Pancreatic Stone Protein as a Biomarker for Sepsis at the Emergency Department of a Large Tertiary Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
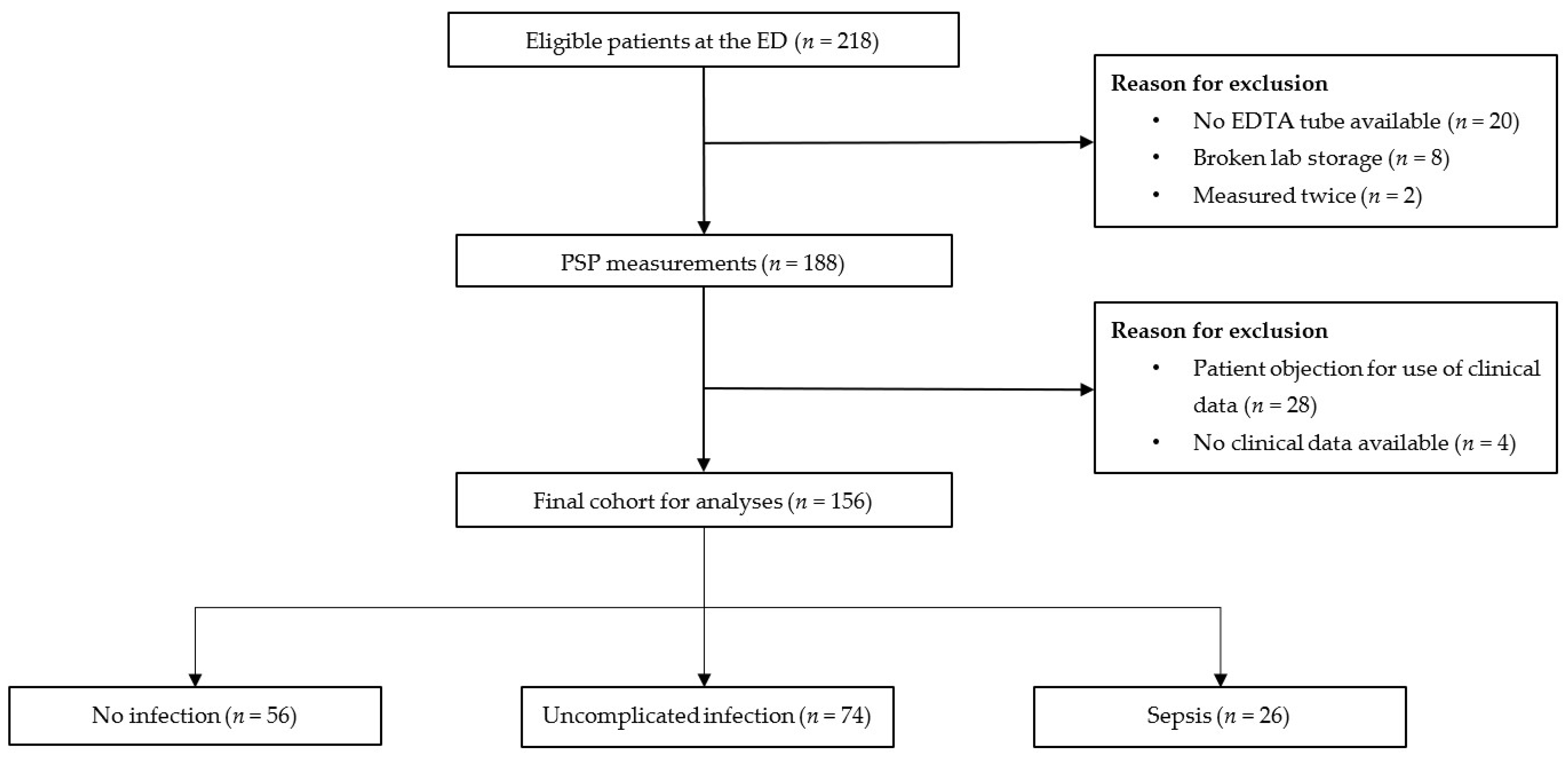
2.2. Study Population and Data Collection
2.3. Laboratory Measurements
2.4. Patient Classification
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Biomarker Distribution
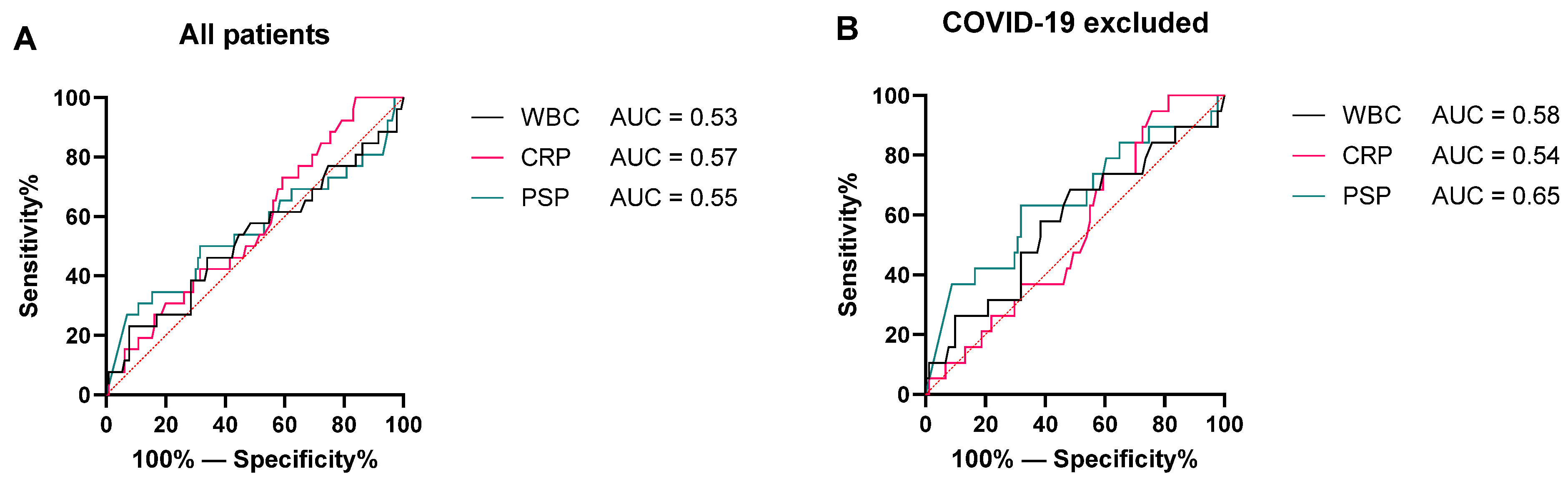
3.3. Discrimination between No Sepsis and Sepsis
3.4. False-Positive and False-Negative Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in Sepsis and Septic Shock in Europe, North America and Australia between 2009 and 2019-Results from a Systematic Review and Meta-Analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Langa, K.M.; Iwashyna, T.J. Readmission Diagnoses after Hospitalization for Severe Sepsis and Other Acute Medical Conditions. JAMA 2015, 313, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Mostel, Z.; Perl, A.; Marck, M.; Mehdi, S.F.; Lowell, B.; Bathija, S.; Santosh, R.; Pavlov, V.A.; Chavan, S.S.; Roth, J. Post-Sepsis Syndrome- An Evolving Entity That Afflicts Survivors of Sepsis. Mol. Med. 2019, 26, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Woznica, E.A.; Inglot, M.; Woznica, R.K.; Lysenko, L. Liver Dysfunction in Sepsis. Adv. Clin. Exp. Med. 2018, 27, 547–551. [Google Scholar] [CrossRef]
- Prowle, J.R. Sepsis-Associated AKI. Clin. J. Am. Soc. Nephrol. 2018, 13, 339–342. [Google Scholar] [CrossRef]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The Global Burden of Sepsis: Barriers and Potential Solutions. Crit. Care 2018, 22, 232. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Seetharaman, S.; Wilson, C.; Landrum, M.; Qasba, S.; Katz, M.; Ladikos, N.; Harris, J.E.; Galiatsatos, P.; Yousem, D.M.; Knight, A.M.; et al. Does Use of Electronic Alerts for Systemic Inflammatory Response Syndrome (SIRS) to Identify Patients with Sepsis Improve Mortality? Am. J. Med. 2019, 132, 862–868. [Google Scholar] [CrossRef]
- El Gkotmi, N.; Kosmeri, C.; Filippatos, T.D.; Elisaf, M.S. Use of Intravenous Fluids/Solutions: A Narrative Review. Curr. Med. Res. Opin. 2017, 33, 459–471. [Google Scholar] [CrossRef]
