Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum
Abstract
1. Introduction
2. Results
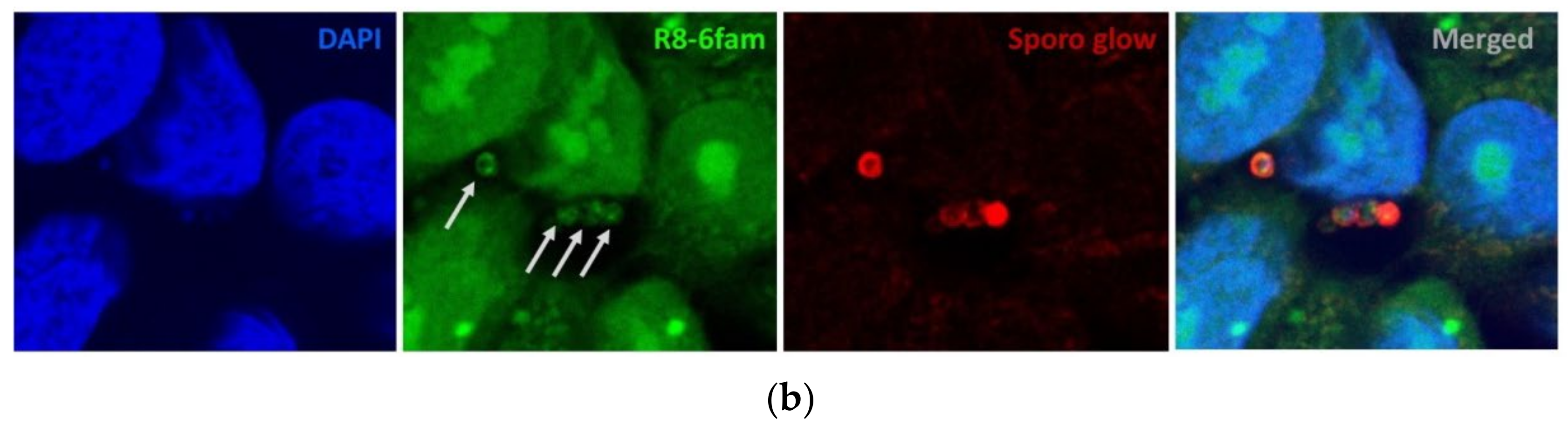
2.1. Uptake of FAM-Labelled Octaarginine by Cryptosporidium parvum
2.2. NTZ-R8 Inhibits Intracellular Cryptosporidium parvum Growth in HCT-8 Cells
3. Discussion
4. Materials and Methods
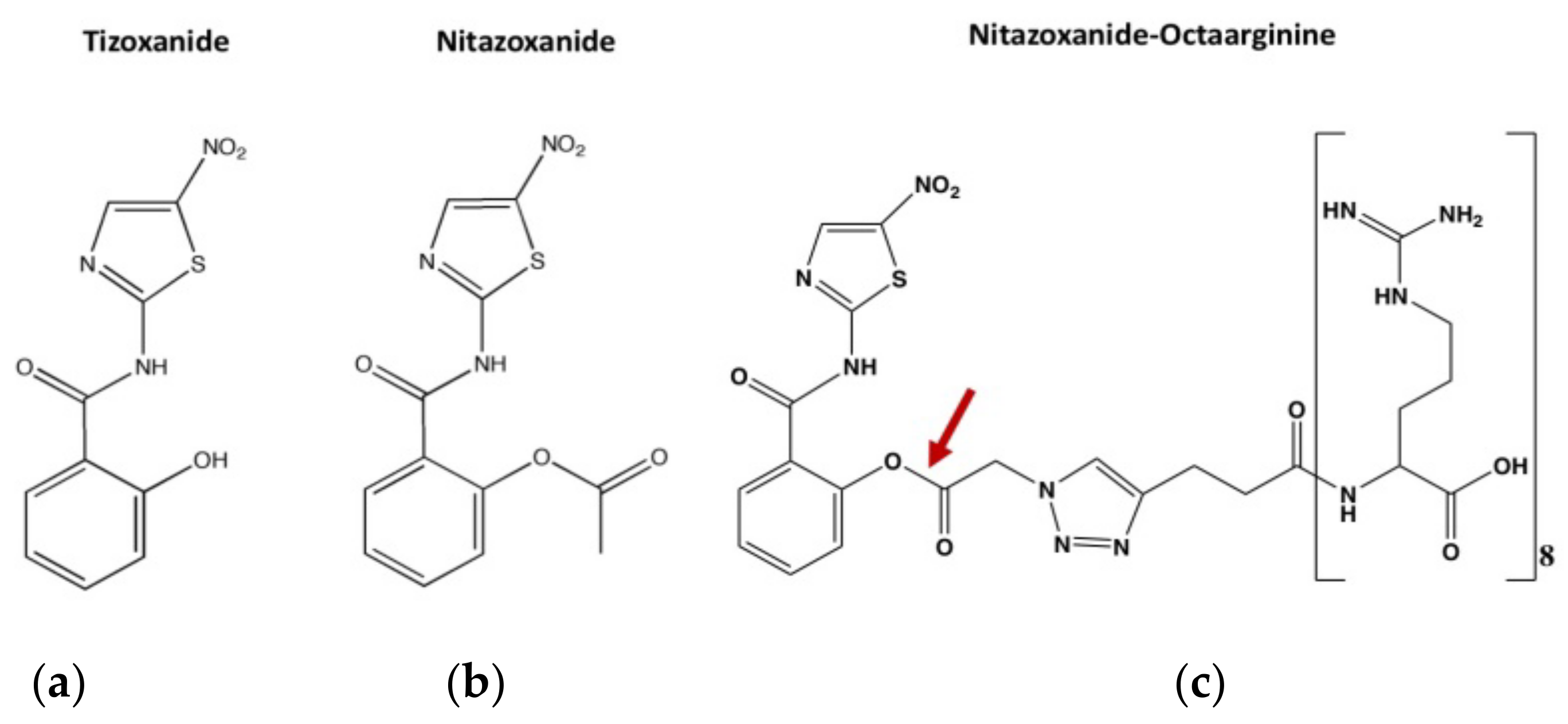
4.1. Compounds
4.2. Synthesis of Octaarginine-6-FAM
4.3. Cell Culture
4.4. Parasites
4.5. Uptake of FAM-Labeled Octaarginine by Excysted Sporozoites and Intracellular Cryptosporidium parvum
4.6. Mitochondrial Toxicity Test (MTT)
4.7. In Vitro Inhibition Assay
4.8. RNA Extraction
4.9. Real-Time PCR
4.10. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leitch, G.J.; He, Q. Cryptosporidiosis-an overview. J. Biomed. Res. 2011, 25, 1–16. [Google Scholar] [CrossRef]
- Raj Mainali, N.; Quinlan, P.; Ukaigwe, A.; Amirishetty, S. Cryptosporidial diarrhea in an immunocompetent adult: Role of nitazoxanide. J. Community Hosp. Intern. Med. Perspect. 2013, 3, 21075. [Google Scholar] [CrossRef]
- Ventura, G.; Cauda, R.; Larocca, L.M.; Riccioni, M.E.; Tumbarello, M.; Lucia, M.B. Gastric cryptosporidiosis complicating HIV infection: Case report and review of the literature. Eur. J. Gastroenterol. Hepatol. 1997, 9, 307–310. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Miao, Y.M.; Gazzard, B.G. Management of protozoal diarrhoea in H1V disease. HIV Med. 2000, 1, 194–199. [Google Scholar] [CrossRef][Green Version]
- Ives, N.J.; Gazzard, B.G.; Easterbrook, P.J. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART) in London clinic. J. Infect. 2001, 42, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Adesiji, Y.O.; Lawal, R.O.; Taiwo, S.S.; Fayemiwo, S.A.; Adeyeba, O.A. Cryptosporidiosis in HIV infected patients with diarrhoea in Osun State Southwestern Nigeria. Eur. J. Gen. Med. 2007, 4, 119–122. [Google Scholar] [CrossRef]
- Amadi, B.; Mwiya, M.; Sianongo, S.; Payne, L.; Watuka, A.; Katubulushi, M.; Kelly, P. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: A randomised controlled trial. BMC Infect. Dis. 2009, 9, 195. [Google Scholar] [CrossRef]
- Kamena, F.; Monnanda, B.; Makou, D.; Capone, S.; Patora-Komisarska, K.; Seebach, D. On the mechanism of eukaryotic cell penetration by α- And β-oligoarginines-Targeting infected erythrocytes. Chem. Biodivers. 2011, 8, 1–12. [Google Scholar] [CrossRef]
