Erythema Multiforme as Early Manifestation of COVID-19: A Case Report
Abstract
:1. Introduction
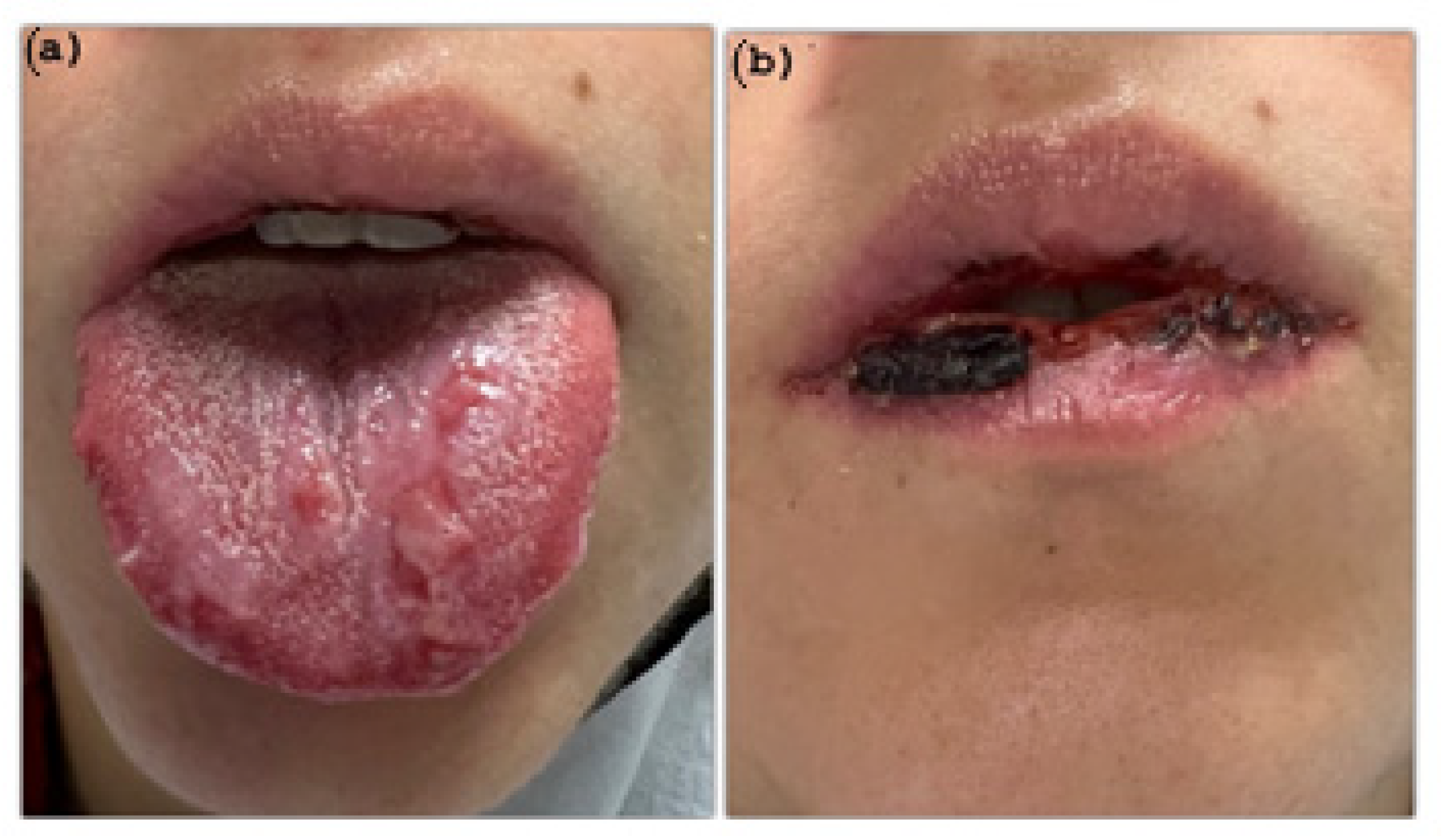
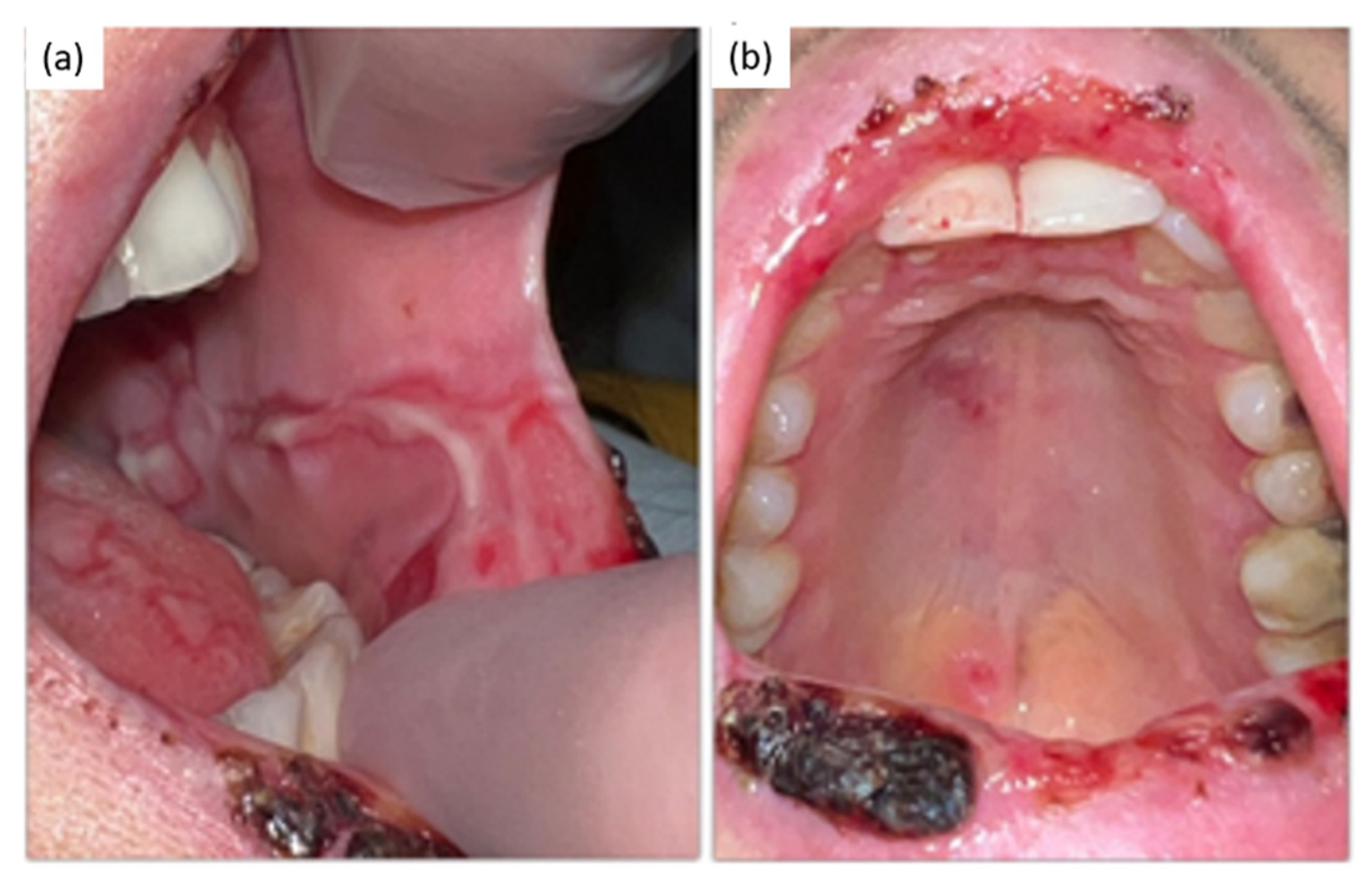
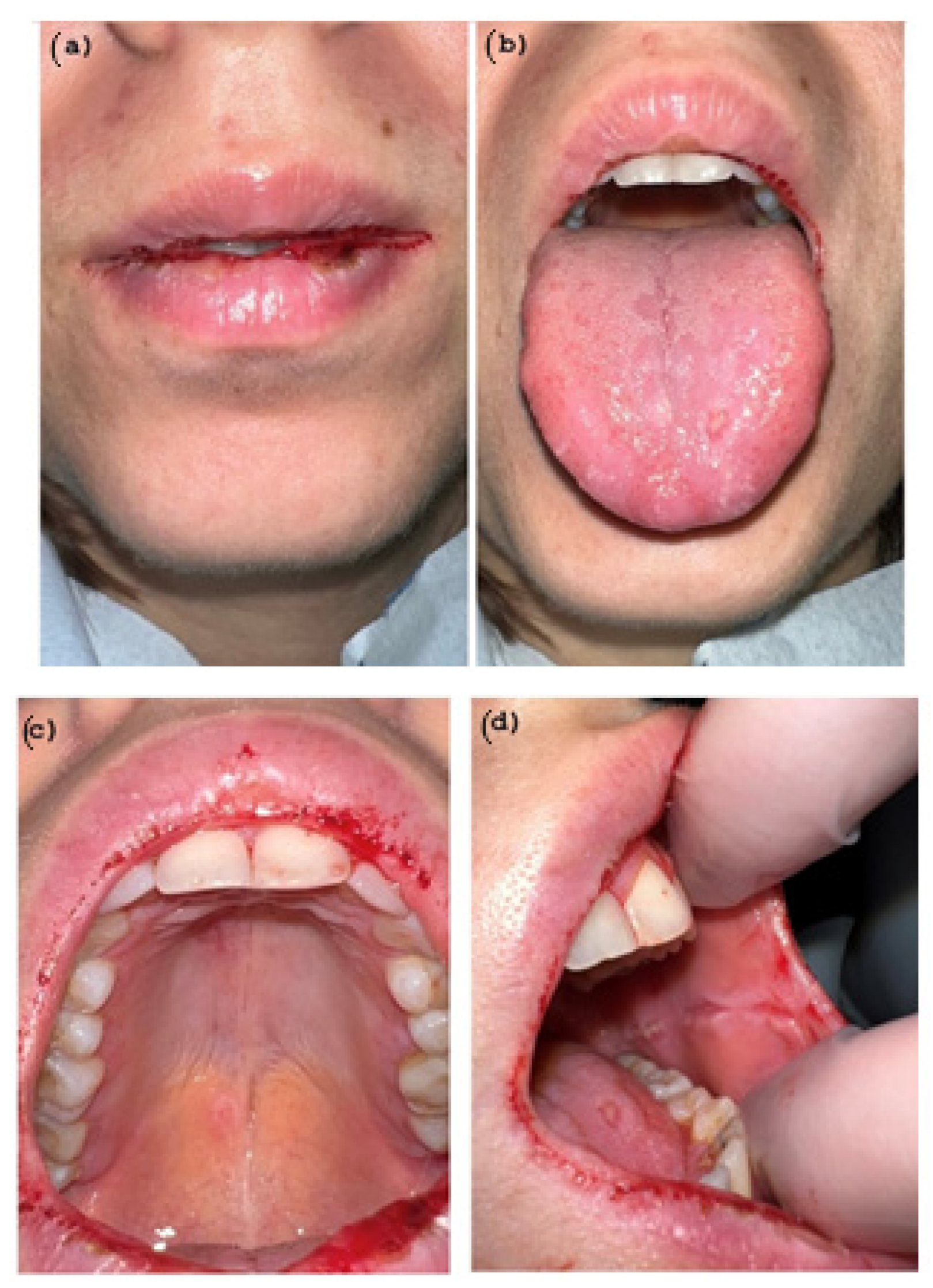
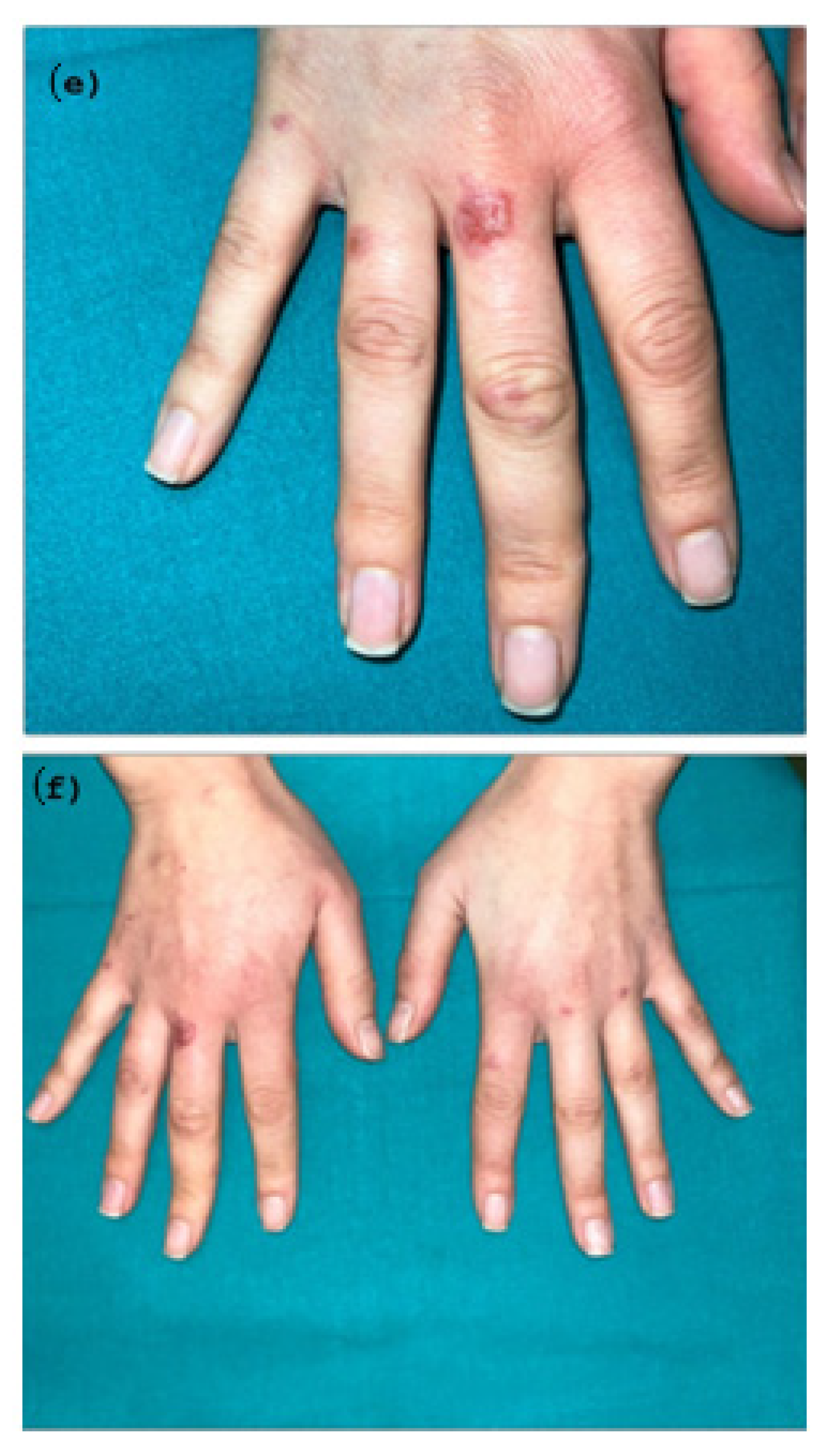
2. Case Presentation
Diagnosis and Treatment
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kusiak, A.; Cichońska, D.; Tubaja, M.; Skorek, A.; Jereczek-Fossa, B.A.; Corrao, G.; Marvaso, G.; Alterio, D. COVID-19 manifestation in the oral cavity–a narrative literature review. Acta Otorhinolaryngol. Ital. 2021, 41, 395. [Google Scholar] [CrossRef] [PubMed]
- Aragoneses, J.; Suárez, A.; Algar, J.; Rodríguez, C.; López-Valverde, N.; Aragoneses, J.M. Oral Manifestations of COVID-19: Updated Systematic Review With Meta-Analysis. Front. Med. 2021, 8, 726753. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cauhe, J.; Ortega-Quijano, D.; Carretero-Barrio, I.; Suarez-Valle, A.; Saceda-Corralo, D.; Moreno-Garcia Del Real, C.; Fernandez-Nieto, D. Erythema multiforme-like eruption in patients with COVID-19 infection: Clinical and histological findings. Clin. Exp. Dermatol. 2020, 45, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Icenogle, T. COVID-19: Infection or Autoimmunity. Front. Immunol. 2020, 11, 2055. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Mariette, X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat. Rev. Rheumatol. 