Vector Surveillance and Pathogen Detection in the Working Areas of Military Working Dogs in Eastern Austria
Abstract
1. Introduction
2. Results
2.1. Ticks
Pathogen Detection in the Ticks
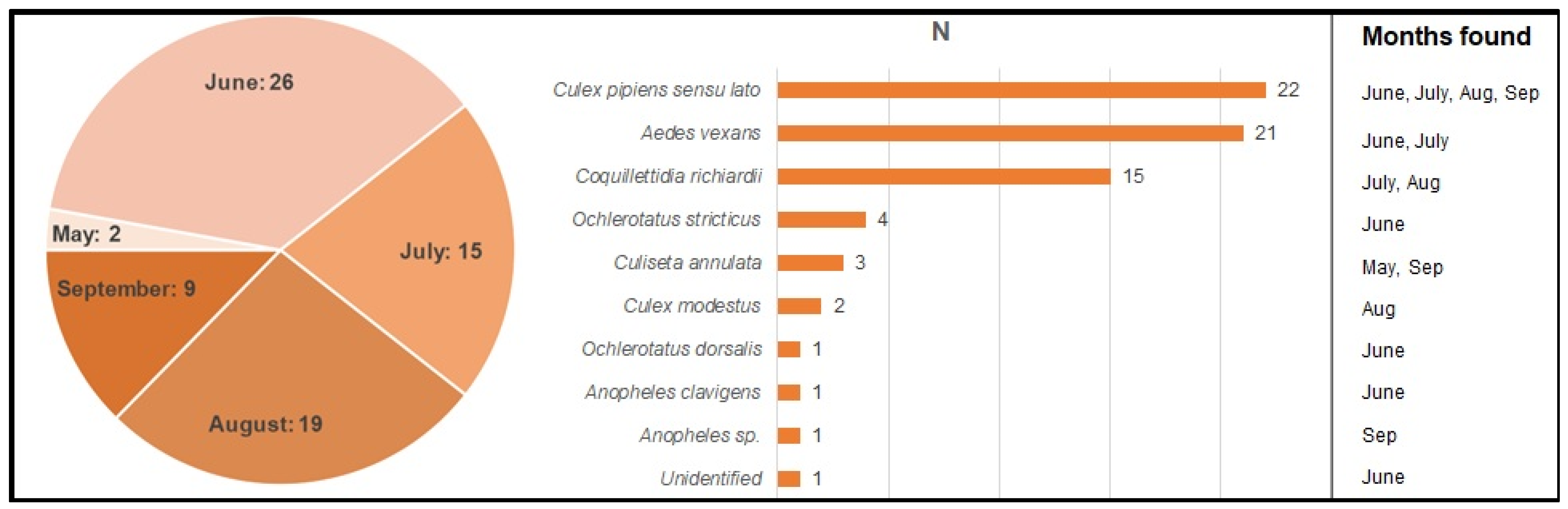
2.2. Mosquitoes
Pathogen Detection in the Mosquitoes
3. Discussion
4. Materials and Methods
4.1. Ticks
Collection and Specification
4.2. Mosquitoes
Collection and Differentiation
4.3. DNA Extraction and Pathogen Detection
4.3.1. DNA Extraction
4.3.2. Pathogen Detection
4.4. Statistics
| Organism | Locus | Primer Sequences | Amplification Protocol [Reference] | Product Size (bp) |
|---|---|---|---|---|
| Rickettsiae | Citrate Synthase | RpCS.877p: 5′-GGGGGCCTGCTCACGGCGG-3′ RpCS.1258n: 5′-ATTGCAAAAAGTACAGTGAACA-3 | 95 °C 5 min; 40×: 95 °C 30 s, 50 °C 30 s, 72 °C 1 min; 72 °C 10 min [54] | 381 |
| Anaplasmataceae | 16s rRNA | EHR16SD_for: 5′-GGTACCYACAGAAGAAGTCC-3′ EHR16SR_rev: 5′-TAGCACATCATCGTTTACAGC-3 | 95 °C 2 min; 35×: 94 °C 1 min, 54 °C 30 s, 72 °C 30 s; 72 °C 5 min [55] | 345 |
| Francisella tularensis | 17 kDa lipoprotein gene | TUL4-435: 5′-GCTGTATCATCATTTAATAAACTGCTG-3′ TUL4-863: 5′-TTGGGAAGCTTGTATCATGGCACT-3 | 94 °C 5 min; 40×: 94 °C 1 min, 51 °C 1 min, 72 °C 1 min; 72 °C 10 min [56] | 400 |
| Piroplasmida | 18S rRNA | Nest 1: BTH-1F: 5′-CCTGAGAAACGGCTACCACATCT-3′ BTH-1R: 5′-TTGCGACCATACTCCCCCCA-3′ Nest 2: G-2_for: 5′-GTCTTGTAATTGGAATGATGG-3′ G-2_rev: 5′-CCAAAGACTTTGATTTCTCTC-3′ | Nest 1: 94 °C 2 min; 40×: 95 °C 30 s, 68 °C 1 min, 72 °C 1 min; 72 °C 10 min Nest 2: 94 °C 2 min; 40×: 95 °C 30 s, 60 °C 1 min, 72 °C 1 min; 72 °C 10 min [57] | Nest 1: 700 Nest 2: 561 |
| Borrelia | 16S rRNA | Borr_allg_for: 5′-ACGCTGGCAGTGCGTCTTAA-3′ Borr_allg_rev: 5′-CTGATATCAACAGATTCCACCC-3′ | 94 °C 5 min; 40×: 94 °C 1.5 min, 63 °C 2 min, 72 °C 2 min; 72 °C 10 min [58] | 674 bp |
| Bartonella | gltA | BhCS.781p: 5′-GGGGACCAGCTCATGGTGG-3′ BhCS.1137n: 5′-AATGCAAAAAGAACAGTAAACA-3′ | 94 °C 5 min; 40×: 94 °C 1 min, 54 °C 1 min, 72 °C 1 min; 72 °C 10 min [59] | 379 bp |
| Filarioid helminths | COI | RpCS.877p: 5′-GGGGGCCTGCTCACGGCGG-3′ RpCS.1258n: 5′-ATTGCAAAAAGTACAGTGAACA-3 | 94 °C 2 min; 8×: 94 °C 45 s, 51 °C 45 s (reduced by 0.5 °C/cycle), 72 °C 1.5 min; 25×: 94° C 45 s, 45 °C 45 s, 72 °C 1.5 min; 72 °C 7 min [60] | 688 bp |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Savić, S.; Vidić, B.; Grgić, Z.; Potkonjak, A.; Spasojevic, L. Emerging vector-borne diseases—Incidence through vectors. Front. Public Health 2014, 2, 267. [Google Scholar] [CrossRef] [PubMed]
- Radda, A.; Burger, I.; Stanek, G.; Wewalka, G. Austrian hard ticks as vectors of Borrelia burgdorferi, overview. Zent. Bakteriol. Mikrobiol. Hyg. B Ser. A Med. Microbiol. Infect. Dis. Virol. Parasitol. 1986, 263, 79–82. [Google Scholar] [CrossRef]
