The Geological Characteristics of the Vadose Zone Influence the Impact of Treated Wastewater on the Groundwater Quality (SCA.Re.S. Project 2019–2020)
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical and Physical Parameters
2.2. Bacteria
2.3. Enteric Viruses
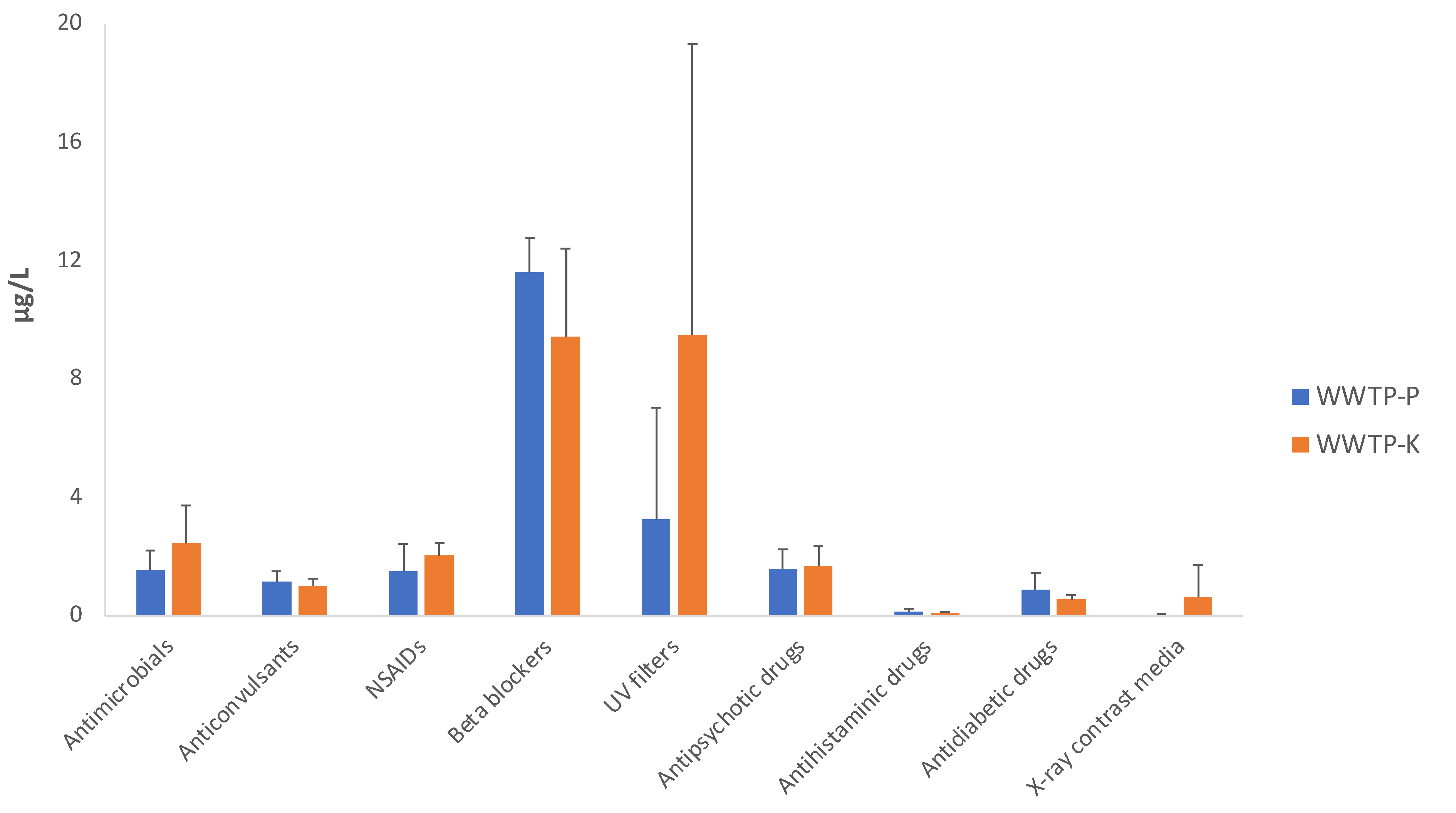
2.4. Contaminants of Emerging Concern
3. Materials and Methods
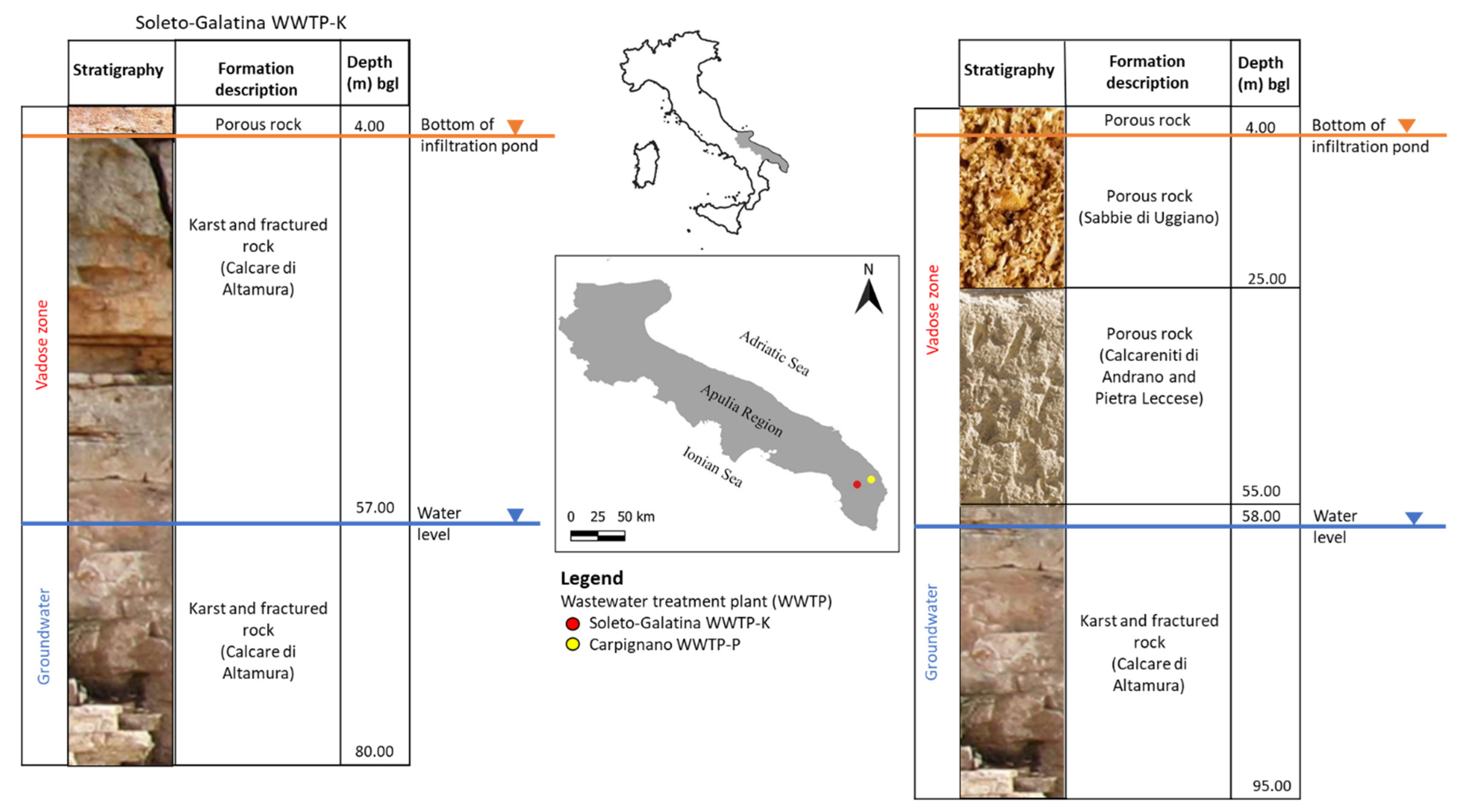
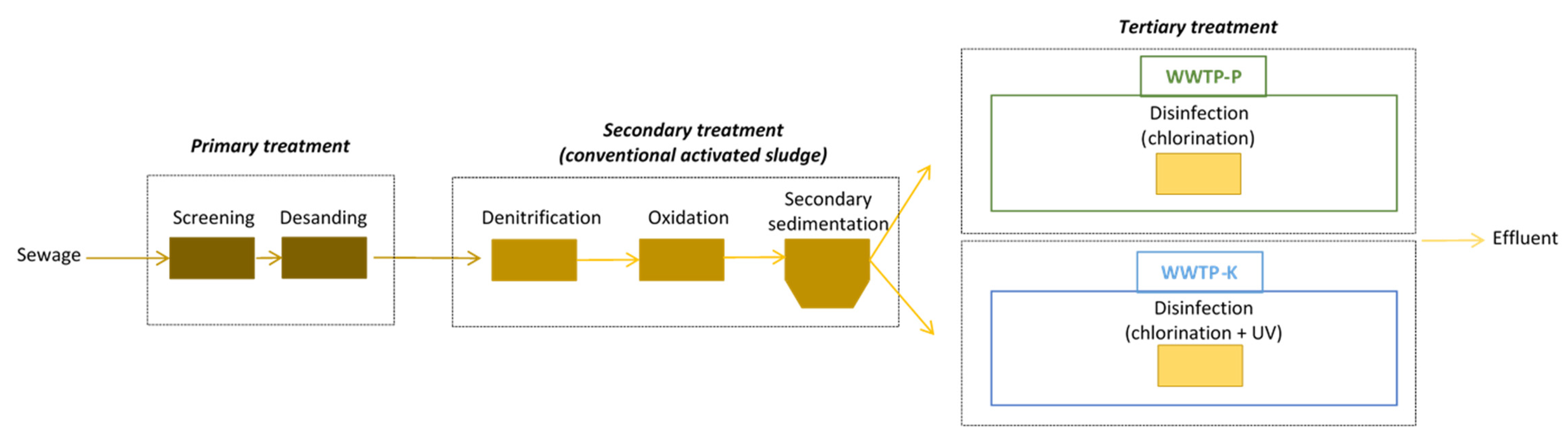
3.1. Study Scenarios
3.2. Sampling
3.3. Detection of Bacterial Indicators
3.4. Detection of Viruses
3.5. Chemical and Physical Parameters
3.6. Contaminants of Emerging Concern
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zektser, I.S.; Everett, L.G. Groundwater Resources of the World and Their Use; UNESCO: Saint-Denis, France, 2004; ISBN 92-9220-007-0. [Google Scholar]
- European Environment Agency 2022. Europe’s Groundwater a Key Resource under Pressure. EN PDF: TH-AM-22-003-EN-N. ISBN: 978-92-9480-459-4. ISSN: 2467-3196. Available online: https://www.eea.europa.eu/downloads/af1493c218ae4218ba0ca0eac8a4b580/1648564905/europes-groundwater.pdf (accessed on 13 April 2022). [CrossRef]
- Lugoli, F.; Leopizzi, M.I.; Bagordo, F.; Grassi, T.; Guido, M.; De Donno, A. Widespread microbiological groundwater contamination in the South-eastern Salento (Puglia-Italy). J. Environ. Monit. 2011, 13, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Portoghese, I.; D’Agostino, D.; Giordano, R.; Scardigno, A.; Apollonio, C.; Vurro, M. An integrated modelling tool to evaluate the acceptability of irrigation constraint measures for groundwater protection. Environ. Modell. Softw. 2013, 46, 90–103. [Google Scholar] [CrossRef]
