Bactericidal Activity of Ceragenin in Combination with Ceftazidime, Levofloxacin, Co-Trimoxazole, and Colistin against the Opportunistic Pathogen Stenotrophomonas maltophilia
Abstract
:1. Introduction
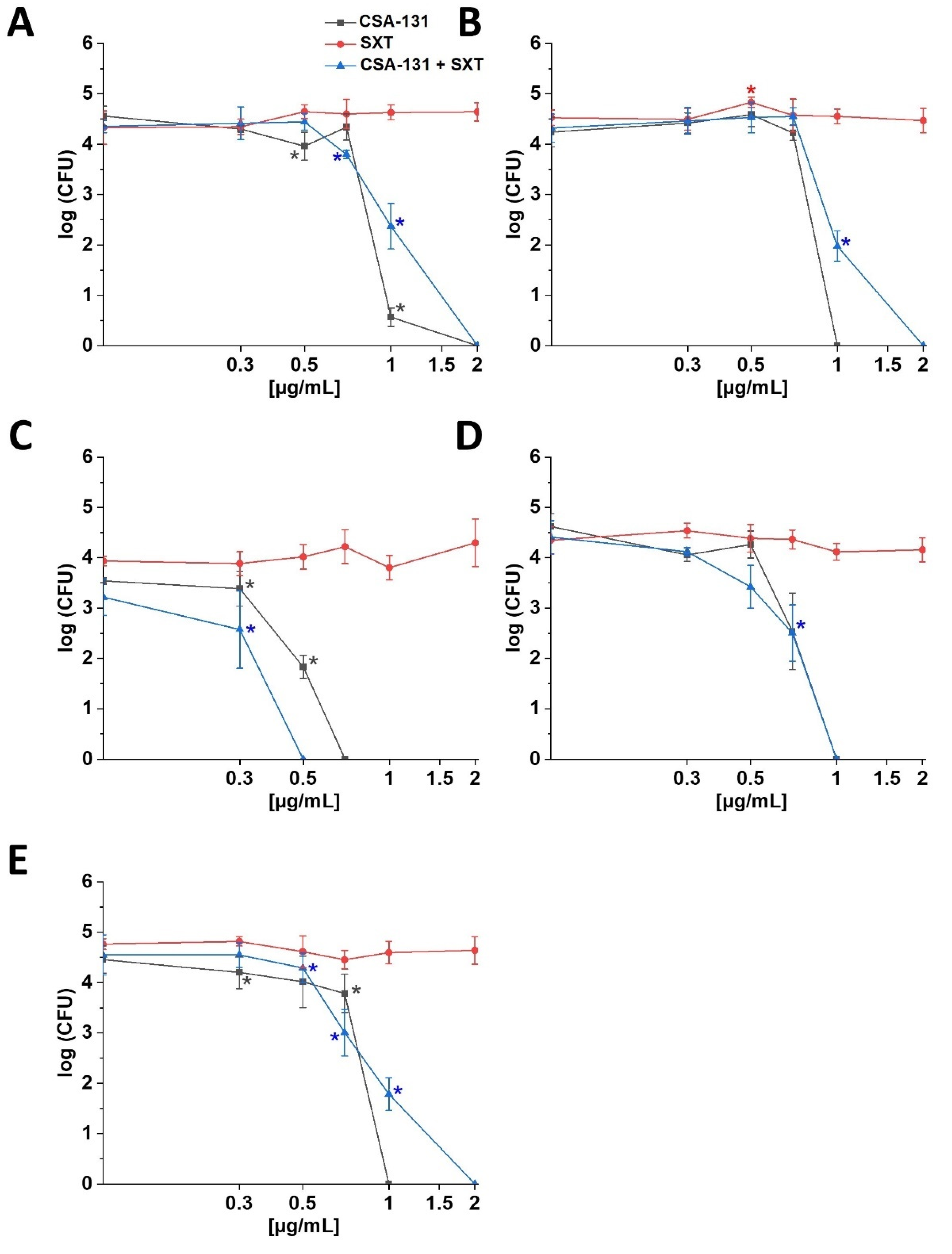
2. Results
2.1. Susceptibility of S. maltophilia Strains to Ceragenins and Conventional Antibiotics
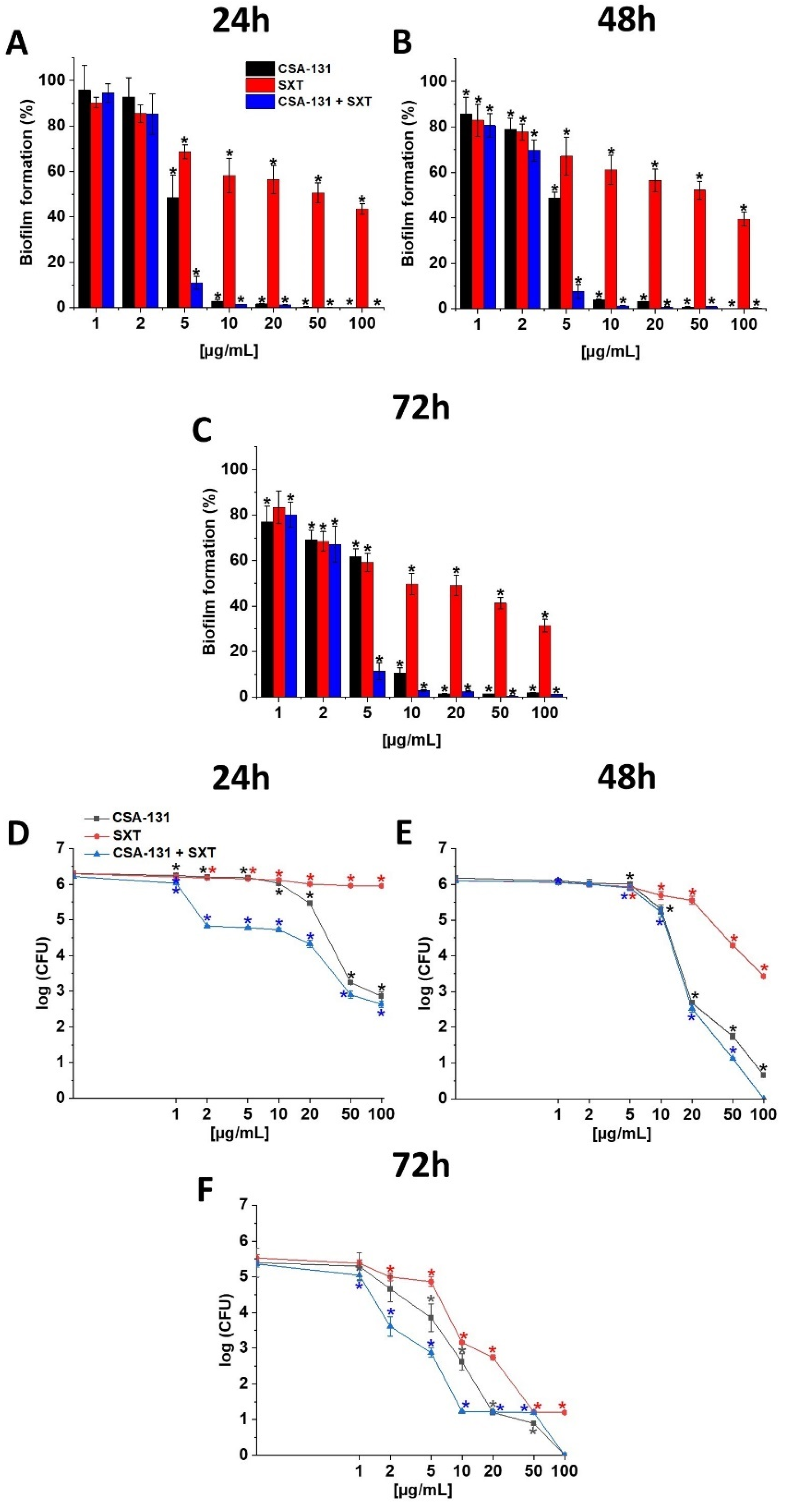
2.2. Ability of CSA-131, SXT and CSA-131 + SXT to Prevent Biofilm Formation and to Disrupt Establish Stationary Biofilm
2.3. Hemolytic Activity of CSA-131 in Combination with SXT
3. Discussion
4. Materials and Methods
4.1. Spectrum of Tested Bacteria
4.2. Determination of MIC and MBC
4.3. Assessment of the Fractional Index of Inhibitory Concentration FICI/FIC
4.4. Killing Assay
4.5. Prevention of Biofilm Formation and Disruption of Established Biofilms
4.6. Hemolytic Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palleroni, N.J.; Bradbury, J.F. Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al. 1983. Int. J. Syst. Bacteriol. 1993, 43, 606–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denet, E.; Vasselon, V.; Burdin, B.; Nazaret, S.; Favre-Bonté, S. Survival and growth of Stenotrophomonas maltophilia in free-living amoebae (FLA) and bacterial virulence properties. PLoS ONE 2018, 13, e0192308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Tong, S. An investigation of Stenotrophomonas maltophilia-positive culture caused by fiberoptic bronchoscope contamination. BMC Infect. Dis. 2019, 19, 1072. [Google Scholar] [CrossRef] [PubMed]
- Singhal, L.; Kaur, P.; Gautam, V. Stenotrophomonas maltophilia: From trivial to grievous. Indian J. Med. Microbiol. 2017, 35, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Bartoszek, D.; Fleischer, M. Znaczenie kliniczne Stenotrophomonas maltophilia. Clinical significance of Stenotrophomonas maltophilia. Forum Zakażeń 2013, 4, 165–171. [Google Scholar] [CrossRef]
- Kim, S.H.; Cha, M.K.; Kang, C.I.; Ko, J.H.; Huh, K.; Cho, S.Y.; Chung, D.R.; Peck, K.R. Pathogenic significance of hemorrhagic pneumonia in hematologic malignancy patients with Stenotrophomonas maltophilia bacteremia: Clinical and microbiological analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 285–295. [Google Scholar] [CrossRef]
- Yero, D.; Huedo, P.; Conchillo-Solé, O.; Martínez-Servat, S.; Mamat, U.; Coves, X.; Llanas, F.; Roca, I.; Vila, J.; Schaible, U.E.; et al. Genetic Variants of the DSF Quorum Sensing System in Stenotrophomonas maltophilia Influence Virulence and Resistance Phenotypes Among Genotypically Diverse Clinical Isolates. Front. Microbiol. 2020, 11, 1160. [Google Scholar] [CrossRef]