- Sparr, C.; Purkayastha, N.; Kolesinska, B.; Gengenbacher, M.; Amulic, B.; Matuschewski, K.; Seebach, D.; Kamena, F. Improved Efficacy of Fosmidomycin against Plasmodium and Mycobacterium Species by Combination with the Cell-Penetrating. Antimicrob. Agents Chemother. 2013, 57, 4689–4698. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ho-Bao, T.; Berberich, M.; Zheng, W.; Seebach, D.; Daugschies, A.; Kamena, F. A simple and efficient transfection protocol for Cryptosporidium parvum using Polyethylenimine (PEI) and Octaarginine. Parasitology 2020, 147, 1065–1070. [Google Scholar] [CrossRef]
- Cabada, M.; White, A.J. Treatment of cryptosporidiosis: Do we know what we think we know? Curr. Opin. Infect. Dis. 2010, 5, 494–499. [Google Scholar] [CrossRef]
- Mead, J.R.; Arrowood, M.J. Treatment of Cryptosporidiosis. Parasite Dis. 2014, 455–486. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Vinayak, S.; Zambriski, J.A.; Chao, A.T.; Sy, T.; Noble, C.G.; Bonamy, G.; Kondreddi, R.R.; Zou, B.; Gedeck, P.; et al. A Cryptosporidium PI (4) K inhibitor is a drug candidate for cryptosporidiosis. Nature 2017, 546, 376–380. [Google Scholar] [CrossRef]
- Fox, L.M.; Saravolatz, L.D. Nitazoxanide: A New Thiazolide Antiparasitic Agent. Clin. Infect. Dis. 2005, 40, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Stalmans, S.; Bracke, N.; Wynendaele, E.; Gevaert, B.; Peremans, K.; Burvenich, C.; Polis, I.; De Spiegeleer, B. Cell-penetrating peptides selectively cross the blood-brain barrier in vivo. PLoS ONE 2015, 10, e0139652. [Google Scholar] [CrossRef]
- Seebach, D.; Namoto, K.; Mahajan, Y.R.; Bindschädler, P.; Sustmann, R.; Kirsch, M.; Ryder, N.S.; Weiss, M.; Sauer, M.; Roth, C.; et al. Chemical and biological investigations of β-oligoarginines. Chem. Biodivers. 2004, 1, 65–97. [Google Scholar] [CrossRef]
- Kristensen, M.; Birch, D.; Nielsen, H.M. Applications and challenges for use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. Int. J. Mol. Sci. 2016, 17, 185. [Google Scholar] [CrossRef]
- Griffiths, J.K.; Balakrishnan, R.; Widmer, G.; Tzipori, S. Paromomycin and geneticin inhibit intracellular Cryptosporidium parvum without trafficking through the host cell cytoplasm: Implications for drug delivery. Infect. Immun. 1998, 66, 3874–3883. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, M.; Hunter, P.R.; Chalmers, R.M.; Tyler, K.M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 2013, 26, 115–134. [Google Scholar] [CrossRef]