- De Giglio, O.; Quaranta, A.; Barbuti, G.; Napoli, C.; Caggiano, G.; Montagna, M.T. Factors influencing groundwater quality: Towards an integrated management approach. Ann. Ig. 2015, 27, 52–57. [Google Scholar] [PubMed]
- De Giglio, O.; Caggiano, G.; Bagordo, F.; Barbuti, G.; Brigida, S.; Lugoli, F.; Grassi, T.; La Rosa, G.; Lucentini, L.; Uricchio, V.F.; et al. Enteric viruses and fecal bacteria indicators to assess groundwater quality and suitability for irrigation. Int. J. Environ. Res. Public Health. 2017, 14, 558. [Google Scholar] [CrossRef]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and consequences of groundwater contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef]
- Bagordo, F.; Migoni, D.; Grassi, T.; Serio, F.; Indolo, A.; Guido, M.; Zaccarelli, N.; Fanizzi, F.P.; De Donno, A. Using the DPSIR framework to identify factors influencing the quality of groundwater in Grecia Salentina (Puglia, Italy). Rend. Lincei. Sci. Fis. Nat. 2016, 27, 113–125. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Calia, C.; Pousis, C.; Triggiano, F.; Murgolo, S.; De Ceglie, C.; Bagordo, F.; Apollonio, F.; Diella, G.; et al. Microbiological and chemical assessment of wastewater discharged by infiltration trenches in fractured and karstified limestone (SCA.Re.S. Project 2019-2020). Pathogens 2020, 9, 1010. [Google Scholar] [CrossRef]
- Reberski, J.L.; Terzic, J.; Maurice, L.D.; Lapworth, D.J. Emerging organic contaminants in karst groundwater: A global level assessment. J. Hydrol. 2022, 604, 127242. [Google Scholar] [CrossRef]
- Kačaroğlu, F. Review of groundwater pollution and protection in karst areas. Water Air Soil Pollut. 1999, 113, 337–356. [Google Scholar] [CrossRef]
