Disturbance Ecology Meets Bovine Tuberculosis (bTB) Epidemiology: A Before-and-After Study on the Association between Forest Clearfelling and bTB Herd Risk in Cattle Herds
Abstract
1. Introduction
bTB in Ireland: A Perfect Storm of Opportunities to Understand the Link between Ecological Disturbance and Bovine Tuberculosis
2. Results
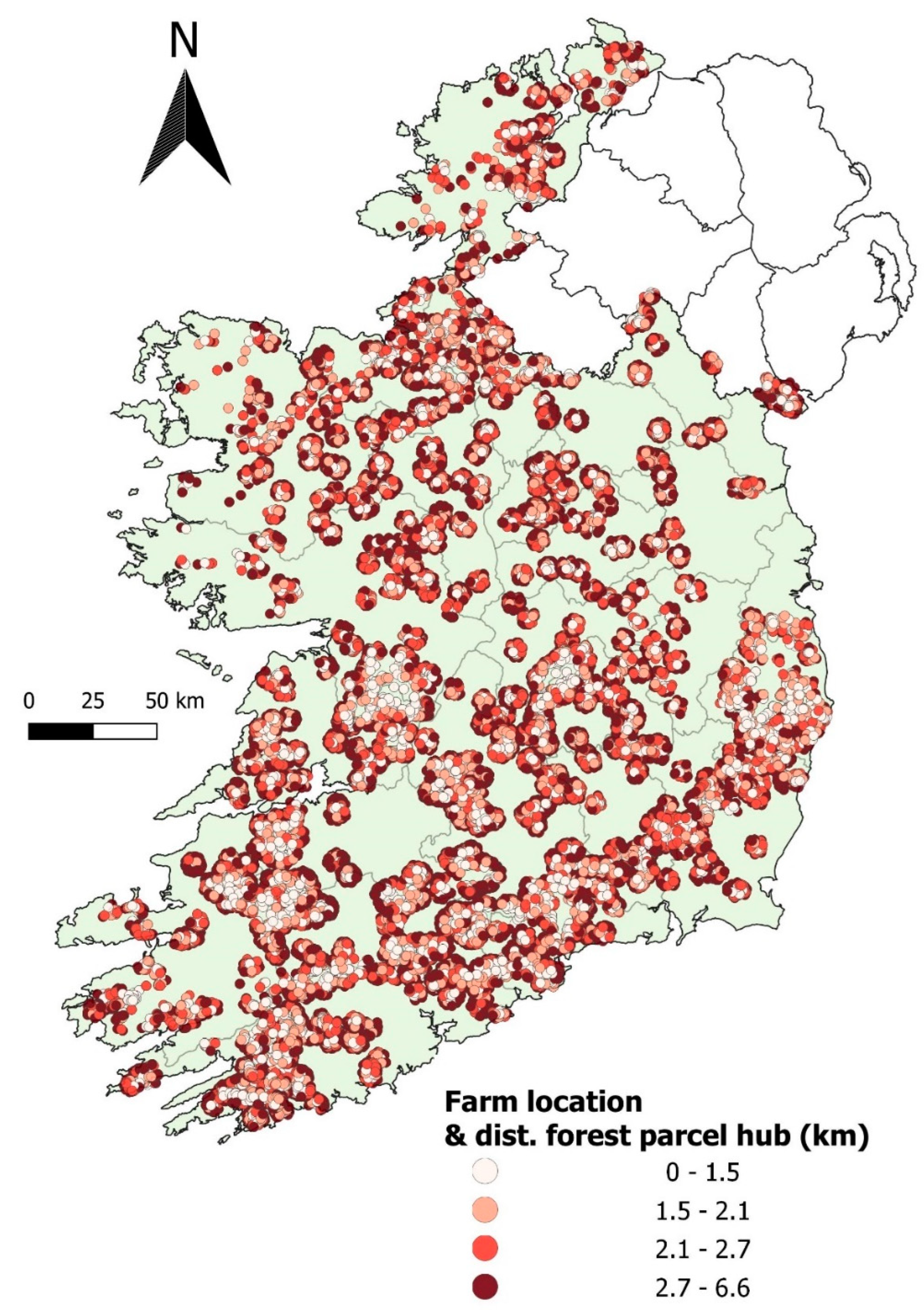
2.1. Sample Sizes and Breakdown Descriptive Statistics
2.2. Multivariable Binomial Model
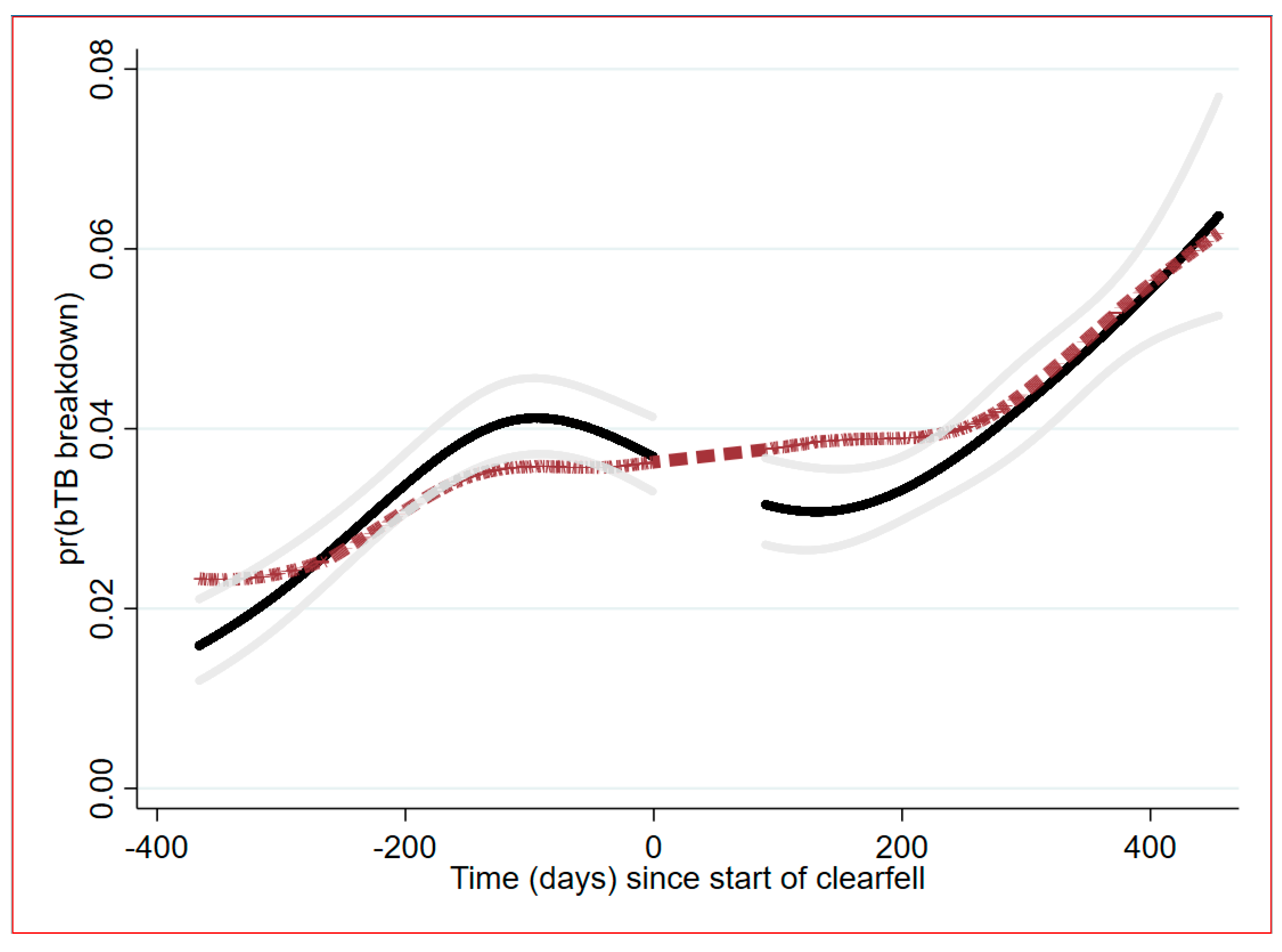
2.3. Temporal Trend Analysis
3. Discussion
4. Conclusions
5. Methods
5.1. Study Design
5.2. Definitions
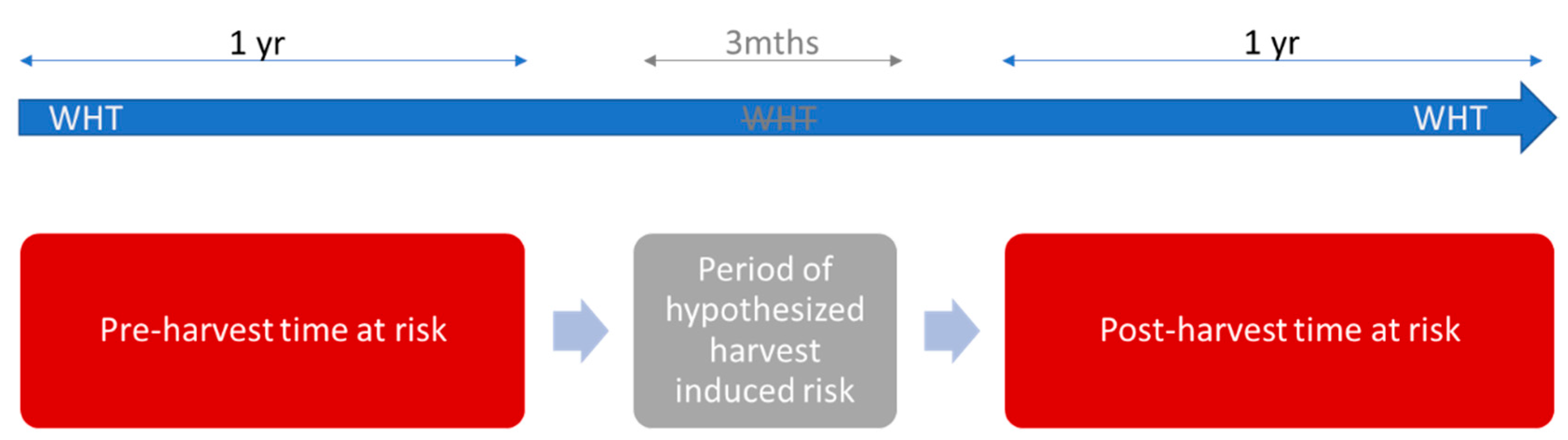
5.3. Time at Risk
- A whole herd test was completed between −366 days and −1 day prior to the first day of the month during which clearfelling occurred (note, clearfelling activities were recorded to the month/year level only);
- a whole herd test was completed between 90 days and 455 days after the first day of the month during which clearfelling occurred (i.e., 1 day to 365 days after the 3-month period of hypothesized harvest induced risk; Figure 4
5.4. Confounders
5.5. Descriptive Analysis
5.6. Modelling bTB Risk: Binomial Regressions
5.7. Modelling bTB Risk: Time-Series Regressions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blitzer, E.J.; Dormann, C.F.; Holzschuh, A.; Klein, A.M.; Rand, T.A.; Tscharntke, T. Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 2012, 146, 34–43. [Google Scholar] [CrossRef]
- Plowright, R.K.; Reaser, J.K.; Locke, H.; Woodley, S.J.; Patz, J.A.; Becker, D.J.; Oppler, G.; Hudson, P.J.; Tabor, G.M. Land use-induced spillover: A call to action to safeguard environmental, animal, and human health. Lancet Planet. Health 2021, 5, e237–e245. [Google Scholar] [CrossRef]
- Tompkins, D.M.; Dunn, A.; Smith, M.J.; Telfer, S. Wildlife diseases: From individuals to ecosystems. J. Anim. Ecol. 2011, 80, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Faust, C.L.; McCallum, H.I.; Bloomfield, L.S.P.; Gottdenker, N.L.; Gillespie, T.R.; Torney, C.J.; Dobson, A.P.; Plowright, R.K. Pathogen spillover during land conversion. Ecol. Lett. 2018, 21, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Brearley, G.; Rhodes, J.; Bradley, A.; Baxter, G.; Seabrook, L.; Lunney, D.; Liu, Y.; McAlpine, C. Wildlife disease prevalence in human-modified landscapes. Biol. Rev. 2013, 88, 427–442. [Google Scholar] [CrossRef]