- Duscher, G.G.; Feiler, A.; Leschnik, M.; Joachim, A. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from eastern Austria and the influence of acaricides/repellents on these parameters. Parasit. Vectors 2013, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany-evidence of a continuing spread of Dermacentor reticulatus. Front. Vet. Sci. 2020, 7, 578220. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G. Büchse der Pandora: Krankheitserreger in Ixodes ricinus-Zecken in Mitteleuropa. Wien. Klin. Wochenschr. 2009, 121, 673–683. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Glass, A.; Topp, A.-K.; Strube, C. Zoonotic tick-borne pathogens in temperate and cold regions of Europe—A review on the prevalence in domestic animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Glass, A.; Probst, J.; Strube, C. Tick-borne zoonoses and commonly used diagnostic methods in human and veterinary medicine. Parasitol. Res. 2021, 120, 4075–4090. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasit. Vectors 2016, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. (Eds.) Ticks of Europe and North Africa: A Guide to Species Identification; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-63759-4. [Google Scholar]
- Estrada-Peña, A.; D’Amico, G.; Fernández-Ruiz, N. Modelling the potential spread of Hyalomma marginatum ticks in Europe by migratory birds. Int. J. Parasitol. 2021, 51, 1–11. [Google Scholar] [CrossRef]
- Duscher, G.G.; Hodžić, A.; Hufnagl, P.; Wille-Piazzai, W.; Schötta, A.-M.; Markowicz, M.A.; Estrada-Peña, A.; Stanek, G.; Allerberger, F. Adult Hyalomma marginatum tick positive for Rickettsia aeschlimannii in Austria, October 2018. Euro Surveill. 2018, 23, 1800595. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Schötta, A.-M.; Müller, A.; Stockinger, H.; Stanek, G. A newly established real-time PCR for detection of Borrelia miyamotoi in Ixodes ricinus ticks. Ticks Tick Borne Dis. 2015, 6, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tobudic, S.; Burgmann, H.; Stanek, G.; Winkler, S.; Schötta, A.-M.; Obermüller, M.; Markowicz, M.; Lagler, H. Human Borrelia miyamotoi infection, Austria. Emerg. Infect. Dis. 2020, 26, 2201–2204. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Rinaldi, L.; Mortarino, M.; Genchi, M.; Cringoli, G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009, 163, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, H.-P.; Auer, H.; Leschnik, M.; Silbermayr, K.; Duscher, G.; Joachim, A. Dirofilaria in humans, dogs, and vectors in Austria (1978–2014) from imported pathogens to the endemicity of Dirofilaria repens. PLoS Negl. Trop. Dis. 2016, 10, e0004547. [Google Scholar] [CrossRef]
- Zittra, C. Mosquito Fauna in Eastern Austria with the Main Focus on Invasive Species—Molecular Phylogeny, Prevalence and Ecology. Ph.D. Thesis, University of Veterinary Medicine Vienna, Vienna, Austria, 2017. [Google Scholar]
