Efficacy of Dry Heat Treatment against Clostridioides difficile Spores and Mycobacterium tuberculosis on Filtering Facepiece Respirators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Inoculums
2.1.1. Preparation of C. difficile Spore Suspension
2.1.2. Preparation of M. tb Solution and Media Used in the Experiment
2.2. Inoculation of FFR Coupons
2.2.1. C. difficile Inoculation
2.2.2. M. tb Inoculation
2.3. Recovery and Visual Assessment of Heat Inactivation Experiment
2.3.1. Recovery of C. difficile Spores
2.3.2. Recovery of M. tb
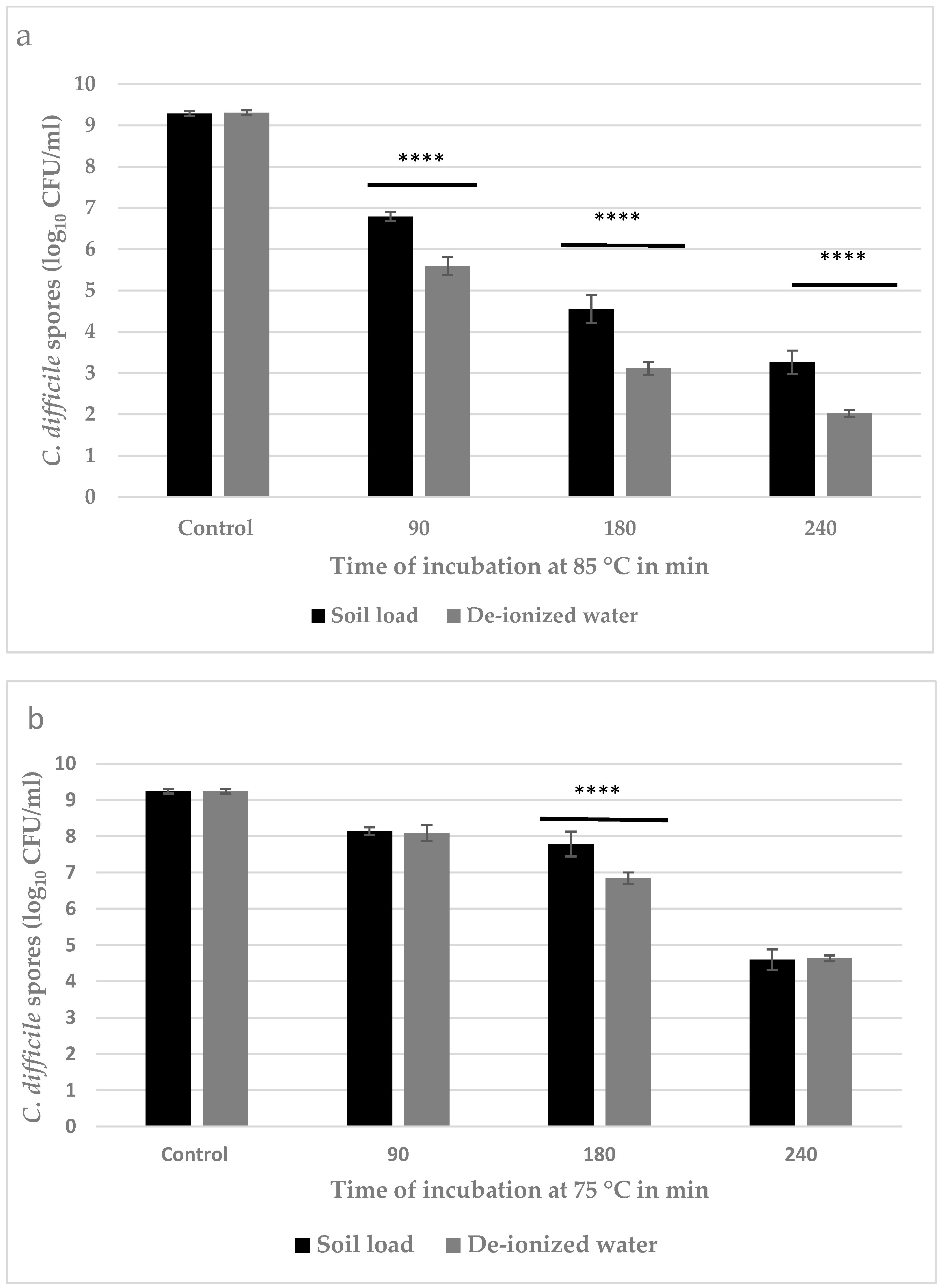
2.4. Quantification of Reduction in C. difficile Spores by Dry Heat Treatment at 75 and 85 °C
2.5. Temperature Monitoring
2.6. Statistical Analysis
3. Results
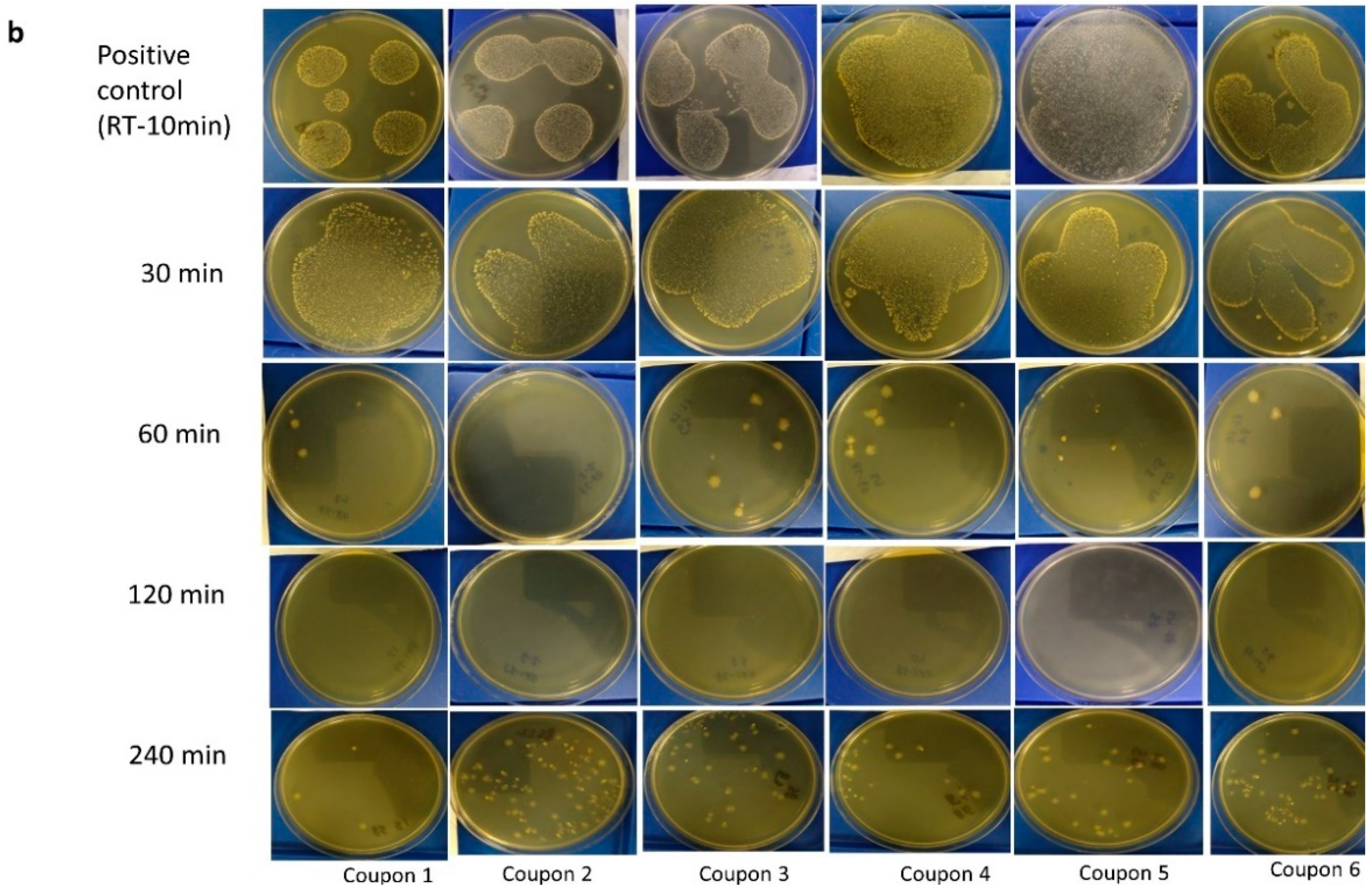