- Rhee, C.; Kadri, S.S.; Danner, R.L.; Suffredini, A.F.; Massaro, A.F.; Kitch, B.T.; Lee, G.; Klompas, M. Diagnosing Sepsis Is Subjective and Highly Variable: A Survey of Intensivists Using Case Vignettes. Crit. Care 2016, 20, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of Sepsis: Time for a Reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef] [PubMed]
- Mellors, J.W.; Kelly, J.J.; Gusberg, R.J.; Horwitz, S.M.; Horwitz, R.I. A Simple Index to Estimate the Likelihood of Bacterial Infection in Patients Developing Fever after Abdominal Surgery. Am. Surg. 1988, 54, 558–564. [Google Scholar] [PubMed]
- Nargis, W.; Ahamed, B.; Ibrahim, M. Procalcitonin versus C-Reactive Protein: Usefulness as Biomarker of Sepsis in ICU Patient. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 195. [Google Scholar] [CrossRef] [Green Version]
- Seigel, T.A.; Cocchi, M.N.; Salciccioli, J.; Shapiro, N.I.; Howell, M.; Tang, A.; Donnino, M.W. Inadequacy of Temperature and White Blood Cell Count in Predicting Bacteremia in Patients with Suspected Infection. J. Emerg. Med. 2012, 42, 254–259. [Google Scholar] [CrossRef]
- De Caro, A.; Lohse, J.; Sarles, H. Characterization of a Protein Isolated from Pancreatic Calculi of Men Suffering from Chronic Calcifying Pancreatitis. Biochem. Biophys. Res. Commun. 1979, 87, 1176–1182. [Google Scholar] [CrossRef]
- Terazono, K.; Yamamoto, H.; Takasawa, S.; Shiga, K.; Yonemura, Y.; Tochino, Y.; Okamoto, H. A Novel Gene Activated in Regenerating Islets. J. Biol. Chem. 1988, 263, 2111–2114. [Google Scholar] [CrossRef]
- Keel, M.; Härter, L.; Reding, T.; Sun, L.K.; Hersberger, M.; Seifert, B.; Bimmler, D.; Graf, R. Pancreatic Stone Protein Is Highly Increased during Posttraumatic Sepsis and Activates Neutrophil Granulocytes. Crit. Care Med. 2009, 37, 1642–1648. [Google Scholar] [CrossRef]