- Auer, H.; Susani, M. Der erste autochthone Fall einer subkutanen Dirofilariose in Osterreich. Wien. Klin. Wochenschr. 2008, 120, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Silbermayr, K.; Eigner, B.; Joachim, A.; Duscher, G.G.; Seidel, B.; Allerberger, F.; Indra, A.; Hufnagl, P.; Fuehrer, H.-P. Autochthonous Dirofilaria repens in Austria. Parasit. Vectors 2014, 7, 226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonnberger, B.W.; Graf, B.; Straubinger, R.K.; Rackl, D.; Obwaller, A.G.; Peschke, R.; Shahi Barogh, B.; Joachim, A.; Fuehrer, H.-P. Vector-borne pathogens in clinically healthy military working dogs in eastern Austria. Parasitol. Int. 2021, 84, 102410. [Google Scholar] [CrossRef]
- Duscher, G.G.; Hodžić, A.; Weiler, M.; Vaux, A.G.C.; Rudolf, I.; Sixl, W.; Medlock, J.M.; Versteirt, V.; Hubálek, Z. First report of Rickettsia raoultii in field collected Dermacentor reticulatus ticks from Austria. Ticks Tick Borne Dis. 2016, 7, 720–722. [Google Scholar] [CrossRef]
- Vogelgesang, J.R.; Walter, M.; Kahl, O.; Rubel, F.; Brugger, K. Long-term monitoring of the seasonal density of questing ixodid ticks in Vienna (Austria): Setup and first results. Exp. Appl. Acarol. 2020, 81, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Huber, D.; Polkinghorne, A.; Kurilj, A.G.; Benko, V.; Mrljak, V.; Reljić, S.; Kusak, J.; Reil, I.; Beck, R. The prevalence and impact of Babesia canis and Theileria sp. in free-ranging grey wolf (Canis lupus) populations in Croatia. Parasit. Vectors 2017, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Meli, M.L.; Perreten, A.; Farkas, R.; Willi, B.; Beugnet, F.; Lutz, H.; Hofmann-Lehmann, R. Molecular investigation of hard ticks (Acari: Ixodidae) and fleas (Siphonaptera: Pulicidae) as potential vectors of rickettsial and mycoplasmal agents. Vet. Microbiol. 2010, 140, 98–104. [Google Scholar] [CrossRef]
- Heglasová, I.; Rudenko, N.; Golovchenko, M.; Zubriková, D.; Miklisová, D.; Stanko, M. Ticks, fleas and rodent-hosts analyzed for the presence of Borrelia miyamotoi in Slovakia: The first record of Borrelia miyamotoi in a Haemaphysalis inermis tick. Ticks Tick Borne Dis. 2020, 11, 101456. [Google Scholar] [CrossRef] [PubMed]
- Schötta, A.-M.; Wijnveld, M.; Stockinger, H.; Stanek, G. Approaches for reverse line blot-based detection of microbial pathogens in Ixodes ricinus ticks collected in Austria and impact of the chosen method. Appl. Environ. Microbiol. 2017, 83, e00489-17. [Google Scholar] [CrossRef] [PubMed]
- Collares-Pereira, M.; Couceiro, S.; Franca, I.; Kurtenbach, K.; Schäfer, S.M.; Vitorino, L.; Gonçalves, L.; Baptista, S.; Vieira, M.L.; Cunha, C. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 2004, 42, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Diza, E.; Papa, A.; Vezyri, E.; Tsounis, S.; Milonas, I.; Antoniadis, A. Borrelia valaisiana in cerebrospinal fluid. Emerg. Infect. Dis. 2004, 10, 1692–1693. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.V.L.; Earnhart, C.G.; Mather, T.N.; Meeus, P.F.M.; Marconi, R.T. Identification of Borrelia burgdorferi ospC genotypes in canine tissue following tick infestation: Implications for Lyme disease vaccine and diagnostic assay design. Vet. J. 2013, 198, 412–418. [Google Scholar] [CrossRef]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef] [PubMed]