- McCallum, H.; Dobson, A. Disease, habitat fragmentation and conservation. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 2041–2049. [Google Scholar] [CrossRef]
- Hing, S.; Narayan, E.J.; Thompson, R.C.A.; Godfrey, S.S. The relationship between physiological stress and wildlife disease: Consequences for health and conservation. Wildl. Res. 2016, 43, 51–60. [Google Scholar] [CrossRef]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Glidden, C.K.; Nova, N.; Kain, M.P.; Lagerstrom, K.M.; Skinner, E.B.; Mandle, L.; Sokolow, S.H.; Plowright, R.K.; Dirzo, R.; De Leo, G.A.; et al. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. Curr. Biol. 2021, 31, R1342–R1361. [Google Scholar] [CrossRef]
- Alexander, K.A.; Sanderson, C.E.; Marathe, M.; Lewis, B.L.; Rivers, C.M.; Shaman, J.; Drake, J.M.; Lofgren, E.; Dato, V.M.; Eisenberg, M.C.; et al. What factors might have led to the emergence of Ebola in West Africa? PLoS Negl. Trop. Dis. 2015, 9, e0003652. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Emerging Infectious Diseases: Threats to Human Health and Global Stability. PLOS Pathog. 2013, 9, e1003467. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.K.; Hitchens, P.; Evans, T.S.; Goldstein, T.; Thomas, K.; Clements, A.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; Karesh, W.; et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci. Rep. 2015, 5, 14830. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Foley, P.; Field, H.E.; Dobson, A.P.; Foley, J.E.; Eby, P.; Daszak, P. Urban habituation, ecological connectivity and epidemic dampening: The emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B Biol. Sci. 2011, 278, 3703–3712. [Google Scholar] [CrossRef]
- Edson, D.; Field, H.; McMichael, L.; Jordan, D.; Kung, N.; Mayer, D.; Smith, C. Flying-Fox Roost Disturbance and Hendra Virus Spillover Risk. PLoS ONE 2015, 10, e0125881. [Google Scholar] [CrossRef]