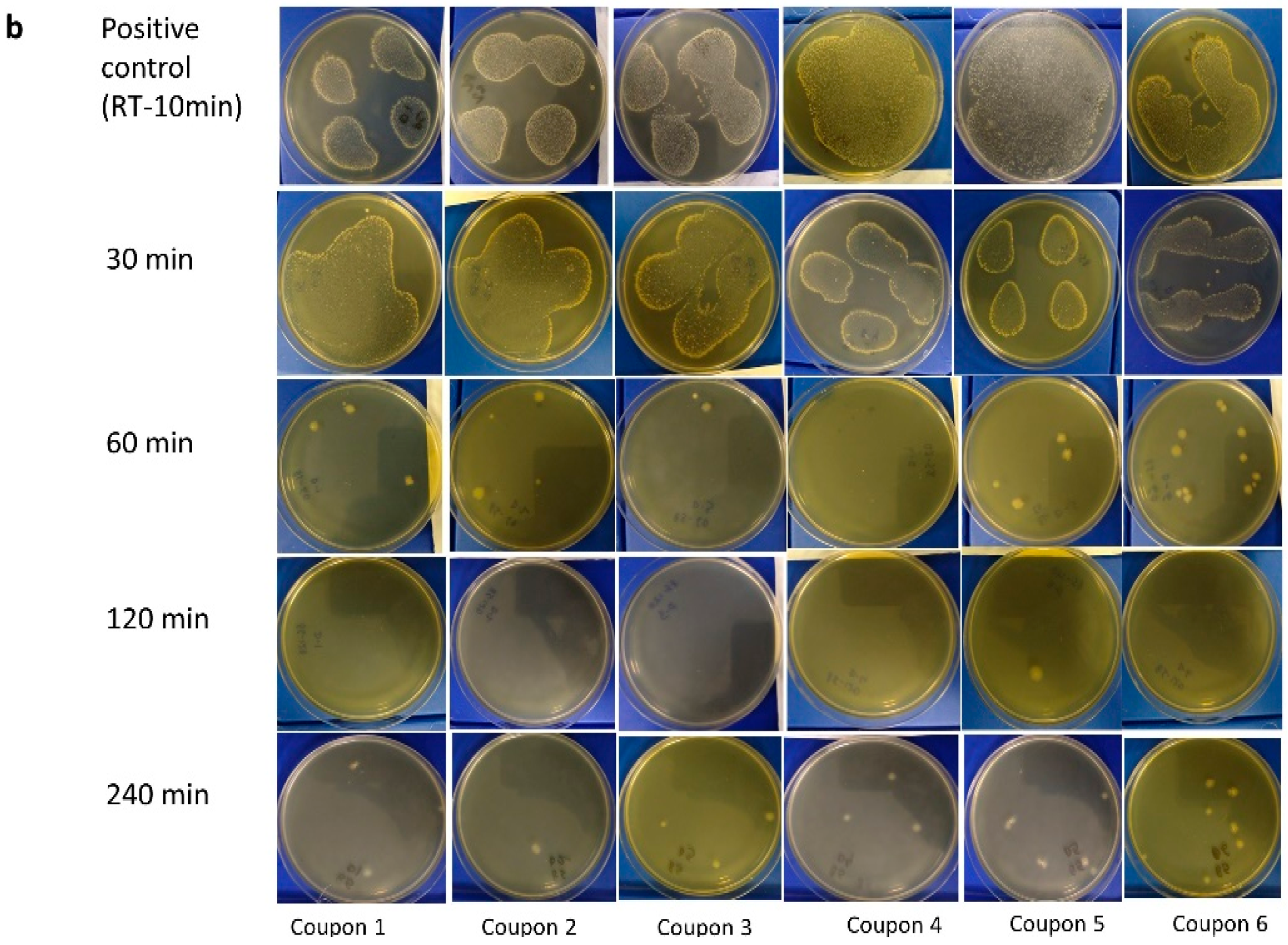
3.1. Visual Assessment of Inactivation of C. difficile at 85 °C
- Dry heat treatment at 85 °C for up to 240 min did not result in complete elimination of the C. difficile spores, although the increase in temperature did indicate some reduction in the density of colonies upon visual inspection, with the presence of soil load having a protective effect.