- Glatz, M.; Müllegger, R.R.; Maurer, F.; Fingerle, V.; Achermann, Y.; Wilske, B.; Bloemberg, G.V. Detection of Candidatus Neoehrlichia mikurensis, Borrelia burgdorferi sensu lato genospecies and Anaplasma phagocytophilum in a tick population from Austria. Ticks Tick Borne Dis. 2014, 5, 139–144. [Google Scholar] [CrossRef]
- Chmielewski, T.; Podsiadly, E.; Karbowiak, G.; Tylewska-Wierzbanowska, S. Rickettsia spp. in ticks, Poland. Emerg. Infect. Dis. 2009, 15, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Hamel, D.; Thiel, C.; Pfister, K.; Pfeffer, M. Spotted fever group rickettsiae in ticks, Germany. Emerg. Infect. Dis. 2011, 17, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, I.; Venclíková, K.; Blažejová, H.; Betášová, L.; Mendel, J.; Hubálek, Z.; Parola, P. First report of Rickettsia raoultii and Rickettsia helvetica in Dermacentor reticulatus ticks from the Czech Republic. Ticks Tick Borne Dis. 2016, 7, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Ouarti, B.; Hamzaoui, B.E.; Stanko, M.; Laroche, M.; Mediannikov, O.; Parola, P.; Sekeyová, Z. Detection of Rickettsia raoultii in Dermacentor reticulatus and Haemaphysalis inermis ticks in Slovakia. Biologia 2021. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.-H.; Huang, Y.; Du, J.; Cui, N.; Yang, Z.-D.; Tang, F.; Fu, F.-X.; Li, X.-M.; Cui, X.-M.; et al. Isolation and identification of Rickettsia raoultii in human cases: A surveillance study in 3 medical centers in China. Clin. Infect. Dis. 2018, 66, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, G.; Socolovschi, C.; Del Giudice, P.; Fournier, P.E.; Raoult, D. Scalp eschar and neck lymphadenopathy after tick bite: An emerging syndrome with multiple causes. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1449–1456. [Google Scholar] [CrossRef]
- Fournier, P.E.; Grunnenberger, F.; Jaulhac, B.; Gastinger, G.; Raoult, D. Evidence of Rickettsia helvetica infection in humans, Eastern France. Emerg. Infect. Dis. 2000, 6, 389–392. [Google Scholar] [CrossRef]
- Jado, I.; Oteo, J.A.; Aldámiz, M.; Gil, H.; Escudero, R.; Ibarra, V.; Portu, J.; Portillo, A.; Lezaun, M.J.; García-Amil, C.; et al. Rickettsia monacensis and human disease, Spain. Emerg. Infect. Dis. 2007, 13, 1405–1407. [Google Scholar] [CrossRef]