- Riojas, M.A.; McGough, K.J.; Rider-Riojas, C.J.; Rastogi, N.; Hazbón, M.H. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int. J. Syst. Evol. 2018, 68, 324–332. [Google Scholar]
- Humblet, M.F.; Boschiroli, M.L.; Saegerman, C. Classification of worldwide bovine tuberculosis risk factors in cattle: A stratified approach. Vet. Res. 2009, 40, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.R.; Skuce, R.A.; Byrne, A. Bovine Tuberculosis in Britain and Ireland—A Perfect Storm? the Confluence of Potential Ecological and Epidemiological Impediments to Controlling a Chronic Infectious Disease. Front. Vet. Sci. 2018, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Skuce, R.A.; Allen, A.R.; McDowell, S. Herd-Level Risk Factors for Bovine Tuberculosis: A Literature Review. Vet. Med. Int. 2012, 2012, 621210. [Google Scholar] [CrossRef]
- Broughan, J.M.; Judge, J.; Ely, E.; Delahay, R.J.; Wilson, G.; Clifton-Hadley, R.S.; Goodchild, A.V.; Bishop, H.; Parry, J.E.; Downs, S.H. A review of risk factors for bovine tuberculosis infection in cattle in the UK and Ireland. Epidemiol. Infect. 2016, 144, 2899–2926. [Google Scholar] [CrossRef]
- Fitzgerald, S.D.; Kaneene, J.B. Wildlife reservoirs of bovine tuberculosis worldwide: Hosts, pathology, surveillance, and control. Vet. Path. 2013, 50, 488–499. [Google Scholar] [CrossRef]
- Gortázar, C.; Delahay, R.J.; Mcdonald, R.A.; Boadella, M.; Wilson, G.J.; Gavier-Widen, D.; Acevedo, P. The status of tuberculosis in European wild mammals. Mammal Rev. 2012, 42, 193–206. [Google Scholar] [CrossRef]
- Didkowska, A.; Orłowska, B.; Witkowski, L.; Olbrych, K.; Brzezińska, S.; Augustynowicz-Kopeć, E.; Krajewska-Wędzina, M.; Bereznowski, A.; Bielecki, W.; Krzysiak, M.; et al. Biopsy and Tracheobronchial Aspirates as Additional Tools for the Diagnosis of Bovine Tuberculosis in Living European Bison (Bison bonasus). Animals 2020, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, B.; Krajewska-Wędzina, M.; Augustynowicz-Kopeć, E.; Kozińska, M.; Brzezińska, S.; Zabost, A.; Didkowska, A.; Welz, M.; Kaczor, S.; Żmuda, P.; et