- Non-selective media were unreliable for the recovery of heat-treated C. difficile spores. Hence, only selective media were used for the subsequent experiments in this study.
3.2. Quantitative Estimation of Inactivation of C. difficile Spores
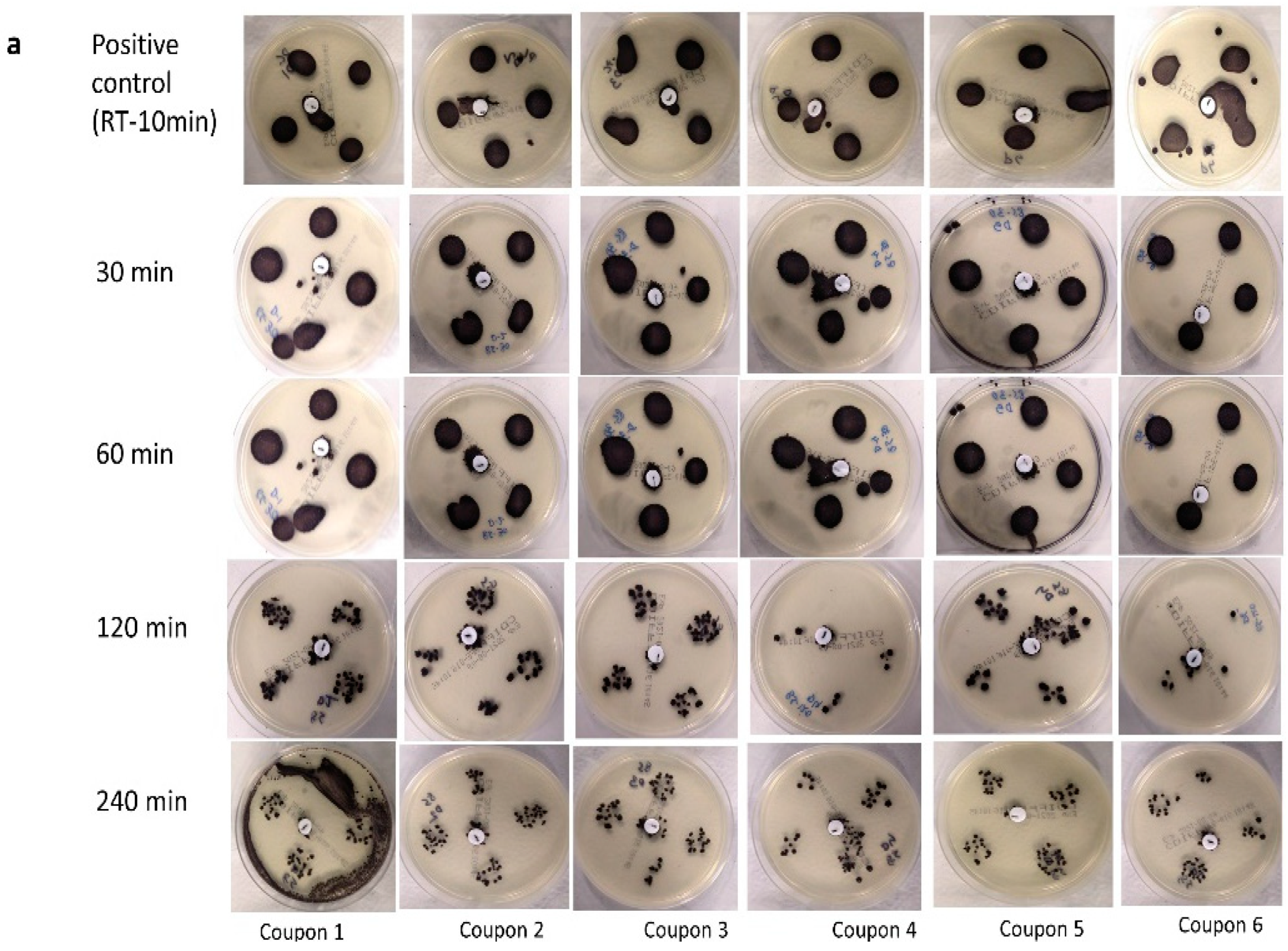
3.3. Visual Assessment of M. tb Inactivation at Room Temperature, 75 °C, and 85 °C on Agar
3.4. MGIT Enrichment Broth
4. Discussion
4.1. Inactivation of C. difficile Spores
4.2. Inactivation of M. tb
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burki, T. Global shortage of personal protective equipment. Lancet Infect. Dis. 2020, 20, 785–786. [Google Scholar] [CrossRef]
- Derraik, J.G.B.; Anderson, W.A.; Connelly, E.A.; Anderson, Y.C. Rapid review of SARS-CoV-1 and SARS-CoV-2 viability, susceptibility to treatment, and the disinfection and reuse of PPE, particularly filtering facepiece respirators. Int. J. Environ. Res. Public Health 2020, 17, 6117. [Google Scholar] [CrossRef] [PubMed]
- Harfoot, R.; Yung, D.B.; Anderson, W.A.; Wild, C.E.; Coetzee, N.; Hernández, L.C.; Lawley, B.; Pletzer, D.; Derraik, J.G.; Anderson, Y.C. Ultraviolet-C irradiation, heat, and storage as potential methods of inactivating SARS-CoV-2 and bacterial pathogens on filtering facepiece respirators. Pathogens 2022, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://stacks.cdc.gov/view/cdc/47378 (accessed on 30 June 2022).
- Shirey, B.T.; Schubert, W.; Benardini, J. An overview of surface heat microbial reduction as a viable microbial reduction modality for spacecraft surfaces. In Proceedings of the 47th International Conference on Environmental Systems (ICES), Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Fox, K.; Eder, B.D. Comparison of survivor curves of Bacillus subtilis spores subjected to wet and dry heat. J. Food. Sci. 1969, 34, 518–521. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setlow, P. Spore resistance properties. Microbiol. Spectr. 