- Eggimann, P.; Que, Y.A.; Rebeaud, F. Measurement of Pancreatic Stone Protein in the Identification and Management of Sepsis. Biomark. Med. 2019, 13, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Klein, H.J.; Niggemann, P.; Buehler, P.K.; Lehner, F.; Schweizer, R.; Rittirsch, D.; Fuchs, N.; Waldner, M.; Steiger, P.; Giovanoli, P.; et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity A Monocentric Observational Study. Ann. Surg. 2021, 274, e1179–e1186. [Google Scholar] [CrossRef]
- Iovanna, J.; Frigerio, J.M.; Dusetti, N.; Ramare, F.; Raibaud, P.; Dagorn, J.C. Lithostathine, an Inhibitor of Cac03crystal Growth in Pancreatic Juice, Induces Bacterial Aggregation. Pancreas 1993, 8, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.J.; Berger, M.; Gregory, M.; Ramaiah, R.; Taylor, A.L.; Curdt, I.; Lajaunias, F.; Graf, R.; Blincko, S.J.; Drage, S.; et al. Sepsis Biomarkers in Unselected Patients on Admission to Intensive or High-Dependency Care. Crit. Care 2013, 17, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Que, Y.A.; Guessous, I.; Dupuis-Lozeron, E.; De Oliveira, C.R.A.; Oliveira, C.F.; Graf, R.; Seematter, G.; Revelly, J.P.; Pagani, J.L.; Liaudet, L.; et al. Prognostication of Mortality in Critically Ill Patients with Severe Infections. Chest 2015, 148, 674–682. [Google Scholar] [CrossRef] [PubMed]
- García de Guadiana-Romualdo, L.; Berger, M.; Jiménez-Santos, E.; Rebollo-Acebes, S.; Jiménez-Sánchez, R.; Esteban-Torrella, P.; Hernando-Holgado, A.; Ortín-Freire, A.; Albaladejo-Otón, M.D. Pancreatic Stone Protein and Soluble CD25 for Infection and Sepsis in an Emergency Department. Eur. J. Clin. Investig. 2017, 47, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, P.; Nora, D.; Coelho, L.; Povoa, P. Pancreatic Stone Protein: Review of a New Biomarker in Sepsis. J. Clin. Med. 2022, 11, 1085. [Google Scholar] [CrossRef]
- García De Guadiana-Romualdo, L.; Jiménez-Santos, E.; Cerezuela-Fuentes, P.; Español-Morales, I.; Berger, M.; Esteban-Torrella, P.; Hernando-Holgado, A.; Albaladejo-Otón, M.D. Analyzing the Capability of PSP, PCT and SCD25 to Support the Diagnosis of Infection in Cancer Patients with Febrile Neutropenia. Clin. Chem. Lab. Med. 2019, 57, 540–548. [Google Scholar] [CrossRef]