- Flores-Treviño, S.; Bocanegra-Ibarias, P.; Camacho-Ortiz, A.; Morfín-Otero, R.; Salazar-Sesatty, H.A.; Garza-González, E. Stenotrophomonas maltophilia biofilm: Its role in infectious diseases. Expert Rev. Anti-Infect. Ther. 2019, 17, 877–893. [Google Scholar] [CrossRef]
- Ezaj, M.M.A.; Haque, M.S.; Syed, S.B.; Khan, M.S.A.; Ahmed, K.R.; Khatun, M.T.; Nayeem, S.M.A.; Rizvi, G.R.; Al-Forkan, M.; Khaleda, L. Comparative proteomic analysis to annotate the structural and functional association of the hypothetical proteins of S. maltophilia k279a and predict potential T and B cell targets for vaccination. PLoS ONE 2021, 16, e0252295. [Google Scholar] [CrossRef]
- Bucki, R.; Durnaś, B.; Wątek, M.; Piktel, E.; Cruz, K.; Wolak, P.; Savage, P.B.; Janmey, P.A. Targeting polyelectrolyte networks in purulent body fluids to modulate bactericidal properties of some antibiotics. Infect. Drug Resist. 2018, 11, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladunjoye, O.O.; Oladunjoye, A.O.; Oladiran, O.; Donato, A.A. Stenotrophomonas maltophilia Infection in a Patient with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD): A Colonizer or True Infection? Am. J. Case Rep. 2020, 21, e924577. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, N.; Boncompagni, S.; Valzano, F.; Cariani, L.; Aliberti, S.; Blasi, F.; Pollini, S.; Rossolini, G.M.; Pallecchi, L. In Vitro Synergism of Colistin and N-acetylcysteine against Stenotrophomonas maltophilia. Antibiotics 2019, 8, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baidya, A.; Kodan, P.; Fazal, F.; Tsering, S.; Menon, P.R.; Jorwal, P.; Chowdhury, U.K. Stenotrophomonas maltophilia: More than Just a Colonizer! Indian J. Crit. Care Med. 2019, 23, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Guerci, P.; Bellut, H.; Mokhtari, M.; Gaudefroy, J.; Mongardon, N.; Charpentier, C.; Louis, G.; Tashk, P.; Dubost, C.; Ledochowski, S.; et al. Outcomes of Stenotrophomonas maltophilia hospital-acquired pneumonia in intensive care unit: A nationwide retrospective study. Crit. Care 2019, 23, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ince, N.; Yekenkurul, D.; Danış, A.; Çalışkan, E.; Akkaş, İ. An evaluation of six-year Stenotrophomonas maltophilia infections in a university hospital. Afr. Health Sci. 2020, 20, 1118–1123. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Stenström, T.A.; Okoh, A.I. Stenotrophomonas maltophilia as an Emerging Ubiquitous Pathogen: Looking Beyond Contemporary Antibiotic Therapy. Front. Microbiol. 2017, 8, 2276. [Google Scholar] [CrossRef] [Green Version]