al. Epidemiological characterization of Mycobacterium caprae strains isolated from wildlife in the Bieszczady Mountains, on the border of Southeast Poland. BMC Vet. Res. 2020, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Biek, R.; O’Hare, A.; Wright, D.; Mallon, T.; McCormick, C.; Orton, R.J.; McDowell, S.; Trewby, H.; Skuce, R.A.; Kao, R.R. Whole genome sequencing reveals local transmission patterns of Mycobacterium bovis in sympatric cattle and badger populations. PLoS Pathogens. 2012, 8, e1003008. [Google Scholar] [CrossRef] [PubMed]
- Akhmetova, A.; Guerrero, J.; McAdam, P.; Salvador, L.C.; Crispell, J.; Lavery, J.; Presho, E.; Kao, R.R.; Biek, R.; Menzies, F.; et al. Genomic epidemiology of Mycobacterium bovis infection in sympatric badger and cattle populations in Northern Ireland. bioRxiv 2021. [Google Scholar] [CrossRef]
- Griffin, J.M.; Williams, D.H.; Kelly, G.E.; Clegg, T.A.; O’Boyle, I.; Collins, J.D.; More, S.J. The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Prev. Vet. Med. 2005, 67, 237–266. [Google Scholar] [CrossRef]
- Donnelly, C.A.; Woodroffe, R.; Cox, D.R.; Bourne, F.J.; Cheeseman, C.L.; Clifton-Hadley, R.S.; Wei, G.; Gettinby, G.; Gilks, P.; Jenkins, H.; et al. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 2006, 439, 843–846. [Google Scholar] [CrossRef]
- Tabachnick, W.J. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J. Exp. Biol. 2010, 213, 946–954. [Google Scholar] [CrossRef]
- Murphy, K.J.; Morera-Pujol, V.; Ryan, E.; Byrne, A.W.; Breslin, P.; Ciuti, S. Habitat availability alters the relative risk of a bovine tuberculosis breakdown in the aftermath of a commercial forest clearfell disturbance. J. Appl. Ecol. 2022. [Google Scholar] [CrossRef]
- Martin, S.; O’Keeffe, J.; Byrne, A.; Rosen, L.; White, P.; McGrath, G. Is moving from targeted culling to BCG-vaccination of badgers (Meles meles) associated with an unacceptable increased incidence of cattle herd tuberculosis in the Republic of Ireland? A practical non-inferiority wildlife intervention study in the Republic of Ireland (2011–2017). Prev. Vet. Med. 2020, 179, 105004. [Google Scholar] [CrossRef]