- Wallace, B.I.; Kenney, B.; Malani, P.N.; Clauw, D.J.; Nallamothu, B.K.; Waljee, A.K. Prevalence of Immunosuppressive Drug Use among Commercially Insured US Adults, 2018–2019. JAMA Netw. Open 2021, 4, e214920. [Google Scholar] [CrossRef]
- Uffen, J.W.; Oomen, P.; De Regt, M.; Oosterheert, J.J.; Kaasjager, K. The Prognostic Value of Red Blood Cell Distribution Width in Patients with Suspected Infection in the Emergency Department. BMC Emerg. Med. 2019, 19, 76. [Google Scholar] [CrossRef]
- Benninga, R.; van den Bogaard, P.; Rebeaud, F. Abionic’s PSP ‘Sepsis Test’ on the AbioSCOPE® Device: 5 Minutes to Save Lives. Abionic SA 2019. [Google Scholar]
- Calandra, T.; Cohen, J. The International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit. Crit. Care Med. 2005, 37, 1538–1548. [Google Scholar] [CrossRef]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC Definitions for Nosocomial Infections, 1988. AJIC Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Subbe, C.P.; Davies, R.G.; Williams, E.; Rutherford, P.; Gemmell, L. Effect of Introducing the Modified Early Warning Score on Clinical Outcomes, Cardio-Pulmonary Arrests and Intensive Care Utilisation in Acute Medical Admissions. Anaesthesia 2003, 58, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Olwal, C.O.; Nganyewo, N.N.; Tapela, K.; Djomkam Zune, A.L.; Owoicho, O.; Bediako, Y.; Duodu, S. Parallels in Sepsis and COVID-19 Conditions: Implications for Managing Severe COVID-19. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Wilhelms, S.B.; Wilhelms, D.B. Emergency Department Admissions to the Intensive Care Unit—A National Retrospective Study. BMC Emerg. Med. 2021, 21, 122. [Google Scholar] [CrossRef]
- Lvovschi, V.; Arnaud, L.; Parizot, C.; Freund, Y.; Juillien, G.; Ghillani-Dalbin, P.; Bouberima, M.; Larsen, M.; Riou, B.; Gorochov, G.; et al. Cytokine Profiles in Sepsis Have Limited Relevance for Stratifying Patients in the Emergency Department: A Prospective Observational Study. PLoS ONE 2011, 6, e28870. [Google Scholar] [CrossRef]
- Federatie Medisch Specialisten Richtlijn Sepsis Fase 1. Available online: https://www.nvmm.nl/media/3614/200116-bijlage-24b-richtlijn-sepsis-fase-1.pdf (accessed on 3 May 2022).