2014, 2, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Sabja, D.; Shen, A.; Sorg, J.A. Clostridium difficile spore biology: Sporulation, germination, and spore structural proteins. Trends Microbiol. 2014, 22, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Tonna, I.; Welsby, P. Pathogenesis and treatment of Clostridium difficile infection. Postgrad. Med. J. 2005, 81, 367. [Google Scholar] [CrossRef]
- Keessen, E.C.; Gaastra, W.; Lipman, L.J.A. Clostridium difficile infection in humans and animals, differences and similarities. Vet. Microbiol. 2011, 153, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Sorg, J.A.; Sun, X. Clostridioides difficile Biology: Sporulation, Germination, and Corresponding Therapies for C. difficile Infection. Front. Cell Infect. Microbiol. 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, F.; Lagos-Moraga, S.; Calderón-Romero, P.; Pizarro-Guajardo, M.; Paredes-Sabja, D. Updates on Clostridium difficile spore biology. Anaerobe 2017, 45, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.G.; Rousseau, J.; Willey, B.M.; Low, D.E.; Staempfli, H.; McGeer, A.; Weese, J.S. Use of a selective enrichment broth to recover Clostridium difficile from stool swabs stored under different conditions. J. Clin. Microbiol. 2005, 43, 5341–5343. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Palacios, A.; Staempfli, H.R.; Duffield, T.; Weese, J.S. Clostridium difficile in retail ground meat, Canada. Emerg. Infect. Dis. 2007, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Janezic, S.; Potocnik, M.; Zidaric, V.; Rupnik, M. Highly divergent Clostridium difficile strains isolated from the environment. PLoS ONE 2016, 11, e0167101. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [Green Version]
- Global Tuberculosis Report Geneva: World Health Organization; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 30 June 2022).