- More, S.J. Can bovine TB be eradicated from the Republic of Ireland? Could this be achieved by 2030? Ir. Vet. J. 2019, 72, 3. [Google Scholar] [CrossRef]
- Byrne, A.W.; White, P.W.; McGrath, G.; Martin, S.W. Risk of tuberculosis cattle herd breakdowns in Ireland: Effects of badger culling effort, density and historic large-scale interventions. Vet. Res. 2014, 45, 109. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Byrne, A.W.; Menzies, F.D.; McBride, K.R.; McCormick, C.M.; Scantlebury, M.; Reid, N. Interspecific visitation of cattle and badgers to fomites: A transmission risk for bovine tuberculosis? Ecol. Evolution. 2019, 9, 8479–8489. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Gormley, E.; Collins, D.M.; McGrath, G.; Sovsic, E.; Costello, E.; Corner, L.A. Tuberculosis in cattle herds are sentinels for Mycobacterium bovis infection in European badgers (Meles meles): The Irish Greenfield Study. Vet. Microbiol. 2011, 151, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.W.; Kenny, K.; Fogarty, U.; O’keeffe, J.J.; More, S.J.; McGrath, G.; Teeling, M.; Martin, S.W.; Dohoo, I.R. Spatial and temporal analyses of metrics of tuberculosis infection in badgers (Meles meles) from the Republic of Ireland: Trends in apparent prevalence. Prev. Vet. Med. 2015, 122, 345–354. [Google Scholar] [CrossRef]
- Crispell, J.; Cassidy, S.; Kenny, K.; McGrath, G.; Warde, S.; Cameron, H.; Rossi, G.; MacWhite, T.; White, P.C.L.; Lycett, S.; et al. Mycobacterium bovis genomics reveals transmission of infection between cattle and deer in Ireland. Microb. Genom. 2020, 6, e000388. [Google Scholar] [CrossRef]
- DAFM. Forest Statistics Ireland. 2020. Available online: https://www.teagasc.ie/media/website/crops/forestry/advice/Forest-Statistics-Ireland-2020.pdf (accessed on 1 June 2022).
- DAFM. Standards for Felling and Reforestation. 2019. Available online: https://www.teagasc.ie/media/website/crops/forestry/advice/Standards-for-Felling-and-Reforestation.pdf (accessed on 1 June 2022).