- Capaldo, C.; Beauruelle, C.; Saliou, P.; Rault, G.; Ramel, S.; Héry-Arnaud, G. Investigation of Stenotrophomonas maltophilia epidemiology in a French cystic fibrosis center. Respir. Med. Res. 2020, 78, 100757. [Google Scholar] [CrossRef]
- Cruz-Córdova, A.; Mancilla-Rojano, J.; Luna-Pineda, V.M.; Escalona-Venegas, G.; Cázares-Domínguez, V.; Ormsby, C.; Franco-Hernández, I.; Zavala-Vega, S.; Hernández, M.A.; Medina-Pelcastre, M.; et al. Molecular Epidemiology, Antibiotic Resistance, and Virulence Traits of Stenotrophomonas maltophilia Strains Associated with an Outbreak in a Mexican Tertiary Care Hospital. Front. Cell Infect. Microbiol. 2020, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Oyardi, O.; Savage, P.B.; Erturan, Z.; Bozkurt-Guzel, C. In vitro assessment of CSA-131 and CSA-131 poloxamer form for the treatment of Stenotrophomonas maltophilia infections in cystic fibrosis. J. Antimicrob. Chemother. 2021, 76, 443–450. [Google Scholar] [CrossRef]
- Hankiewicz-Ziołkowska, K.; Gospodarek, E. Mechanizmy oporności na antybiotyki i chemioteraputyki pałeczek stenotrophomonas maltophilia. Postępy Mikrobiol. 2014, 53, 135–140. [Google Scholar]
- Al-Anazi, K.A.; Al-Jasser, A.M. Infections Caused by Stenotrophomonas maltophilia in Recipients of Hematopoietic Stem Cell Transplantation. Front. Oncol. 2014, 4, 232. [Google Scholar] [CrossRef] [Green Version]
- Zha, L.; Zhang, D.; Pan, L.; Ren, Z.; Li, X.; Zou, Y.; Li, S.; Luo, S.; Yang, G.; Tefsen, B. Tigecycline in the Treatment of Ventilator-Associated Pneumonia Due to Stenotrophomonas maltophilia: A Multicenter Retrospective Cohort Study. Infect. Dis. Ther. 2021, 10, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Nys, C.; Cherabuddi, K.; Venugopalan, V.; Klinker, K.P. Clinical and Microbiologic Outcomes in Patients with Monomicrobial Stenotrophomonas maltophilia Infections. Antimicrob. Agents Chemother. 2019, 63, e00788-19. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Lin, C.Y.; Chen, Y.H.; Hsueh, P.-R. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 2015, 6, 893. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Oota, M.; Matsumoto, S.; Sato, T.; Yamano, Y. In Vitro Activity and In Vivo Efficacy of Cefiderocol against Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2021, 65, e01436-20. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef] [Green Version]