- Umemura, Y.; Yamakawa, K.; Kiguchi, T.; Nishida, T.; Kawada, M.; Fujimi, S. Hematological Phenotype of COVID-19-Induced Coagulopathy: Far from Typical Sepsis-Induced Coagulopathy. J. Clin. Med. 2020, 9, 2875. [Google Scholar] [CrossRef]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe Immunosuppression and Not a Cytokine Storm Characterizes COVID-19 Infections. JCI Insight 2020, 5, e140329. [Google Scholar] [CrossRef]
- Pugin, J.; Daix, T.; Pagani, J.L.; Morri, D.; Giacomucci, A.; Dequin, P.F.; Guitton, C.; Que, Y.A.; Zani, G.; Brealey, D.; et al. Serial Measurement of Pancreatic Stone Protein for the Early Detection of Sepsis in Intensive Care Unit Patients: A Prospective Multicentric Study. Crit. Care 2021, 25, 151. [Google Scholar] [CrossRef]
- Wu, C.C.; Lan, H.M.; Han, S.T.; Chaou, C.H.; Yeh, C.F.; Liu, S.H.; Li, C.H.; Blaney, G.N.; Liu, Z.Y.; Chen, K.F. Comparison of Diagnostic Accuracy in Sepsis between Presepsin, Procalcitonin, and C-Reactive Protein: A Systematic Review and Meta-Analysis. Ann. Intensive Care 2017, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zhu, X.; Lin, H.; Liu, D.; Dai, Y.; Su, X.; Li, L. Association of Serum PSP/REG i with Renal Function in Type 2 Diabetes Mellitus. J. Diabetes Res. 2020, 2020, 9787839. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Minamiya, Y.; Takahashi, N.; Nakagawa, T.; Katayose, Y.; Ito, A.; Saito, H.; Motoyama, S.; Ogawa, J.I. REG1A Expression Is an Independent Factor Predictive of Poor Prognosis in Patients with Breast Cancer. Ann. Surg. Oncol. 2008, 15, 3244–3251. [Google Scholar] [CrossRef]
- Astrosini, C.; Roeefzaad, C.; Dai, Y.Y.; Dieckgraefe, B.K.; Jöns, T.; Kemmner, W. REG1A Expression Is a Prognostic Marker in Colorectal Cancer and Associated with Peritoneal Carcinomatosis. Int. J. Cancer 2008, 123, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, M.; Nozawa, K.; Kawasaki, M.; Yamaguchi, A.; Iwabuchi, K.; Yanagida, M.; Suzuki, F.; Miyazawa, K.; Fukui, H.; Kaneko, K.; et al. Regenerating Gene (REG) 1 Alpha Promotes Pannus Progression in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 2012, 22, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Jia, J.; Sheng, J.; Zhang, S.; Huang, K.; Li, H.; He, F. Protective and Anti-Inflammatory Role of REG1A in Inflammatory Bowel Disease Induced by JAK/STAT3 Signaling Axis. Int. Immunopharmacol. 2021, 92, 107304. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 156) | No Infection (n = 56) | Uncomplicated Infection (n = 74) | Sepsis (n = 26) | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 60.0 (44.5–73.0) | 55.0 (47.0–68.0) | 66.0 (48.0–74.0) | 54.0 (27.0–77.3) |
| Sex (M) (%) | 82 (52.6) | 28 (50.0) | 42 (56.8) | 12 (46.2) |
| Vital parameters | ||||
| Temperature (°C) | 38.0 (37.0–38.9) | 37.4 (36.9–38.4) | 37.9 (36.8–38.4) | 39.2 (38.9–39.6) |
| Heart rate (/min) | 95.0 (82.0–110.0) | 92.5 (80.0–107.5) | 89.5 (80.0–105.0) | 111.5 (104.5–117.8) |
| Respiratory rate (/min) | 18.0 (15.8–22.0) | 16.0 (14.0–19.0) | 18.0 (15.0–22.0) | 22.0 (19.5–30.5) |