- Byrne, A.W.; Sleeman, P.D.; O’Keeffe, J.; Davenport, J. The ecology of the European badger (Meles meles) in Ireland: A review. Biol. Environ. Proc. R. Irish Acad. 2012, 112, 105–132. [Google Scholar] [CrossRef]
- Carden, R.F.; Carlin, C.M.; Marnell, F.; Mcelholm, D.; Hetherington, J.; Gammell, M.P. Distribution and range expansion of deer in Ireland. Mammal Rev. 2011, 41, 313–325. [Google Scholar] [CrossRef]
- Liu, Y.; Mccullagh, A.; Nieuwenhuis, M. What factors affect national-scale deer population dynamics in the Republic of Ireland? Scand. J. For. Res. 2018, 33, 535–549. [Google Scholar] [CrossRef]
- Morera-Pujol, V.; Mostert, P.S.; Murphy, K.; Burkitt, T.; Coad, B.; McMahon, B.J.; Nieuwenhuis, M.; Morelle, K.; Ward, A.; Ciuti, S. Bayesian species distribution models integrate presence-only and presence-absence data to predict deer distribution and relative abundance. bioRxiv 2022. Available online: https://www.biorxiv.org/content/10.1101/2022.05.23.493051v1.abstract (accessed on 1 June 2022).
- Potvin, F.; Bélanger, L.; Lowell, K. Marten Habitat Selection in a Clearcut Boreal Landscape. Conserv. Biol. 2000, 14, 844–857. [Google Scholar] [CrossRef]
- Fuller, A.K.; Harrison, D.J.; Lachowski, H.J. Stand scale effects of partial harvesting and clearcutting on small mammals and forest structure. For. Ecol. Manag. 2004, 191, 373–386. [Google Scholar] [CrossRef]
- Wäber, K.; Spencer, J.; Dolman, P.M. Achieving landscape-scale deer management for biodiversity conservation: The need to consider sources and sinks. J. Wildl. Manag. 2013, 77, 726–736. [Google Scholar] [CrossRef]
- Prentice, J.C.; Fox, N.J.; Hutchings, M.; White, P.C.L.; Davidson, R.; Marion, G. When to kill a cull: Factors affecting the success of culling wildlife for disease control. J. R. Soc. Interface 2019, 16, 20180901. [Google Scholar] [CrossRef] [PubMed]
- Borremans, B.; Faust, C.; Manlove, K.R.; Sokolow, S.; Lloyd-Smith, J.O. Cross-species pathogen spillover across ecosystem boundaries: Mechanisms and theory. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180344. [Google Scholar] [CrossRef]
- Barroso, P.; Breslin, P.; McGrath, G.; Madden, J.M.; Tratalos, J.A.; More, S.J.; Ryan, E.; Byrne, A.W.; Barrett, D. Is there an association between road building and bovine tuberculosis herd risk? A three time-point study in Ireland, 2011–2019. Prev. Vet. Med. 2022, 198, 105542. [Google Scholar] [CrossRef]
- van Tonder, A.J.; Thornton, M.J.; Conlan, A.J.K.; Jolley, K.A.; Goolding, L.; Mitchell, A.P.; Dale, J.; Palkopoulou, E.; Hogarth, P.J.; Hewinson, R.G.; et al. Inferring Mycobacterium bovis transmission between cattle and badgers using isolates from the Randomised Badger Culling Trial. PLOS Pathog. 2021, 17, e1010075. [Google Scholar] [CrossRef]
- O’Corry-Crowe, G.; Eves, J.; Hayden, T.J. Sett distribution, territory size and population density of badgers (Meles meles L.) in East Offaly. Badger 1993, 35, 56. [Google Scholar]
- Riordan, P.; Delahay, R.J.; Cheeseman, C.; Johnson, P.J.; Macdonald, D.W. Culling-Induced Changes in Badger (Meles meles) Behaviour, Social Organisation and the Epidemiology of Bovine Tuberculosis. PLoS ONE 2011, 6, e28904. [Google Scholar] [CrossRef]
- Gaughran, A.; Mullen, E.; MacWhite, T.; Maher, P.; Kelly, D.J.; Kelly, R.; Good, M.; Marples, N.M. Badger territoriality maintained despite disturbance of major road construction. PLoS ONE 2021, 16, e0242586. [Google Scholar] [CrossRef]