- Surel, U.; Niemirowicz-Laskowska, K.; Marzec, M.; Savage, P.; Bucki, R. Ceragenins—A new weapon to fight multidrug resistant bacterial infections. Med. Stud. 2014, 3, 207–213. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Surel, U.; Wilczewska, A.Z.; Mystkowska, J.; Piktel, E.; Gu, X.; Namiot, Z.; Kułakowska, A.; Savage, P.B.; Bucki, R. Bactericidal activity and biocompatibility of ceragenin-coated magnetic nanoparticles. J. Nanobiotechnol. 2015, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Chmielewska, S.J.; Skłodowski, K.; Depciuch, J.; Deptuła, P.; Piktel, E.; Fiedoruk, K.; Kot, P.; Paprocka, P.; Fortunka, K.; Wollny, T.; et al. Bactericidal Properties of Rod-, Peanut-, and Star-Shaped Gold Nanoparticles Coated with Ceragenin CSA-131 against Multidrug-Resistant Bacterial Strains. Pharmaceutics 2021, 13, 425. [Google Scholar] [CrossRef]
- Dao, A.; Mills, R.J.; Kamble, S.; Savage, P.B.; Little, D.G.; Schindeler, A. The application of ceragenins to orthopedic surgery and medicine. J. Orthop. Res. 2020, 38, 1883–1894. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal Activity of Ceragenin CSA-13 in Cell Culture and in an Animal Model of Peritoneal Infection. Antimicrob. Agents Chemother. 2015, 59, 6274–6282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piktel, E.; Markiewicz, K.H.; Wilczewska, A.Z.; Daniluk, T.; Chmielewska, S.; Niemirowicz-Laskowska, K.; Mystkowska, J.; Paprocka, P.; Savage, P.B.; Bucki, R. Quantification of Synergistic Effects of Ceragenin CSA-131 Combined with Iron Oxide Magnetic Nanoparticles Against Cancer Cells. Int J Nanomed. 2020, 15, 4573–4589. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0, (01.01.2022). Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 4 May 2022).
- Chmielarczyk, A.; Pobiega, M.; Ziółkowski, G.; Pomorska-Wesołowska, M.; Romaniszyn, D.; Krawczyk, L.; Wójkowska-Mach, J. Severe infections caused by multidrug-resistant non-fermentative bacilli in southern Poland. Adv. Clin. Exp. Med. 2018, 27, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.-F.; Chen, G.-S.; Kong, Q.-X.; Gao, L.-P.; Chen, X.; Ye, Y.; Li, J.-B. Increase in the Prevalence of Resistance Determinants to Trimethoprim/Sulfamethoxazole in Clinical Stenotrophomonas maltophilia Isolates in China. PLoS ONE 2016, 11, e0157693. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gautam, V.; Tewari, R. Distribution of Class 1 Integrons, sul1 and sul2 Genes Among Clinical Isolates of Stenotrophomonas maltophilia from a Tertiary Care Hospital in North India. Microb. Drug Resist. 2015, 21, 380–385. [Google Scholar] [CrossRef]