- O’Toole, R.F.; Shukla, S.D.; Walters, E.H. TB meets COPD: An emerging global co-morbidity in human lung disease. Tuberculosis 2015, 95, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Jørstad, M.D.; Aẞmus, J.; Marijani, M.; Sviland, L.; Mustafa, T. Diagnostic delay in extrapulmonary tuberculosis and impact on patient morbidity: A study from Zanzibar. PLoS ONE 2018, 13, e0203593. [Google Scholar] [CrossRef] [Green Version]
- Pai, M.; Kasaeva, T.; Swaminathan, S. Covid-19’s devastating effect on tuberculosis care—A path to recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef]
- Koupaei, M.; Naimi, A.; Moafi, N.; Mohammadi, P.; Tabatabaei, F.S.; Ghazizadeh, S.; Heidary, M.; Khoshnood, S. Clinical Characteristics, Diagnosis, Treatment, and Mortality Rate of TB/COVID-19 Coinfectetd Patients: A Systematic Review. Front. Med. 2021, 8, 2188. [Google Scholar] [CrossRef] [PubMed]
- Bostanghadiri, N.; Jazi, F.M.; Razavi, S.; Fattorini, L.; Darban-Sarokhalil, D. Mycobacterium tuberculosis and SARS-CoV-2 Coinfections: A Review. Front. Microbiol. 2021, 12, 747827. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 2003, 83, 91–97. [Google Scholar] [CrossRef]
- Brill, F.H.H.; Lenz, J.; Lach, C.; Radischat, N.; Paßvogel, L.; Goroncy-Bermes, P.; Gabriel, H.; Steinmann, J.; Steinhauer, K. Improved method for tuberculocidal and mycobactericidal activity testing of disinfectants based on the European Standard EN 14348. J. Hosp. Infect. 2021, 111, 176–179. [Google Scholar] [CrossRef]
- Bemer-Melchior, P.; Drugeon, H.B. Inactivation of Mycobacterium tuberculosis for DNA typing analysis. J. Clin. Microbiol. 1999, 37, 2350–2351. [Google Scholar] [CrossRef] [Green Version]
- Doig, C.; Seagar, A.L.; Watt, B.; Forbes, K.J. The efficacy of the heat killing of Mycobacterium tuberculosis. J. Clin. Pathol. 2002, 55, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palacios, A.; Lejeune, J.T. Moist-heat resistance, spore aging, and superdormancy in Clostridium difficile. Appl. Environ. Microbiol. 2011, 77, 3085–3091. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, C.; Mu, Y.; Houston, H.; Martinez-Smith, M.; Noble-Wang, J.; Coulliette-Salmond, A.; Rose, L. Persistence of bacteriophage phi 6 on porous and nonporous surfaces and the potential for its use as an Ebola virus or coronavirus surrogate. Appl. Environ. Microbiol. 2020, 86, e01482-20. [Google Scholar] [CrossRef] [PubMed]
- Pfyffer, G.E.; Welscher, H.M.; Kissling, P.; Cieslak, C.; Casal, M.J.; Gutierrez, J.; Rüsch-Gerdes, S. Comparison of the mycobacteria growth indicator tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli. J. Clin. Microbiol. 1997, 35, 364–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: The evolution of culture media in clinical microbiology. New Microbes New Infect 2020, 34, 100622. [Google Scholar] [CrossRef]
- Espina, L.; García-Gonzalo, D.; Pagán, R. Detection of Thermal Sublethal Injury in Escherichia coli via the Selective Medium Plating Technique: Mechanisms and Improvements. Front. Microbiol. 2016, 7, 1376. [Google Scholar] [CrossRef]