- Theodos, C.M.; Griffiths, J.K.; D’onfro, J.; Fairfield, A.; Tzipori, S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 1998, 42, 1959–1965. [Google Scholar] [CrossRef]
- Chen, X.M.; O’Hara, S.P.; Huang, B.Q.; Splinter, P.L.; Nelson, J.B.; LaRusso, N.F. Localized glucose and water influx facilitates Cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proc. Natl. Acad. Sci. USA 2005, 102, 6338–6343. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Dyachenko, V.; Obwaller, A.; Unglaube, S.; Daugschies, A. Combination of cell culture and quantitative PCR for screening of drugs against Cryptosporidium parvum. Vet Parasitol. 2009, 162, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef]
- Sonzogni-Desautels, K.; Renteria Flores, A.; Vasquez Camargo, F.; Di Lenardo, T.Z.; Mikhail, A.; Arrowood, M.J.; Fortin, A.; Ndao, M. Oleylphosphocholine (OlPC) arrests Cryptosporidium parvum growth in vitro and prevents lethal infection in interferon gamma receptor knock-out mice. Front. Microbiol. 2015, 6, 973. [Google Scholar] [CrossRef] [PubMed]
- Najdrowski, M.; Heckeroth, A.R.; Wackwitz, C.; Gawlowska, S.; Mackenstedt, U.; Kliemt, D.; Daugschies, A. Development and validation of a cell culture based assay for in vitro assessment of anticryptosporidial compounds. Parasitol. Res. 2007, 101, 161–167. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G. Quantitative RT-PCR assay for high-throughput screening (HTS) of drugs against the growth of Cryptosporidium parvum in vitro. Front. Microbiol. 2015, 6, 991. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen-Ho-Bao, T.; Ambe, L.A.; Berberich, M.; Hermosilla, C.; Taubert, A.; Daugschies, A.; Kamena, F. Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum. Pathogens 2022, 11, 653. https://doi.org/10.3390/pathogens11060653
Nguyen-Ho-Bao T, Ambe LA, Berberich M, Hermosilla C, Taubert A, Daugschies A, Kamena F. Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum. Pathogens. 2022; 11(6):653. https://doi.org/10.3390/pathogens11060653
Chicago/Turabian StyleNguyen-Ho-Bao, Tran, Lum A. Ambe, Maxi Berberich, Carlos Hermosilla, Anja Taubert, Arwid Daugschies, and Faustin Kamena. 2022. "Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum" Pathogens 11, no. 6: 653. https://doi.org/10.3390/pathogens11060653
APA StyleNguyen-Ho-Bao, T., Ambe, L. A., Berberich, M., Hermosilla, C., Taubert, A., Daugschies, A., & Kamena, F. (2022). Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum. Pathogens, 11(6), 653. https://doi.org/10.3390/pathogens11060653