- Vial, F.; Donnelly, C.A. Localized reactive badger culling increases risk of bovine tuberculosis in nearby cattle herds. Biol. Lett. 2012, 8, 50–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Hagan, M.J.H.; Gordon, A.W.; McCormick, C.M.; Collins, S.F.; Trimble, N.A.; McGeown, C.F.; McHugh, G.E.; McBride, K.R.; Menzies, F.D. Effect of selective removal of badgers (Meles meles) on ranging behaviour during a ‘Test and Vaccinate or Remove’ intervention in Northern Ireland. Epidemiol. Infect. 2021, 149, e125. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.R.; Milne, G.; McCormick, C.; Collins, S.; O’Hagan, M.; Skuce, R.; Trimble, N.; Harwood, R.; Menzies, F.; Byrne, A.W. European badger (Meles meles) responses to low-intensity, selective culling: Using mark recapture and relatedness data to assess social perturbation. Ecol. Solut. Evid. 2022. [Google Scholar] [CrossRef]
- Wright, D.M.; Reid, N.; Ian Montgomery, W.; Allen, A.R.; Skuce, R.A.; Kao, R.R. Herd-level bovine tuberculosis risk factors: Assessing the role of low-level badger population disturbance. Sci. Rep. 2015, 5, 13062. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.G.; Evans, K.E.; Vineer, H.R.; van Wyk, J.A.; Morgan, E.R. Prediction and attenuation of seasonal spillover of parasites between wild and domestic ungulates in an arid mixed-use system. J. Appl. Ecol. 2018, 55, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.; Byrne, A.W.; Campbell, E.; Graham, J.; McGrath, J.; Kirke, R.; McMaster, W.; Zimmermann, J.; Adenuga, A.H. Quantifying Land Fragmentation in Northern Irish Cattle Enterprises. Land 2022, 11, 402. [Google Scholar] [CrossRef]
- Milne, G.; Graham, J.; McGrath, J.; Kirke, R.; McMaster, W.; Byrne, A.W. Investigating Farm Fragmentation as a Risk Factor for Bovine Tuberculosis in Cattle Herds: A Matched Case-Control Study from Northern Ireland. Pathogens 2022, 11, 299. [Google Scholar] [CrossRef]
- Tsairidou, S.; Allen, A.; Banos, G.; Coffey, M.; Anacleto, O.; Byrne, A.W.; Skuce, R.A.; Glass, E.J.; Woolliams, J.A.; Doeschl-Wilson, A.B. Can we breed cattle for lower bovine TB infectivity? Front. Vet. Sci. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- Walker, B.; Sánchez-Vizcaíno, F.; Barker, E.N. Effect of an antimicrobial stewardship intervention on the prescribing behaviours of companion animal veterinarians: A pre–post study. Vet. Rec. 2022, 190, e1485. [Google Scholar] [CrossRef]
- Buxton, R.T.; Lendrum, P.E.; Crooks, K.R.; Wittemyer, G. Pairing camera traps and acoustic recorders to monitor the ecological impact of human disturbance. Glob. Ecol. Conserv. 2018, 16, e00493. [Google Scholar] [CrossRef]
- Sosa, S.; Sueur, C.; Puga-Gonzalez, I. Network measures in animal social network analysis: Their strengths, limits, interpretations and uses. Methods Ecol. Evol. 2021, 12, 10–21. [Google Scholar] [CrossRef]
- Abdou, M.; Frankena, K.; O’Keeffe, J.; Byrne, A.W. Effect of culling and vaccination on bovine tuberculosis infection in a European badger (Meles meles) population by spatial simulation modelling. Prev. Vet. Med. 2016, 125, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Ciuti, S.; Kane, A. An introduction to agent-based models as an accessible surrogate to field-based research and teaching. Ecol. Evol. 2020, 10, 12482–12498. [Google Scholar] [CrossRef]
- Thiese, M.S. Observational and interventional study design types; an overview. Biochem. Med. 2014, 24, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lahuerta-Marin, A.; Milne, M.G.; McNair, J.; Skuce, R.A.; McBride, S.H.; Menzies, F.D.; McDowell, S.J.W.; Byrne, A.W.; Handel, I.G.; Bronsvoort, B.D.C. Bayesian latent class estimation of sensitivity and specificity parameters of diagnostic tests for bovine tuberculosis in chronically infected herds in Northern Ireland. Vet. J. 2018, 238, 15–21. [Google Scholar] [CrossRef]