| Systolic blood pressure (mmHg) | 127.0 (112.0–144.0) | 126.0 (113.5–142.0) | 128.0 (112.0–148.0) | 128.0 (105.0–153.3) |
| Diastolic blood pressure (mmHg) | 71.0 (62.0–80.0) | 74.0 (65.0–80.0) | 69.0 (62.0–80.0) | 69.0 (49.8–77.8) |
| Glasgow Coma Scale (EMV) | 15.0 (15.0–15.0) | 15.0 (15.0–15.0) | 15.0 (15.0–15.0) | 15.0 (14.0–15.0) |
| qSOFA score | ||||
| qSOFA = 0 (%) | 102 (65.4) | 41 (73.2) | 52 (70.3) | 9 (34.6) |
| qSOFA = 1 (%) | 39 (25.0) | 12 (21.4) | 18 (24.3) | 9 (34.6) |
| qSOFA = 2 (%) | 14 (9.0) | 3 (5.4) | 3 (4.1) | 8 (30.8) |
| qSOFA = 3 (%) | 1 (0.6) | 0 | 1 (1.4) | 0 |
| Hospitalization characteristics | ||||
| Admitted to hospital (%) | 114 (73.1) | 35 (62.5) | 57 (77.0) | 22 (84.6) |
| Length of stay (days) | 4.0 (3.0–8.0) | 4.0 (2.0–6.0) | 5.0 (3.0–8.0) | 4.0 (2.3–8.8) |
| Immunocompromised (%) | 60 (38.5) | 22 (39.3) | 25 (34.2) | 13 (50.0) |
| Charlson Comorbidity Index | 4.0 (2.0–6.8) | 3.0 (2.0–6.0) | 5.0 (3.0–7.0) | 3.0 (1.0–6.3) |
| Specialism | ||||
| General internal medicine (%) | 54 (34.6) | 15 (26.8) | 28 (37.8) | 11 (42.3) |
| Nephrology (%) | 24 (15.4) | 8 (14.3) | 12 (16.2) | 4 (15.4) |
| Hematology (%) | 27 (17.3) | 10 (17.9) | 13 (17.6) | 4 (15.4) |
| Oncology (%) | 30 (19.2) | 12 (21.4) | 16 (21.6) | 2 (7.7) |
| Rheumatology/Immunology (%) | 8 (5.1) | 5 (8.9) | 1 (1.4) | 2 (7.7) |
| Other (%) | 13 (8.3) | 6 (10.7) | 4 (5.4) | 3 (11.5) |
| Infection | ||||
| Lower respiratory tract (%) | 18 (11.5) | - | 9 (12.2) | 4 (15.4) |
| Intra-abdominal (%) | 20 (12.8) | - | 12 (16.2) | 1 (3.8) |
| Urinary (%) | 26 (16.7) | - | 16 (21.6) | 7 (26.9) |
| Skin/soft tissue (%) | 7 (4.5) | - | 2 (2.7) | 1 (3.8) |
| Viral systemic infection (%) | 39 (25.0) | - | 24 (32.4) | 9 (34.6) |
| Cardiovascular (%) | 4 (2.6) | - | 2 (2.7) | 2 (7.7) |
| Other (%) | 16 (10.3) | - | 9 (12.2) | 2 (7.7) |
| Patient No. | Gender | Specialism | Age | Immunocompromised | Final Diagnosis | WBC (×109/L) | CRP (mg/mL) | PSP (ng/mL) |
|---|---|---|---|---|---|---|---|---|
| 1. | Male | Oncology | 87 | No | Auto-immune pneumonitis | 14.10 | 85 | 202 |
| 2. | Male | Nephrology | 73 | Yes | Unknown | 15.00 | 11 | 403 |
| 3. | Male | Oncology | 67 | No | Auto-immune gastroenteritis | 14.10 | 31 | 472 |
| 4. | Male | Nephrology | 57 | Yes | Pericarditis | 6.50 | 286 | 367 |
| 5. | Female | Hematology | 61 | Yes | Graft versus host disease | 1.70 | 81 | 500 |
| 6. | Male | Hematology | 53 | Yes | Graft versus host disease | 9.50 | 179 | 247 |
| 7. | Female | Nephrology | 48 | Yes | Unknown | 4.10 | 7 | 242 |
| 8. | Male | Infectiology | 50 | No | Unknown | 10.80 | 24 | 577 |
| 9. | Male | Nephrology | 63 | Yes | Unknown | 13.60 | 49 | 320 |
| 10. | Female | Oncology | 52 | Yes | Unknown | 5.90 | 159 | 287 |
| 11. | Female | Oncology | 53 | No | Auto-immune ileocolitis | 20.10 | 114 | 528 |
| 12. | Female | Nephrology | 58 | Yes | Unknown | 8.90 | 3 | 206 |
| 13. | Male | Oncology | 81 | No | Auto-immune pneumonitis | 8.70 | 140 | 422 |
