | 29/34 85.3% | |
| FlaB(211–223) | Positive | 1/64 1.6% | 0/64 0.0% | 1/64 1.6% | 0/44 0.0% | 2/44 4.5% | 2/44 4.5% | 0/34 0.0% | 2/34 5.9% | 2/34 5.9% |
| Equivocal | 2/64 3.1% | 2/64 3.1% | 3/64 4.7% | 1/44 2.3% | 1/44 2.3% | 2/44 4.5% | 1/34 2.9% | 3/34 8.8% | 4/34 11.8% | |
| Negative | 61/64 95.3% | 62/64 96.9% | 60/64 93.8% | 43/44 97.7% | 41/44 93.2% | 40/44 90.9% | 33/34 97.1% | 29/34 85.3% | 28/34 82.4% | |
| pFlaB-mV | Positive | 2/64 3.1% | 2/64 3.1% | 4/64 6.3% | 2/40 4.2% | 1/40 2.1% | 3/40 6.3% | 1/32 3.1% | 1/32 3.1% | 2/32 6.3% |
| Equivocal | 1/64 1.6% | 2/64 3.1% | 3/64 4.7% | 5/40 10.4% | 1/40 2.1% | 6/40 12.5% | 1/32 3.1% | 1/32 3.1% | 2/32 6.3% | |
| Negative | 61/64 95.3% | 60/64 93.7% | 57/64 89.1% | 41/40 85.4% | 46/40 95.8% | 39/40 81.3% | 30/32 93.7% | 30/32 93.7% | 28/32 87.5% | |
| mVlsE(275–291) (mV) | Positive | 0/61 0.0% | 1/61 1.6% | 1/61 1.6% | 0/30 0.0% | 0/30 0.0% | 0/30 0.0% | 0/20 0.0% | 4/20 20.0% | 4/20 20.0% |
| Equivocal | 4/61 6.6% | 2/61 3.3% | 5/61 8.2% | 0/30 0.0% | 0/30 0.0% | 0/30 0.0% | 0/20 0.0% | 3/20 15.0% | 3/20 15.0% | |
| Negative | 57/61 93.4% | 58/61 95.1% | 55/61 90.2% | 30/30 100.0% | 30/30 100.0% | 30/30 100.0% | 20/20 100.0% | 13/20 65.0% | 13/20 65.0% | |
| IgM | IgG | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| p35(101–115) | 13.8% | 99.3% | 6.9% | 96.4% |
| pp35-mV | 35.3% | 99.3% | 54.0% | 95.6% |
| ErpP(51–65) | 24.1% | 98.6% | 12.5% | 99.3% |
| pErpP-mV | 43.2% | 94.9% | 51.8% | 98.5% |
| FlaB(211–223) | 37.6% | 98.6% | 17.3% | 97.2% |
| pFlaB-mV | 56.2% | 96.5% | 54.7% | 97.9% |
| mVlsE(275–291) (mV) | 12.1% | 99.1% | 26.5% | 95.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnaboldi, P.M.; Katseff, A.S.; Sambir, M.; Dattwyler, R.J. Linear Peptide Epitopes Derived from ErpP, p35, and FlaB in the Serodiagnosis of Lyme Disease. Pathogens 2022, 11, 944. https://doi.org/10.3390/pathogens11080944
Arnaboldi PM, Katseff AS, Sambir M, Dattwyler RJ. Linear Peptide Epitopes Derived from ErpP, p35, and FlaB in the Serodiagnosis of Lyme Disease. Pathogens. 2022; 11(8):944. https://doi.org/10.3390/pathogens11080944
Chicago/Turabian StyleArnaboldi, Paul M., Adiya S. Katseff, Mariya Sambir, and Raymond J. Dattwyler. 2022. "Linear Peptide Epitopes Derived from ErpP, p35, and FlaB in the Serodiagnosis of Lyme Disease" Pathogens 11, no. 8: 944. https://doi.org/10.3390/pathogens11080944
APA StyleArnaboldi, P. M., Katseff, A. S., Sambir, M., & Dattwyler, R. J. (2022). Linear Peptide Epitopes Derived from ErpP, p35, and FlaB in the Serodiagnosis of Lyme Disease. Pathogens, 11(8), 944. https://doi.org/10.3390/pathogens11080944