- Panda, B.; Chidambaram, S. Influence of the vadose zone on groundwater pollution—A review. Int. J. Civ. Environ. Agric. Eng. 2019, 1, 41–44. [Google Scholar] [CrossRef]
- Dahan, O. Vadose zone monitoring as a key to groundwater protection. Front. Water 2020, 2, 599569. [Google Scholar] [CrossRef]
- Li, J.; Xi, B.; Cai, W.; Yang, Y.; Yongfeng, J.; Xiang, L.; Yonggao, L.; Ningqing, L.; Huan, H.; Jinjin, Y. Identification of dominating factors affecting vadose zone vulnerability by a simulation method. Sci. Rep. 2017, 7, 45955. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ju, F.; Zhang, T. Tracking human sewage microbiome in a municipal wastewater treatment plant. Appl. Microbiol. Biotechnol. 2014, 98, 3317–3326. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustainability 2018, 10, 86. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, T.; Li, M.; Liu, X. Organic contaminants in the effluent of Chinese wastewater treatment plants. Environ. Sci. Pollut. Res. 2018, 25, 26852–26860. [Google Scholar] [CrossRef] [PubMed]
- Triggiano, F.; Calia, C.; Diella, G.; Montagna, M.T.; De Giglio, O.; Caggiano, G. The Role of urban wastewater in the environmental transmission of antimicrobial resistance: The current situation in Italy (2010–2019). Microorganisms 2020, 8, 1567. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Yin, L.F.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total Environ. 2021, 754, 142122. [Google Scholar] [CrossRef]
- Legislative Decree 3 April 2006. No. 152. Environmental Standards (G.U. n.88 of 14 April 2006). Available online: https://www.gazzettaufficiale.it/dettaglio/codici/materiaAmbientale (accessed on 4 March 2022).