- Byrne, A.W.; Quinn, J.L.; O’Keeffe, J.J.; Green, S.; Sleeman, D.P.; Martin, S.W.; Davenport, J. Large-scale movements in European badgers: Has the tail of the movement kernel been underestimated? J. Anim. Ecol. 2014, 83, 991–1001. [Google Scholar] [CrossRef]
- Byrne, A.W.; O’Keeffe, J.; Buesching, C.D.; Newman, C. Push and pull factors driving movement in a social mammal: Context dependent behavioral plasticity at the landscape scale. Curr. Zool. 2019, 65, 517–525. [Google Scholar] [CrossRef]
- More, S.J.; Clegg, T.A.; McGrath, G.; Collins, J.D.; Corner, L.A.L.; Gormley, E. Does reactive badger culling lead to an increase in tuberculosis in cattle? Vet. Rec. 2007, 161, 208–209. [Google Scholar] [CrossRef]
- Giller, P.S.; Johnson, M.; O’Halloran, J. Managing the Impacts of Forest Clearfelling on Stream Environments; COFORD: Dublin, Ireland, 2002. [Google Scholar]
- Ramírez-Villaescusa, A.; Medley, G.; Mason, S.; Green, L. Herd and individual animal risks associated with bovine tuberculosis skin test positivity in cattle in herds in south west England. Prev. Vet. Med. 2009, 92, 188–198. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]

| ≥1 Reactor Threshold | >2 Reactor Threshold | |||
|---|---|---|---|---|
| Breakdown status | Pre- | Post- | Pre- | Post- |
| 0 | 15,811 | 15,711 | 16,126 | 16,084 |
| % | 96.53 | 95.92 | 98.45 | 98.19 |
| 1 | 569 | 669 | 254 | 296 |
| % | 3.47 | 4.08 | 1.55 | 1.81 |
| Total | 16,380 | 16,380 | 16,380 | 16,380 |
| Parameter | OR | SE | Z | p | Upper 95%CI | Lower 95%CI |
|---|---|---|---|---|---|---|
| Pre/Post | 1.204 | 0.074 | 3.020 | 0.003 | 1.067 | 1.357 |
| Log(Herd Size) | 1.758 | 0.072 | 13.750 | 0.000 | 1.622 | 1.905 |
| Beef (Ref) | ||||||
| Dairy | 1.403 | 0.163 | 2.920 | 0.004 | 1.118 | 1.761 |
| Other | 0.483 | 0.092 | −3.830 | 0.000 | 0.332 | 0.701 |
| Suckler | 1.002 | 0.097 | 0.020 | 0.981 | 0.829 | 1.212 |
| Proportion Forestry | 2.282 | 0.878 | 2.140 | 0.032 | 1.074 | 4.851 |
| Constant | 0.002 | 0.000 | −28.880 | 0.000 | 0.001 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, A.W.; Barrett, D.; Breslin, P.; O’Keeffe, J.; Murphy, K.J.; Conteddu, K.; Morera-Pujol, V.; Ryan, E.; Ciuti, S. Disturbance Ecology Meets Bovine Tuberculosis (bTB) Epidemiology: A Before-and-After Study on the Association between Forest Clearfelling and bTB Herd Risk in Cattle Herds. Pathogens 2022, 11, 807. https://doi.org/10.3390/pathogens11070807
Byrne AW, Barrett D, Breslin P, O’Keeffe J, Murphy KJ, Conteddu K, Morera-Pujol V, Ryan E, Ciuti S. Disturbance Ecology Meets Bovine Tuberculosis (bTB) Epidemiology: A Before-and-After Study on the Association between Forest Clearfelling and bTB Herd Risk in Cattle Herds. Pathogens. 2022; 11(7):807. https://doi.org/10.3390/pathogens11070807
Chicago/Turabian StyleByrne, Andrew W., Damien Barrett, Philip Breslin, James O’Keeffe, Kilian J. Murphy, Kimberly Conteddu, Virginia Morera-Pujol, Eoin Ryan, and Simone Ciuti. 2022. "Disturbance Ecology Meets Bovine Tuberculosis (bTB) Epidemiology: A Before-and-After Study on the Association between Forest Clearfelling and bTB Herd Risk in Cattle Herds" Pathogens 11, no. 7: 807. https://doi.org/10.3390/pathogens11070807
APA StyleByrne, A. W., Barrett, D., Breslin, P., O’Keeffe, J., Murphy, K. J., Conteddu, K., Morera-Pujol, V., Ryan, E., & Ciuti, S. (2022). Disturbance Ecology Meets Bovine Tuberculosis (bTB) Epidemiology: A Before-and-After Study on the Association between Forest Clearfelling and bTB Herd Risk in Cattle Herds. Pathogens, 11(7), 807. https://doi.org/10.3390/pathogens11070807