- Michael, K.; No, D.; Dankoff, J.; Lee, K.; Lara-Crawford, E.; Roberts, M.C. Clostridium difficile environmental contamination within a clinical laundry facility in the USA. FEMS Microbiol. Lett. 2016, 363, fnw236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, J.; Springthorpe, V.S.; Sattar, S.A. Clospore: A liquid medium for producing high titers of semi-purified spores of Clostridium difficile. J. AOAC Int. 2011, 94, 618–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, D.A.; Heap, J.T.; Minton, N.P. SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J. Bacteriol. 2010, 192, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.D.; Asir, K.; Halimi, D.; Orenga, S.; Dale, J.; Payne, M.; Carlton, R.; Evans, J.; Gould, F.K. Evaluation of a chromogenic culture medium for isolation of Clostridium difficile within 24 hours. J. Clin. Microbiol. 2010, 48, 3852–3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharmasena, M.; Wei, T.; Bridges, W.C., Jr.; Jiang, X. Thermal resistance of Clostridium difficile endospores in dairy compost upon exposure to wet and dry heat treatments. J. Appl. Microbiol. 2019, 127, 274–283. [Google Scholar] [CrossRef] [PubMed]
- International, A. Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Adhikari, A.; Reponen, T.; Yermakov, M.; Grinshpun, S.A. Association between increased DNA mutational frequency and thermal inactivation of aerosolized Bacillus spores exposed to dry heat. Aerosol Sci. Technol. 2011, 45, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Salas-Pacheco, J.M.; Setlow, B.; Setlow, P.; Pedraza-Reyes, M. Role of the Nfo (YqfS) and ExoA apurinic/apyrimidinic endonucleases in protecting Bacillus subtilis spores from DNA damage. J. Bacteriol. 2005, 187, 7374–7381. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.T.; Setlow, B.; Conlon, E.M.; Lyon, J.L.; Imamura, D.; Sato, T.; Setlow, P.; Losick, R.; Eichenberger, P. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 2006, 358, 16–37. [Google Scholar] [CrossRef]
- Jain, S.; Smyth, D.; O’Hagan, B.M.G.; Heap, J.T.; McMullan, G.; Minton, N.P.; Ternan, N.G. Inactivation of the dnaK gene in Clostridium difficile 630 Δerm yields a temperature-sensitive phenotype and increases biofilm-forming ability. Sci. Rep. 2017, 7, 17522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldfless, S.J.; Morag, A.S.; Belisle, K.A.; Sutera, V.A., Jr.; Lovett, S.T. DNA repeat rearrangements mediated by DnaK-dependent replication fork repair. Mol. Cell 2006, 21, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Solano, M.; Burson, D.E.; Thippareddi, H. Thermal Resistance of Clostridium difficile Spores in Peptone Water and Pork Meat. J. Food Prot. 2016, 79, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Sorg, J.A.; Sonenshein, A.L. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J. Bacteriol. 2010, 192, 4983–4990. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, S.; Yamakawa, K.; Ogura, H.; Nakamura, S. Recovery of spores of Clostridium difficile altered by heat or alkali. J. Med. Microbiol. 1989, 28, 217–221. [Google Scholar] [CrossRef]
- Eckert, C.; Burghoffer, B.; Lalande, V.; Barbut, F. Evaluation of the chromogenic agar chromID C. difficile. J. Clin. Microbiol. 2013, 51, 1002–1004. [Google Scholar] [CrossRef] [Green Version]
- Bechler, J.; Bermudez, L.E. Investigating the role of mucin as frontline defense of mucosal surfaces against Mycobacterium avium subsp. hominissuis. J. Pathog. 2020, 2020, 9451591. [Google Scholar] [CrossRef]
- Whalen, J.J. Environmental Control for Tuberculosis; Basic Upper-Room Ultraviolet Germicidal Irradiation Guidelines for Healthcare Settings Guide. 2009. Available online: https://stacks.cdc.gov/view/cdc/5306 (accessed on 30 June 2022).