- Commission Implementing Decision (EU) 2020/1161 of 4 August 2020 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document Number C(2020) 5205) (Text with EEA Relevance) OJ L 257 06.08.2020, p. 32. Available online: http://data.europa.eu/eli/dec_impl/2020/1161/oj (accessed on 4 March 2022).
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Kumar Parida, V.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Kumar Gupta, A. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Masciopinto, C.; Caputo, M.C. Modeling unsaturated-saturated flow and nickel transport in fractured rocks. Vadose Zone J. 2011, 10, 1045–1057. [Google Scholar] [CrossRef]
- Medici, G.; West, L.J.; Chapman, P.J.; Banwart, S.A. Prediction of contaminant transport in fractured carbonate aquifer types: A case study of the Permian Magnesian Limestone Group (NE England, UK). Environ. Sci. Pollut. Res. 2019, 26, 24863–24884. [Google Scholar] [CrossRef] [PubMed]
- Masciopinto, C.; De Giglio, O.; Scrascia, M.; Fortunato, F.; La Rosa, G.; Suffredini, E.; Pazzani, C.; Prato, R.; Montagna, M.T. Human health risk assessment for the occurrence of enteric viruses in drinking water from wells: Role of flood runoff injections. Sci. Total Environ. 2019, 666, 559–571. [Google Scholar] [CrossRef]
- Walczak, J.J.; Bardy, S.L.; Feriancikova, L.; Xu, S. Influence of tetracycline resistance on the transport of manure-derived Escherichia coli in saturated porous media. Water Res. 2011, 45, 1681–1690. [Google Scholar] [CrossRef][Green Version]
- Bhavna, A.; Dipankar, D.; Boris, F.; Raghavendra, B.J.; Wainwright, H.M. Understanding and predicting vadose zone processes. Rev. Mineral. Geochem. 2019, 85, 303–328. [Google Scholar]
- Lee, J.H.; Lee, B.J.; Yun, U.; Koh, D.C.; Kim, S.J.; Han, D.; Unno, T. In-situ microbial colonization and its potential contribution on biofilm formation in subsurface sediments. J. Appl. Biol. Chem. 2019, 62, 51–56. [Google Scholar] [CrossRef]
- Vivaldi, G.A.; Camposeo, S.; Caponio, G.; Lopriore, G.; Discipio, F.; Apollonio, F.; Triggiano, F.; De Giglio, O.; Montagna, M.T. Irrigation of olives with reclaimed wastewaters and deficit strategies affect pathogenic bacteria contamination of water and soil. Pathogens 2022, 11, 488. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bryant, A.E. The role of clostridial toxins in the pathogenesis of gas gangrene. Clin. Infect. Dis. 2002, 35, S93–S100. [Google Scholar] [CrossRef]
- Kadzielska, J.; Obuch-Woszczatynski, P.; Pituch, H.; Młynarczyk, G. Clostridium perfringens as the etiological agent of antibiotic associated diarrhoea. Postep. Microbiol. 2012, 51, 17–25. [Google Scholar]
- Ghorpade, K.B.; Suryawanshi, M.; Shinde, S.M. Elimination of Pseudomonas aeruginosa from water systems: A review. J. Biomed. Pharm. Res. 2019, 8, 124–127. [Google Scholar] [CrossRef][Green Version]
- Jamieson, R.C.; Gordon, R.; Sharples, K.E.; Madani, G. Movement and persistence of fecal bacteria in agricultural soils and subsurface drainage water: A review. Can. Agric. Eng. 2002, 44, 1.1–1.9. [Google Scholar]
- Stevik, T.K.; Aa, K.; Ausland, G.; Hanssen, J.F. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: A review. Water Res. 2004, 38, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G.; Harvey, R.W. Transport of pathogens through soils and aquifers. Environ. Microbiol. 1992, 19, 103–123. [Google Scholar]
- Engström, E.; Thunvik, R.; Kulabako, R.; Balfors, B. Water transport, retention, and survival of escherichia coli in unsaturated porous media: A comprehensive review of processes, models, and factors. Crit. Rev. Environ. Sci. 2015, 45, 1–100. [Google Scholar] [CrossRef]
- Lance, J.C.; Gerba, C.P. Virus movement in soil during saturated and unsaturated flow. Appl. Environ. Microb. 1984, 47, 335–337. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; Anto’nio, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Ladhari, A.; La Mura, G.; Di Marino, C.; Di Fabio, G.; Zarrelli, A. Sartans: What they are for, how they degrade, where they are found and how they transform. Sustain. Chem. Pharm. 2021, 20, 100409. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Soulsby, C.; Cheng, Q.; Binley, A.; Jiang, R.; Tao, M. Characterizing the heterogeneity of karst critical zone and its hydrological function: An integrated approach. Hydrol. Process. 2018, 32, 2932–2946. [Google Scholar] [CrossRef]
- Caputo, M.C.; De Carlo, L.; Masciopinto, C.; Nimmo, J.R. Measurement of field-saturated hydraulic conductivity on fractured rock outcrops near Altamura (Southern Italy) with an adjustable large ring infiltrometer. Environ. Earth Sci. 2010, 60, 583–590. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Grenni, P.; Caputo, M.C.; Ancona, V.; Uricchio, V.F. Pharmaceutical waste disposal: Assessment of its effects on bacterial communities in soil and groundwater. Chem. Ecol. 2011, 27, 43–51. [Google Scholar] [CrossRef]
- UNI EN ISO 9308-1:2017; Qualità dell’acqua-Conta di Escherichia Coli e Batteri Coliformi-Parte 1: Metodo per Filtrazione su Membrana per Acque Contraddistinte da una Ridotta Flora Batterica di Fondo. Available online: http://store.uni.com/catalogo/uni-en-iso-9308-1-2017?josso_back_to=http://store.uni.com/josso-security-check.php&josso_cmd=login_optional&josso_partnerapp_host=store.uni.com (accessed on 4 March 2022).