- Hodžić, A.; Zörer, J.; Duscher, G.G. Dermacentor reticulatus, a putative vector of Babesia cf. microti (syn. Theileria annae) piroplasm. Parasitol. Res. 2017, 116, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.M.; Kumar, S.; Krause, P.J. Persistence of Babesia microti infection in humans. Pathogens 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Bohacsova, M.; Mediannikov, O.; Kazimirova, M.; Raoult, D.; Sekeyova, Z. Arsenophonus nasoniae and Rickettsiae infection of Ixodes ricinus due to parasitic wasp Ixodiphagus hookeri. PLoS ONE 2016, 11, e0149950. [Google Scholar] [CrossRef]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe—New distribution trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef] [PubMed]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef]
- Kronefeld, M.; Kampen, H.; Sassnau, R.; Werner, D. Molecular detection of Dirofilaria immitis, Dirofilaria repens and Setaria tundra in mosquitoes from Germany. Parasit. Vectors 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Übleis, S.S.; Cuk, C.; Nawratil, M.; Butter, J.; Schoener, E.; Obwaller, A.G.; Zechmeister, T.; Duscher, G.G.; Rubel, F.; Lebl, K.; et al. Xenomonitoring of Mosquitoes (Diptera: Culicidae) for the presence of filarioid helminths in Eastern Austria. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 9754695. [Google Scholar] [CrossRef]
- Fuehrer, H.-P.; Morelli, S.; Unterköfler, M.S.; Bajer, A.; Bakran-Lebl, K.; Dwużnik-Szarek, D.; Farkas, R.; Grandi, G.; Heddergott, M.; Jokelainen, P.; et al. Dirofilaria spp. and Angiostrongylus vasorum: Current risk of spreading in central and northern Europe. Pathogens 2021, 10, 1268. [Google Scholar] [CrossRef]
- Sonnberger, K.; Fuehrer, H.-P.; Sonnberger, B.W.; Leschnik, M. The Incidence of Dirofilaria immitis in shelter dogs and mosquitoes in Austria. Pathogens 2021, 10, 550. [Google Scholar] [CrossRef]
- Hillyard, P. Ticks of North-West Europe: Keys and Notes for Identification of The Species; Field Studies Council: Shrewsbury, UK, 1996; ISBN 1851532579. [Google Scholar]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Minoo, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany; Dordrecht, The Netherlands, 2010; ISBN 3540928731. [Google Scholar]
- Răileanu, C.; Tauchmann, O.; Vasić, A.; Wöhnke, E.; Silaghi, C. Borrelia miyamotoi and Borrelia burgdorferi (sensu lato) identification and survey of tick-borne encephalitis virus in ticks from north-eastern Germany. Parasit. Vectors 2020, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Biggerstaff, B. PooledInfRate software. Vector Borne Zoonotic Dis. 2005, 5, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Roux, V.; Camicas, J.-L.; Baradji, I.; Brouqui, P.; Raoult, D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 707–708. [Google Scholar] [CrossRef]
- Sjöstedt, A.; Eriksson, U.; Berglund, L.; Tärnvik, A. Detection of Francisella tularensis in ulcers of patients with tularemia by PCR. J. Clin. Microbiol. 1997, 35, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Zintl, A.; Finnerty, E.J.; Murphy, T.M.; de Waal, T.; Gray, J.S. Babesias of red deer (Cervus elaphus) in Ireland. Vet. Res. 2011, 42, 7. [Google Scholar] [CrossRef]