- Pompilio, A.; Savini, V.; Fiscarelli, E.; Gherardi, G.; Di Bonaventura, G. Clonal Diversity, Biofilm Formation, and Antimicrobial Resistance among Stenotrophomonas maltophilia Strains from Cystic Fibrosis and Non-Cystic Fibrosis Patients. Antibiotics 2020, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Said, M.S.; Tirthani, E.; Lesho, E. Stenotrophomonas Maltophilia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Biagi, M.; Lamm, D.; Meyer, K.; Vialichka, A.; Jurkovic, M.; Patel, S.; Mendes, R.E.; Bulman, Z.P.; Wenzler, E. Activity of Aztreonam in Combination with Avibactam, Clavulanate, Relebactam, and Vaborbactam against Multidrug-Resistant Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2020, 64, e00297-20. [Google Scholar] [CrossRef]
- Ismail, N.; Zam, Z.; Hassan, S.A.; Rahman, Z.A. A Combination of Trimethoprim-sulfamethoxazole and Ceftazidime Showed Good In Vitro Activity against Stenotrophomonas maltophilia. Malays. J. Med. Sci. 2017, 24, 21–27. [Google Scholar] [CrossRef]
- Paprocka, P.; Durnaś, B.; Mańkowska, A.; Skłodowski, K.; Król, G.; Zakrzewska, M.; Czarnowski, M.; Kot, P.; Fortunka, K.; Góźdź, S.; et al. New β-Lactam Antibiotics and Ceragenins—A Study to Assess Their Potential in Treatment of Infections Caused by Multidrug-Resistant Strains of Pseudomonas aeruginosa. Infect. Drug Resist. 2021, 14, 5681–5698. [Google Scholar] [CrossRef]
- Nainu, F.; Permana, A.D.; Djide, N.J.N.; Anjani, Q.K.; Utami, R.N.; Rumata, N.R.; Zhang, J.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical Approaches on Antimicrobial Resistance: Prospects and Challenges. Antibiotics 2021, 10, 981. [Google Scholar] [CrossRef]
- Ozbek-Celik, B.; Damar-Celik, D.; Mataraci-Kara, E.; Bozkurt-Guzel, C.; Savage, P.B. Comparative In Vitro Activities of First and Second-Generation Ceragenins Alone and in Combination with Antibiotics Against Multidrug-Resistant Klebsiella pneumoniae Strains. Antibiotics 2019, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, J.N.; Jones, R.N.; Sader, H.S.; Savage, P.B.; Rybak, M.J. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J. Antimicrob. Chemother. 2008, 61, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozkurt-Guzel, C.; Inci, G.; Oyardi, O.; Savage, P.B. Synergistic Activity of Ceragenins Against Carbapenem-Resistant Acinetobacter baumannii Strains in Both Checkerboard and Dynamic Time-Kill Assays. Curr. Microbiol. 