- EN ISO 7899-2, 2003; Water Quality-Detection and Enumeration of Intestinal Enterococci-Part 2: Membrane Filtration Method. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/14854.html (accessed on 13 April 2022).
- APAT CNR IRSA 7080, Man 29/2003. Salmonella spp. Available online: http://www.irsa.cnr.it/Docs/Capitoli/7080.pdf (accessed on 13 April 2022).
- UNI EN ISO 16266:2008; Water Quality-Detection and Enumeration of Pseudomonas aeruginosa by Membrane Filtration. Available online: https://www.iso.org/standard/70091.html (accessed on 13 April 2022).
- APAT CNR IRSA 7060 B Man 29 2003. Spore di Clostridi Solfito Riduttori (Acque Superficiali, di Fiume, di Lago, Acque Reflue anche Sottoposte a Trattamento). Available online: http://www.irsa.cnr.it/Docs/Capitoli/7060.pdf (accessed on 13 April 2022).
- World Health Organization. Guidelines for Environmental Surveillance of Poliovirus Circulation. 2003. Available online: http://polioeradication.org/wp-content/uploads/2016/07/WHO_V-B_03.03_eng.pdf (accessed on 4 March 2022).
- Iaconelli, M.; Muscillo, M.; Della Libera, S.; Fratini, M.; Meucci, L.; De Ceglia, M.; Giacosa, D.; La Rosa, G. One-year surveillance of human enteric viruses in raw and treated wastewaters, downstream river waters, and drinking waters. Food Environ. Virol. 2017, 9, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bonanno Ferraro, G.; Suffredini, E.; Mancini, P.; Veneri, C.; Iaconelli, M.; Bonadonna, L.; Montagna, M.T.; De Giglio, O.; La Rosa, G. Pepper mild mottle virus as indicator of pollution: Assessment of prevalence and concentration in different water environments in Italy. Food Environ. Virol. 2021, 13, 117–125. [Google Scholar] [CrossRef]
- BLAST. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 13 April 2022).
- Ottaviani, M.; Bonadonna, L. (Eds.) Rapporti ISTISAN 2007/31. Metodi Analitici di Riferimento per le Acque Destinate al Consumo Umano ai Sensi del D.L. 31/2001; Metodi Chimici. Met ISS BCA 023; 2007; p. 328. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2277_allegato.pdf (accessed on 13 April 2022).
- APAT CNR IRSA 2030 Man 29 2003. Metodi Analitici per le Acque. Available online: http://www.irsa.cnr.it/Docs/Capitoli/1000.pdf (accessed on 13 April 2022).
- UNI EN 872:2005; Qualità Dell’acqua-Determinazione dei Solidi Sospesi-Metodo per Filtrazione Attraverso Filtri di Fibra di Vetro. Available online: http://wwwstore.uni.com/catalogo/uni-en-872-2005 (accessed on 13 April 2022).
- APHA 22nd ed 2012 5210, D. Respirometric Method. Available online: https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/scientific/technical-documents/white-papers/apha-biochemical-oxygen-demand-white-paper.pdf (accessed on 13 April 2022).
- ISO 15705:2002; Water Quality-Determination of the Chemical Oxygen Demand Index (ST-COD)-Small-Scale Sealed-Tube Method. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/28778.html (accessed on 13 April 2022).
- UNI 11759:2019; Determinazione dell’azoto Totale-Metodo Mediante Spettrometria UV Dopo Digestione Ossidativa con Persolfato di Sodio Utilizzando una Apparecchiatura Che Opera in Sequenza Analitica Coordinata. Available online: http://store.uni.com/catalogo/uni-11759-2019 (accessed on 13 April 2022).