| 14. | Female | Nephrology | 54 | Yes | Unknown | 18.40 | 140 | 601 |
| 15. | Female | Nephrology | 59 | Yes | Adverse effect eplerenon | 21.30 | 0 | 601 |
| Patient No. | Gender | Specialism | Age | Immunocompromised | Final Diagnosis | Microbiological Culture | WBC (×109/L) | CRP (mg/mL) | PSP (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Female | Infectiology | 47 | No | Viral systemic | Coronavirus (Throatswab) | 7.10 | 233 | 48 |
| 2. | Female | Hematology | 20 | Yes | Viral systemic | Coronavirus (Throatswab) | 6.70 | 136 | 63 |
| 3. | Female | Immunology | 21 | Yes | Lower respiratory tract | - | 8.60 | 71 | 42 |
| 4. | Female | Hematology | 27 | Yes | Lower respiratory tract | - | 1.50 | 172 | 74 |
| 5. | Male | General internal medicine | 24 | No | Viral systemic infection | Coronavirus (Throatswab) | 3.10 | 13 | 44 |
| 6. | Male | General internal medicine | 22 | No | Other | Streptococcus pneumonia (Blood) | 35.10 | 169 | 92 |
| 7. | Female | General internal medicine | 29 | Yes | Viral systemic | Coronavirus (Throatswab) | 4.20 | 46 | 26 |
| 8. | Female | Oncology | 41 | No | Urinary tract | Escherichia coli (Urine) | 6.10 | 31 | 134 |
| 9. | Male | Hematology | 68 | Yes | Urinary tract | Escherichia coli (Blood) | 0.00 | 189 | 111 |
| 10. | Female | General internal medicine | 78 | No | Cardiovascular | Staphylococcus aureus (Blood) | 9.90 | 159 | 23 |
| 11. | Female | Infectiology | 35 | Yes | Viral systemic | Cytomegalovirus (Blood) | 6.00 | 126 | 141 |
| 12. | Male | General internal medicine | 84 | No | Viral systemic | Coronavirus (Throatswab) | 9.40 | 99 | 164 |
| 13. | Male | General internal medicine | 59 | No | Cardiovascular | Staphylococcus aureus (Blood) | 19.70 | 224 | 126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Hond, T.A.P.; Oosterheert, J.J.; van Hemert-Glaubitz, S.J.M.; Musson, R.E.A.; Kaasjager, K.A.H. Pancreatic Stone Protein as a Biomarker for Sepsis at the Emergency Department of a Large Tertiary Hospital. Pathogens 2022, 11, 559. https://doi.org/10.3390/pathogens11050559
de Hond TAP, Oosterheert JJ, van Hemert-Glaubitz SJM, Musson REA, Kaasjager KAH. Pancreatic Stone Protein as a Biomarker for Sepsis at the Emergency Department of a Large Tertiary Hospital. Pathogens. 2022; 11(5):559. https://doi.org/10.3390/pathogens11050559
Chicago/Turabian Stylede Hond, Titus A. P., Jan Jelrik Oosterheert, Susan J. M. van Hemert-Glaubitz, Ruben E. A. Musson, and Karin A. H. Kaasjager. 2022. "Pancreatic Stone Protein as a Biomarker for Sepsis at the Emergency Department of a Large Tertiary Hospital" Pathogens 11, no. 5: 559. https://doi.org/10.3390/pathogens11050559
APA Stylede Hond, T. A. P., Oosterheert, J. J., van Hemert-Glaubitz, S. J. M., Musson, R. E. A., & Kaasjager, K. A. H. (2022). Pancreatic Stone Protein as a Biomarker for Sepsis at the Emergency Department of a Large Tertiary Hospital. Pathogens, 11(5), 559. https://doi.org/10.3390/pathogens11050559