2020, 77, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Durnaś, B.; Fiedoruk, K.; Cieśluk, M.; Deptuła, P.; Król, G.; Piktel, E.; Savage, P.B.; Bucki, R. Lysozyme increases bactericidal activity of ceragenin CSA-13 against Bacillus subtilis. Med. Stud./Studia Med. 2019, 35, 1–9. [Google Scholar] [CrossRef]
- Alcaraz, E.; García, C.; Friedman, L.; de Rossi, B.P. The rpf/DSF signalling system of Stenotrophomonas maltophilia positively regulates biofilm formation, production of virulence-associated factors and β-lactamase induction. FEMS Microbiol. Lett. 2019, 366, fnz069. [Google Scholar] [CrossRef]
- Pompilio, A.; Ranalli, M.; Piccirilli, A.; Perilli, M.; Vukovic, D.; Savic, B.; Krutova, M.; Drevinek, P.; Jonas, D.; Fiscarelli, E.V.; et al. Biofilm Formation among Stenotrophomonas maltophilia Isolates Has Clinical Relevance: The ANSELM Prospective Multicenter Study. Microorganisms 2021, 9, 49. [Google Scholar] [CrossRef]
- Hacioglu, M.; Oyardi, O.; Bozkurt-Guzel, C.; Savage, P.B. Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms. J. Antibiot. 2020, 73, 455–462. [Google Scholar] [CrossRef]
- Bozkurt Güzel, Ç.; Hacioğlu, M.; Inci, G.; Savage, P.B. Antibacterial and Antibiofilm Activities of Ceragenins against Pseudomonas aeruginosa Clinical Isolates. Turk. J. Pharm. Sci. 2019, 16, 444–449. [Google Scholar] [CrossRef]
- Damar-Çelik, D.; Mataracı-Kara, E.; Savage, P.B.; Özbek-Çelik, B. Antibacterial and antibiofilm activities of ceragenins against Achromobacter species isolated from cystic fibrosis patients. J. Chemother. 2021, 33, 216–227. [Google Scholar] [CrossRef]
- Chmielewska, S.J.; Skłodowski, K.; Piktel, E.; Suprewicz, Ł.; Fiedoruk, K.; Daniluk, T.; Wolak, P.; Savage, P.B.; Bucki, R. NDM-1 Carbapenemase-Producing Enterobacteriaceae are Highly Susceptible to Ceragenins CSA-13, CSA-44, and CSA-131. Infect. Drug Resist. 2020, 13, 3277–3294. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Holden, S.B.; Durnaś, B.; Bucki, R.; Savage, P. Ceragenins as Mimics of Endogenous Antimicrobial Peptides. J. Antimicrob. Agents 2017, 3, 2472-1212. [Google Scholar] [CrossRef] [Green Version]
- EUCAST. Antimicrobial Susceptibility Testing (AST) of Bacteria. MIC Determination of Non-Fastidious and Fastidious Organisms. V6 (01.01.2020). Available online: https://www.eucast.org/ast_of_bacteria/mic_determination/?no_cache=1 (accessed on 4 May 2022).
- CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents Approved Guideline. CLSI Document M26-A, Wayne. September 1999. Available online: https://clsi.org/media/1462/m26a_sample.pdf (accessed on 4 May 2022).
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbusińska, A.; Szliszka, E. The activity of antimicrobial drugs used in combination against Gram-negative bacteria in vitro. Postępy Nauk Med. 2017, 7, 427–433. [Google Scholar]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnol. 2020, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska, S.J.; Depciuch, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Zakrzewska, M.; Czarnowski, M.; Cieśluk, M.; Durnaś, B.; et al. Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents. Pharmaceutics 2021, 13, 1940. [Google Scholar] [CrossRef]

| STRAIN NUMBER | Antibiotic | MIC (µg/mL) | MBC (µg/mL) | Ceragenins | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|---|---|---|
| Strain 1 Sm (ATCC 13636) | CAZ 1 | 16 | 32 | CSA-13 | 1 | 2 |
| LVX 2 | 0.5 | 2 | CSA-44 | 4 | 8 | |
| SXT 3 | 4 | 4 | CSA-131 | 2 | 2 | |
| COL | 16 | 16 | ||||
| Strain 2 | CAZ | 256 | >256 | CSA-13 | 2 | 4 |
| LVX | 4 | 16 | CSA-44 | 2 | 8 | |
| SXT | 2 | 8 | CSA-131 | 1 | 1 | |
| COL | 2 | 8 | ||||
| Strain 3 | CAZ | 1 | 4 | CSA-13 | 1 | 2 |
| LVX | 0.25 | 0.5 | CSA-44 | 1 | 4 | |
| SXT | 0.5 | 0.5 | CSA-131 | 0.5 | 2 | |
| COL | 4 | 16 | ||||
| Strain 4 | CAZ | 64 | 64 | CSA-13 | 0.5 | 2 |
| LVX | 1 | 1 | CSA-44 | 0.5 | 2 | |
| SXT | 0.5 | 2 | CSA-131 | 0.5 | 1 | |
| COL | 1 | 4 | ||||
| Strain 5 | CAZ | 16 | 32 | CSA-13 | 1 | 4 |
| LVX | 1 | 1 | CSA-44 | 2 | 8 | |
| SXT | 1 | 1 | CSA-131 | 0.5 | 1 | |
| COL | 4 | 16 | ||||
| Strain 6 | CAZ | 4 | 16 | CSA-13 | 2 | 4 |
| LVX | 0.25 | 0.5 | CSA-44 | 2 | 8 | |
| SXT | 0.25 | 0.5 | CSA-131 | 1 | 4 | |
| COL | 0.5 | 2 | ||||
| Strain 7 | CAZ | 32 | 128 | CSA-13 | 2 | 8 |
| LVX | 1 | 4 | CSA-44 | 2 | 8 | |
| SXT | 0.5 | 2 | CSA-131 | 2 | 8 | |
| COL | 2 | 16 |
| STRAIN | CAZ/ CSA-13 MIC (µg/mL) | CAZ/ CSA-44 MIC (µg/mL) | CAZ/ CSA-131 MIC (µg/mL) | LVX/ CSA-13 MIC (µg/mL) | LVX/ CSA-44 MIC (µg/mL) | LVX/ CSA-131 MIC (µg/mL) | SXT/ CSA-13 MIC (µg/mL) | SXT/ CSA-44 MIC (µg/mL) | SXT/ CSA-131 MIC (µg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 1 Sm (ATCC 13636) | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 2 | 2 | 2 |
| 2 | 4 | 2 | 2 | 1 | 1 | 0.5 | 1 | 1 | 0.5 |
| 3 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.125 | 0.125 | 0.25 |