- UNI EN ISO 10304-1:2009; Qualità Dell’acqua-Determinazione di Anioni Disciolti Mediante Cromatografia Ionica in Fase Liquida-Parte 1: Determinazione di Bromuri, Cloruri, Fluoruri, Nitrati, Nitriti, Fosfati e Solfati. Available online: http://store.uni.com/catalogo/uni-en-iso-10304-1-2009 (accessed on 13 April 2022).
- UNICHIM 2252:2008; Qualità dell’acqua: Determinazione del Fosfato Solubile e del Fosforo Totale-Metodo Colorimetrico Dell’ammonio Fosfoantimonilmolibdato Dopo Sequenza Analitica Coordinata. Available online: https://www.unichim.it/metodi/ (accessed on 13 April 2022).
- Lorimer, M.F.; Kiermeier, A. Analysing microbiological data: Tobit or not Tobit? Int. J. Food Microbiol. 2007, 116, 313–318. [Google Scholar] [CrossRef]
| Parameters | Unit of Measure | Limit Value | WWTP-P | WWTP-K | ||||
|---|---|---|---|---|---|---|---|---|
| TW | SW | TW | MW | SW | TW | MW | ||
| pH | 6–8 | 7.7 ± 0.3 | 7.8 ± 0.2 | 7.4 ± 0.1 | 7.7 ± 0.3 | 7.4 ± 0.3 | 7.4 ± 0.3 | |
| Conductivity | (μS/cm) | - | 1745 ± 264 | 1318 ± 319 | 568 ± 36 | 1699 ± 210 | 1080 ± 265 | 1163 ± 35 |
| BOD5 | (mg/L) | 20 | 640 ± 207 | 11.3 ± 4.6 | 0 | 580 ± 142 | 10.3 ± 2.5 | 0 |
| COD | (mg/L) | 100 | 935 ± 267 | 42.9 ± 45.5 | 0 | 859 ± 215 | 27.5 ± 6.5 | 2.5 ± 3.1 |
| TN | (mg/L) | 15 | 106.4 ± 16.7 | 14.0 ± 17.3 | 27.1 ± 2.2 * | 74.1 ± 11.6 | 14.4 ± 9.2 | 26.4 ± 2.3 * |
| TP | (mg/L) | 2 | 12.3 ± 4.0 | 3.4 ± 3.7 | ND | 10.7 ± 3.6 | 2.1 ± 0.7 | ND |
| Bacteria | Unit of Measure | WWTP-P | WWTP-K | ||
|---|---|---|---|---|---|
| TW | MW | TW | MW | ||
| E. coli | CFU/100 mL (P/T) | 893 ± 1138 (8/8) | <1 (0/8) | 6 ± 5 (7/7) | <1 (0/7) |
| Enterococci | CFU/100 mL (P/T) | 16.1 ± 30 (8/8) | <1 (0/8) | 5 ± 11 (7/7) | <1 (0/7) |
| C. perfringens | CFU/100 mL (P/T) | 3 ± 3 (8/8) | 2 ± 5 (1/8) | 5 ± 2 (7/7) | <1 (0/7) |
| P. aeruginosa | CFU/250 mL (P/T) | 3 ± 6 (8/8) | 463 ± 1228 (8/8) | 34.0 ± 89 (7/7) | 5750 ± 7599 (7/7) |
| WWTP-P | WWTP-K | |||||
|---|---|---|---|---|---|---|
| Virus | SW N (%) | TW N (%) | MW N (%) | SW N (%) | TW N (%) | MW N (%) |
| AdV | 0 (0) | 0 (0) | 0 (0) | 5 (71.4) | 3 (42.9) | 1 (14.3) |
| NoV-GI | 1 (12.5) | 0 (0) | 0 (0) | 2 (28.6) | 0 (0) | 0 (0) |
| NoV-GII | 1 (12.5) | 1 (12.5) | 0 (0) | 5 (71.4) | 3 (42.9) | 0 (0) |
| EV | 1 (12.5) | 0 (0) | 0 (0) | 4 (57.1) | 0 (0) | 0 (0) |
| HEV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HAV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| RoV | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PMMoV | 4 (50) | 5 (62.5) | 0 (0) | 5 (71.4) | 6 (85.7) | 3 (42.9) |
| Name | WWTP-P | WWTP-K | ||||
|---|---|---|---|---|---|---|
| TW (μg/L) | MW (μg/L) | RC (%) | TW (μg/L) | MW (μg/L) | RC (%) | |
| Antimicrobials | 1.53 ± 0.69 | 0.01 ± 0.01 | 1.41 ± 2.43 | 2.46 ± 1.27 | 0.49 ± 0.11 | 41.29 ± 52.73 |