2021, 17, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Winchester, N.; Calabrese, C.; Calabrese, L.H. The intersection of COVID-19 and autoimmunity: What is our current understanding? Pathog. Immun. 2021, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Lao, W.P.; Imam, S.A.; Nguyen, S.A. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J. Otorhinolaryngol. Head Neck Surg. 2020, 17, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Capocasale, G.; Nocini, R.; Faccioni, P.; Donadello, D.; Bertossi, D.; Albanese, M.; Zotti, F. How to deal with coronavirus disease 2019: A comprehensive narrative review about oral involvement of the disease. Clin. Exp. Dent Res. 2021, 7, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Eghbali Zarch, R.; Hosseinzadeh, P. COVID-19 from the perspective of dentists: A case report and brief review of more than 170 cases. Dermatol. Ther. 2021, 34, e14717. [Google Scholar] [CrossRef] [PubMed]
- Brandão, T.B.; Gueiros, L.A.; Melo, T.S.; Prado-Ribeiro, A.C.; Nesrallah, A.C.F.A.; Prado, G.V.B.; Santos-Silva, A.R.; Migliorati, C.A. Oral lesions in patients with SARS-CoV-2 infection: Could the oral cavity be a target organ? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, e45–e51. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Y.; Hoseini, E.; Mottaghi, R. Erythema multiform-like lesions in a patient infected with SARS-CoV-2: A case report. Future Virol. 2021, 16, 157–160. [Google Scholar] [CrossRef]