- Turner, R.D.; Chiu, C.; Churchyard, G.J.; Esmail, H.; Lewinsohn, D.M.; Gandhi, N.R.; Fennelly, K.P. Tuberculosis infectiousness and host susceptibility. J. Infect. Dis. 2017, 216, S636–S643. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Temperature | Time (min) | Type of Media | Inoculum Type | ||
|---|---|---|---|---|---|
| Soil Load | Deionized Water | NC | |||
| PC | 10 | Non-selective | 6/6 | 6/6 | 0/6 |
| Selective | 6/6 | 6/6 | 0/6 | ||
| 85 °C | 30 | Non-selective | 6/6 | 6/6 | 0/6 |
| Selective | 6/6 | 6/6 | 0/6 | ||
| 60 | Non-selective | 5/6 | 5/6 | 0/6 | |
| Selective | 6/6 | 6/6 | 0/6 | ||
| 120 | Non-selective | 0/6 | 1/6 | 0/6 | |
| Selective | 6/6 | 6/6 | 0/6 | ||
| 240 | Non-selective | 6/6 | 6/6 | 0/6 | |
| Selective | 6/6 | 6/6 | 0/6 | ||
| Temperature | Time (min) | Inoculum Type | ||
|---|---|---|---|---|
| M. tb. Only | Soil Load | NC | ||
| PC | 10 | 6/6 | 6/6 | 0/6 |
| 75 °C | 30 | 4/6 | 0/6 | 0/6 |
| 60 | 2/6 | 0/6 | 0/6 | |
| 90 | 0/6 | 0/6 | 0/6 | |
| 85 °C | 30 | 0/5 † | 0/6 | 0/6 |
| 60 | 0/6 | 0/5 † | 0/6 | |
| 90 | 0/6 | 0/6 | 0/6 | |
| Temperature | Duration (min) | Inoculum Type | ||
|---|---|---|---|---|
| M. tb. Alone | Soil Load | NC | ||
| PC | 10 | 6/6 | 6/6 | 0/6 |
| 75 °C | 30 | 5/6 | 4/6 | 0/6 |
| 60 | 4/6 | 5/6 | 0/6 | |
| 90 | 1/6 | 1/6 | 0/6 | |
| 85 °C | 30 | 3/6 | 5/6 | 0/6 |
| 60 | 2/6 | 3/6 | 0/6 | |
| 90 | 0/6 | 0/6 | 0/6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, A.; Parlane, N.A.; Khan, F.; Derraik, J.G.B.; Wild, C.E.K.; Anderson, Y.C.; Brightwell, G. Efficacy of Dry Heat Treatment against Clostridioides difficile Spores and Mycobacterium tuberculosis on Filtering Facepiece Respirators. Pathogens 2022, 11, 871. https://doi.org/10.3390/pathogens11080871
Soni A, Parlane NA, Khan F, Derraik JGB, Wild CEK, Anderson YC, Brightwell G. Efficacy of Dry Heat Treatment against Clostridioides difficile Spores and Mycobacterium tuberculosis on Filtering Facepiece Respirators. Pathogens. 2022; 11(8):871. https://doi.org/10.3390/pathogens11080871
Chicago/Turabian StyleSoni, Aswathi, Natalie A. Parlane, Farina Khan, José G. B. Derraik, Cervantée E. K. Wild, Yvonne C. Anderson, and Gale Brightwell. 2022. "Efficacy of Dry Heat Treatment against Clostridioides difficile Spores and Mycobacterium tuberculosis on Filtering Facepiece Respirators" Pathogens 11, no. 8: 871. https://doi.org/10.3390/pathogens11080871
APA StyleSoni, A., Parlane, N. A., Khan, F., Derraik, J. G. B., Wild, C. E. K., Anderson, Y. C., & Brightwell, G. (2022). Efficacy of Dry Heat Treatment against Clostridioides difficile Spores and Mycobacterium tuberculosis on Filtering Facepiece Respirators. Pathogens, 11(8), 871. https://doi.org/10.3390/pathogens11080871