| Clarithromycin | 0.04 ± 0.05 | 0.00 ± 0.01 | 3.33 ± 8.16 | 0.10 ± 0.05 | 0.00 ± 0.01 | 1.66 ± 4.39 |
| Climbazol | 0.02 ± 0.03 | 0.00 | 0.00 ± 0.00 | 0.08 ± 0.03 | 0.04 ± 0.05 | 52.25 ± 36.76 |
| Fluconazole | 0.62 ± 0.28 | 0.01 ± 0.01 | 2.12 ± 2.54 | 0.38 ± 0.14 | 0.43 ± 0.11 | 122.16 ± 33.04 |
| Levofloxacin | 0.84 ± 0.60 | 0.00 | 0.00 ± 0.00 | 1.89 ± 1.19 | 0.01 ± 0.02 | 0.89 ± 1.87 |
| Anticonvulsants | 1.14 ± 0.35 | 0.02 ± 0.01 | 1.41 ± 1.22 | 1.02 ± 0.25 | 0.86 ± 0.12 | 78.53 ± 67.88 |
| Carbamazepine | 0.39 ± 0.08 | 0.01 ± 0.01 | 2.34 ± 2.66 | 0.32 ± 0.07 | 0.49 ± 0.08 | 162.19 ± 55.75 |
| Carbamazepine-10,11- Epoxide | 0.11 ± 0.10 | 0.00 ± 0.01 | 0.31 ± 0.89 | 0.07 ± 0.02 | 0.04 ± 0.01 | 59.32 ± 25.46 |
| Gabapentin | 0.23 ± 0.18 | 0.00 | 0.00 ± 0.00 | 0.25 ± 0.10 | 0.00 ± 0.01 | 0.64 ± 1.70 |
| Lamotrigine | 0.41 ± 0.19 | 0.01 ± 0.01 | 2.42 ± 1.90 | 0.38 ± 0.14 | 0.33 ± 0.09 | 91.97 ± 33.01 |
| Nonsteroidal anti-inflammatory drugs | 1.49 ± 0.92 | 0.001 ± 0.002 | 0.12 ± 0.35 | 2.04 ± 0.43 | 0.11 ± 0.05 | 19.60 ± 43.52 |
| Diclofenac | 1.20 ± 0.71 | 0.00 | 0.00 ± 0.00 | 1.52 ± 0.42 | 0.00 | 0.00 ± 0.00 |
| Ketoprofen | 0.07 ± 0.13 | 0.00 | 0.00 ± 0.00 | 0.19 ± 0.08 | 0.00 | 0.00 ± 0.00 |
| Niflumic Acid | 0.04 ± 0.03 | 0.00 | 0.00 ± 0.00 | 0.07 ± 0.03 | 0.00 ± 0.01 | 1.19 ± 3.15 |
| Tramadol | 0.18 ± 0.09 | 0.00 ± 0.01 | 0.37 ± 1.04 | 0.25 ± 0.20 | 0.11 ± 0.05 | 77.23 ± 57.85 |
| Beta-adrenoceptor blocking agents | 11.62 ± 1.14 | 0.01 ± 0.01 | 0.05 ± 0.05 | 9.44 ± 2.98 | 1.87 ± 0.54 | 9.30 ± 14.21 |
| Atenolol | 0.02 ± 0.03 | 0.00 | 0.00 ± 0.00 | 0.08 ± 0.03 | 0.00 ± 0.01 | 4.80 ± 9.08 |
| Bisoprolol | 0.07 ± 0.07 | 0.00 | 0.00 ± 0.00 | 0.15 ± 0.07 | 0.01 ± 0.01 | 4.20 ± 5.40 |
| Clopidrogel | 0.02 ± 0.01 | 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.00 | 0.00 ± 0.00 |
| Fenofibric Acid | 0.04 ± 0.10 | 0.00 | 0.00 ± 0.00 | 0.09 ± 0.08 | 0.00 | 0.00 ± 0.00 |
| Flecainide | 1.76 ± 0.88 | 0.00 ± 0.01 | 0.17 ± 0.47 | 0.99 ± 0.22 | 0.07 ± 0.03 | 6.84 ± 3.05 |
| Irbesartan | 3.36 ± 0.76 | 0.00 | 0.01 ± 0.02 | 1.98 ± 0.75 | 0.14 ± 0.10 | 6.65 ± 4.37 |
| Irbesartan 446 | 0.67 ± 0.16 | 0.00 ± 0.01 | 0.19 ± 0.35 | 0.89 ± 0.56 | 0.24 ± 0.10 | 32.36 ± 16.48 |
| Losartan | 0.06 ± 0.07 | 0.00 | 0.00 ± 0.00 | 0.05 ± 0.03 | 0.00 | 0.00 ± 0.00 |
| Metoprolol | 0.08 ± 0.05 | 0.00 | 0.00 ± 0.00 | 0.08 ± 0.04 | 0.01 ± 0.01 | 17.68 ± 11.79 |
| Metoprolol Acid | 0.18 ± 0.14 | 0.00 | 0.00 ± 0.00 | 0.45 ± 0.23 | 0.03 ± 0.02 | 7.76 ± 5.07 |
| Olmesartan | 3.75 ± 0.74 | 0.01 ± 0.01 | 0.31 ± 0.23 | 3.73 ± 1.35 | 1.36 ± 0.35 | 39.57 ± 11.69 |
| Sotalol | 0.19 ± 0.13 | 0.00 | 0.00 ± 0.00 | 0.14 ± 0.04 | 0.01 ± 0.02 | 7.64 ± 14.18 |
| Telmisartan | 1.11 ± 0.44 | 0.00 | 0.00 ± 0.00 | 0.25 ± 0.15 | 0.00 | 0.00 ± 0.00 |
| Valsartan | 0.31 ± 0.51 | 0.00 | 0.00 ± 0.00 | 0.55 ± 0.58 | 0.00 | 0.00 ± 0.00 |