- Dalipi, Z.S.; Dragidella, F. Oral Manifestations of Exudative Erythema Multiforme in a Patient with COVID-19. Case Rep. Dent. 2021, 8, 1148945. [Google Scholar] [CrossRef] [PubMed]
- Bouabdella, S.; Benkaraache, M.; Almheirat, Y.; Zizi, N.; Dikhaye, S. Erythema multiforme eruption due to SARS-COV 2: Case report. Ann. Med. Surg. 2021, 68, 102591. [Google Scholar] [CrossRef] [PubMed]
- Tahir, D.; Souliman, M.; De La Rosa, A.M.; Al-Jobory, O.; Naguib, T. Erythema Multiforme-Like Presentation in an Asymptomatic COVID-19 Patient. Cureus 2021, 13, e20814. [Google Scholar] [CrossRef] [PubMed]
- Elboraey, M.O.; Essa, E.F. Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: A case report. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 132, e139–e142. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Mardani, S.; Asadi Kani, Z.; Hakamifard, A. An extremely rare mucocutaneous adverse reaction following COVID-19 vaccination: Toxic epidermalnecrolysis. Dermatol. Ther. 2022, 35, e15416. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, A.; Dilucca, M.; Squillace, F.; Guarnieri, R.; Polimeni, A.; Galluccio, G. Psychological and Physical Distress in Italian People during COVID-19 Pandemic: One Year Later. Int. J. Environ. Res. Public Health 2021, 18, 12525. [Google Scholar] [CrossRef] [PubMed]