- Liebisch, G.; Sohns, B.; Bautsch, W. Detection and typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks attached to human skin by PCR. J. Clin. Microbiol. 1998, 36, 3355–3358. [Google Scholar] [CrossRef]
- Norman, A.F.; Regnery, R.; Jameson, P.; Greene, C.; Krause, D.C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 1995, 33, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Hamer, G.L.; Anderson, T.K.; Berry, G.E.; Makohon-Moore, A.P.; Crafton, J.C.; Brawn, J.D.; Dolinski, A.C.; Krebs, B.L.; Ruiz, M.O.; Muzzall, P.M.; et al. Prevalence of filarioid nematodes and trypanosomes in American robins and house sparrows. Int. J. Parasitol. Parasites Wildl. 2013, 2, 42–49. [Google Scholar] [CrossRef]



| Pathogen | I. ricinus | D. reticulatus | H. inermis | H. concinna |
|---|---|---|---|---|
| Borrelia afzelii (OM033637) | 29.38 | 0.00 | 0.00 | 0.00 |
| Borrelia miyamotoi (OM033638) | 7.37 | 0.00 | 0.00 | 0.00 |
| Borrelia garinii (OM033640, OM033643, OM033644) | 6.32 | 0.00 | 0.00 | 0.00 |
| Borrelia burgdorferi s. s. (OM033638, OM033645) | 5.26 | 0.00 | 0.00 | 0.00 |
| Borrelia lustinaniae (OM033641) | 3.68 | 0.00 | 0.00 | 0.00 |
| Borrelia valesiana (OM033642) | 0.53 | 0.00 | 0.00 | 0.00 |
| Rickettsia helvetica (OM039458) | 51.05 | 0.00 | 0.00 | 0.00 |
| Rickettsia monacensis (OM039462) | 0.53 | 0.00 | 0.00 | 0.00 |
| Rickettsia raoultii (OM039459) | 0.00 | 60.98 | 0.00 | 0.00 |
| Rickettsia sp. (OM039460, OM039461) | 1.05 | 2.44 | 64.58 | 60.00 |
| Bartonella spp. (OL690436–OL690472) | 1.58 | 2.44 | 62.50 | 60.00 |
| Babesia microti (OL960636) | 0.53 | 0.00 | 0.00 | 0.00 |
| Arsenophonus sp. (OM001650) | 3.68 | 0.00 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonnberger, B.W.; Wortha, L.N.; Rackl, D.; Obwaller, A.G.; Joachim, A.; Fuehrer, H.-P. Vector Surveillance and Pathogen Detection in the Working Areas of Military Working Dogs in Eastern Austria. Pathogens 2022, 11, 506. https://doi.org/10.3390/pathogens11050506
Sonnberger BW, Wortha LN, Rackl D, Obwaller AG, Joachim A, Fuehrer H-P. Vector Surveillance and Pathogen Detection in the Working Areas of Military Working Dogs in Eastern Austria. Pathogens. 2022; 11(5):506. https://doi.org/10.3390/pathogens11050506
Chicago/Turabian StyleSonnberger, Bernhard W., Licha N. Wortha, Dietmar Rackl, Adelheid G. Obwaller, Anja Joachim, and Hans-Peter Fuehrer. 2022. "Vector Surveillance and Pathogen Detection in the Working Areas of Military Working Dogs in Eastern Austria" Pathogens 11, no. 5: 506. https://doi.org/10.3390/pathogens11050506
APA StyleSonnberger, B. W., Wortha, L. N., Rackl, D., Obwaller, A. G., Joachim, A., & Fuehrer, H.-P. (2022). Vector Surveillance and Pathogen Detection in the Working Areas of Military Working Dogs in Eastern Austria. Pathogens, 11(5), 506. https://doi.org/10.3390/pathogens11050506