| UV filters | ||||||
| 2-Phenyl-5-BenzimidazolesulfonicAcid | 3.28 ± 3.77 | 0.00 | 0.00 ± 0.00 | 9.50 ± 9.82 | 0.13 ± 0.23 | 2.74 ± 4.45 |
| Antipsychotic drugs | 1.57 ± 0.66 | 0.001 ± 0.002 | 0.04 ± 0.11 | 1.67 ± 0.68 | 0.60 ± 0.19 | 38.58 ± 34.41 |
| Amisulpride | 0.14 ± 0.07 | 0.00 | 0.00 ± 0.00 | 0.33 ± 0.19 | 0.12 ± 0.05 | 46.44 ± 26.93 |
| EDDP | 0.83 ± 0.38 | 0.00 | 0.00 ± 0.00 | 0.86 ± 0.42 | 0.25 ± 0.14 | 31.37 ± 14.04 |
| Metamphetamine | 0.00 | 0.00 | - | 0.07 ± 0.04 | 0.00 | 0.00 ± 0.00 |
| Sulpride | 0.27 ± 0.14 | 0.001 ± 0.010 | 0.15 ± 0.42 | 0.21 ± 0.07 | 0.14 ± 0.04 | 73.72 ± 38.02 |
| Venlafaxine | 0.31 ± 0.17 | 0.00 | 0.00 ± 0.00 | 0.19 ± 0.09 | 0.09 ± 0.03 | 52.37 ± 24.57 |
| Antihistaminic drugs | ||||||
| Cetirizine | 0.13 ± 0.12 | 0.00 | 0.00 ± 0.00 | 0.10 ± 0.04 | 0.02 ± 0.02 | 22.35 ± 13.14 |
| Antidiabetic drugs | ||||||
| Sitagliptin | 0.87 ± 0.55 | 0.00 | 0.00 ± 0.00 | 0.57 ± 0.12 | 0.11 ± 0.06 | 18.69 ± 8.88 |
| X-ray contrast media | ||||||
| Iopromide | 0.02 ± 0.04 | 0.00 | 0.00 ± 0.00 | 0.62 ± 1.11 | 0.00 ± 0.01 | 0.05 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giglio, O.; Triggiano, F.; Apollonio, F.; Pousis, C.; Calia, C.; Diella, G.; Bagordo, F.; Murgolo, S.; Grassi, T.; De Ceglie, C.; et al. The Geological Characteristics of the Vadose Zone Influence the Impact of Treated Wastewater on the Groundwater Quality (SCA.Re.S. Project 2019–2020). Pathogens 2022, 11, 677. https://doi.org/10.3390/pathogens11060677
De Giglio O, Triggiano F, Apollonio F, Pousis C, Calia C, Diella G, Bagordo F, Murgolo S, Grassi T, De Ceglie C, et al. The Geological Characteristics of the Vadose Zone Influence the Impact of Treated Wastewater on the Groundwater Quality (SCA.Re.S. Project 2019–2020). Pathogens. 2022; 11(6):677. https://doi.org/10.3390/pathogens11060677
Chicago/Turabian StyleDe Giglio, Osvalda, Francesco Triggiano, Francesca Apollonio, Chrysovalentinos Pousis, Carla Calia, Giusy Diella, Francesco Bagordo, Sapia Murgolo, Tiziana Grassi, Cristina De Ceglie, and et al. 2022. "The Geological Characteristics of the Vadose Zone Influence the Impact of Treated Wastewater on the Groundwater Quality (SCA.Re.S. Project 2019–2020)" Pathogens 11, no. 6: 677. https://doi.org/10.3390/pathogens11060677
APA StyleDe Giglio, O., Triggiano, F., Apollonio, F., Pousis, C., Calia, C., Diella, G., Bagordo, F., Murgolo, S., Grassi, T., De Ceglie, C., Brigida, S., La Rosa, G., Mancini, P., Bonanno Ferraro, G., De Donno, A., Mascolo, G., Caputo, M. C., & Montagna, M. T. (2022). The Geological Characteristics of the Vadose Zone Influence the Impact of Treated Wastewater on the Groundwater Quality (SCA.Re.S. Project 2019–2020). Pathogens, 11(6), 677. https://doi.org/10.3390/pathogens11060677