| 4 | 1 | 1 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.125 |
| 5 | 1 | 4 | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 |
| 6 | 0.5 | 1 | 0.5 | 0.25 | 0.25 | 0.125 | 0.5 | 0.5 | 0.25 |
| 7 | 4 | 4 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 |
| STRAIN | FICI CAZ/ CSA-13 | FICI CAZ/CSA-44 | FICI CAZ/CSA-131 | FICI LVX/CSA-13 | FICI LVX/CSA-44 | FICI LVX/CSA-131 | FICI SXT/CSA-13 | FICI SXT/CSA-44 | FICI SXT/CSA-131 |
|---|---|---|---|---|---|---|---|---|---|
| 1Sm (ATCC 13636) | 1.06 | 0.31 | 0.56 | 1.5 | 1.13 | 2.5 | 2.5 | 1 | 1.5 |
| 2 | 2.02 | 1 | 2 | 0.75 | 0.75 | 0.63 | 1 | 1 | 0.75 |
| 3 | 1 | 2 | 3 | 2.5 | 2.5 | 3 | 0.38 | 0.38 | 1 |
| 4 | 2.02 | 2.02 | 1 | 3 | 3 | 1.5 | 2 | 1 | 0.5 |
| 5 | 1.06 | 2.25 | 2.06 | 2 | 1.5 | 1.5 | 1 | 0.75 | 1.5 |
| 6 | 0.38 | 0.75 | 0.63 | 1.13 | 1.13 | 0.63 | 2.25 | 2.25 | 1.25 |
| 7 | 2.13 | 2.13 | 0.53 | 1.5 | 1.5 | 1.5 | 1.25 | 1.25 | 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, P.; Mańkowska, A.; Skłodowski, K.; Król, G.; Wollny, T.; Lesiak, A.; Głuszek, K.; Savage, P.B.; Durnaś, B.; Bucki, R. Bactericidal Activity of Ceragenin in Combination with Ceftazidime, Levofloxacin, Co-Trimoxazole, and Colistin against the Opportunistic Pathogen Stenotrophomonas maltophilia. Pathogens 2022, 11, 621. https://doi.org/10.3390/pathogens11060621
Paprocka P, Mańkowska A, Skłodowski K, Król G, Wollny T, Lesiak A, Głuszek K, Savage PB, Durnaś B, Bucki R. Bactericidal Activity of Ceragenin in Combination with Ceftazidime, Levofloxacin, Co-Trimoxazole, and Colistin against the Opportunistic Pathogen Stenotrophomonas maltophilia. Pathogens. 2022; 11(6):621. https://doi.org/10.3390/pathogens11060621
Chicago/Turabian StylePaprocka, Paulina, Angelika Mańkowska, Karol Skłodowski, Grzegorz Król, Tomasz Wollny, Agata Lesiak, Katarzyna Głuszek, Paul B. Savage, Bonita Durnaś, and Robert Bucki. 2022. "Bactericidal Activity of Ceragenin in Combination with Ceftazidime, Levofloxacin, Co-Trimoxazole, and Colistin against the Opportunistic Pathogen Stenotrophomonas maltophilia" Pathogens 11, no. 6: 621. https://doi.org/10.3390/pathogens11060621
APA StylePaprocka, P., Mańkowska, A., Skłodowski, K., Król, G., Wollny, T., Lesiak, A., Głuszek, K., Savage, P. B., Durnaś, B., & Bucki, R. (2022). Bactericidal Activity of Ceragenin in Combination with Ceftazidime, Levofloxacin, Co-Trimoxazole, and Colistin against the Opportunistic Pathogen Stenotrophomonas maltophilia. Pathogens, 11(6), 621. https://doi.org/10.3390/pathogens11060621