| Incubation Time | Oral and Cutaneous Lesions Occurence | Intensity of Pain (Vas) | Covid-19 Symptoms |
|---|---|---|---|
| Day 1 | Oral vesicle in buccal and cheeck mucosa | 4 | None |
| Day 2 | Oral vesicle in buccal and cheeck mucosa | 5 | None |
| Day 3 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue | 7 | None |
| Day 4 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips | 8 | none |
| Day 5 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips -Target lesions in the hands | 8 | None |
| Day 6 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips -Oral ulcer in palate -Target lesions in the hands | 8 | None |
| Day 7 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips -Oral ulcer in palate -Target lesions in the hands | 7 | None |
| Day 8 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips -Oral ulcer in palate -Target lesions in the hands | 6 | None |
| Day 9 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips -Oral ulcer in palate -Target lesions in the hands | 5 | None |
| Day 10 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips -Oral ulcer in palate -Target lesions in the hands | 3 | None |
| Day 11 | -Oral vesicle in buccal and cheeck mucosa -Blisters in the dorsum of the tongue -Oral ulcerations- aphthous like in the vermillion lips -Oral ulcer in palate -Target lesions in the hands | 3 | None |
| Day 12 | -Hemorragic areas in the oral cavity -Target lesions (Partial Healing wound after 5 days of Corticosteroid therapy) | 2 | None |
| Day 13 | -Hemorragic areas in the oral cavity -Target lesions Each lesions are in remission | 2 | Headache |
| Day 14 | Each lesions are in remission | 1 | Fever and headache |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaia, G.; Pernice, E.; Pergolini, D.; Impellizzeri, A.; Migliau, G.; Gambarini, G.; Romeo, U.; Polimeni, A. Erythema Multiforme as Early Manifestation of COVID-19: A Case Report. Pathogens 2022, 11, 654. https://doi.org/10.3390/pathogens11060654
Palaia G, Pernice E, Pergolini D, Impellizzeri A, Migliau G, Gambarini G, Romeo U, Polimeni A. Erythema Multiforme as Early Manifestation of COVID-19: A Case Report. Pathogens. 2022; 11(6):654. https://doi.org/10.3390/pathogens11060654
Chicago/Turabian StylePalaia, Gaspare, Elena Pernice, Daniele Pergolini, Alessandra Impellizzeri, Guido Migliau, Gianluca Gambarini, Umberto Romeo, and Antonella Polimeni. 2022. "Erythema Multiforme as Early Manifestation of COVID-19: A Case Report" Pathogens 11, no. 6: 654. https://doi.org/10.3390/pathogens11060654
APA StylePalaia, G., Pernice, E., Pergolini, D., Impellizzeri, A., Migliau, G., Gambarini, G., Romeo, U., & Polimeni, A. (2022). Erythema Multiforme as Early Manifestation of COVID-19: A Case Report. Pathogens, 11(6), 654. https://doi.org/10.3390/pathogens11060654










