Ceragenin CSA-44 as a Means to Control the Formation of the Biofilm on the Surface of Tooth and Composite Fillings
Abstract
1. Introduction
2. Results
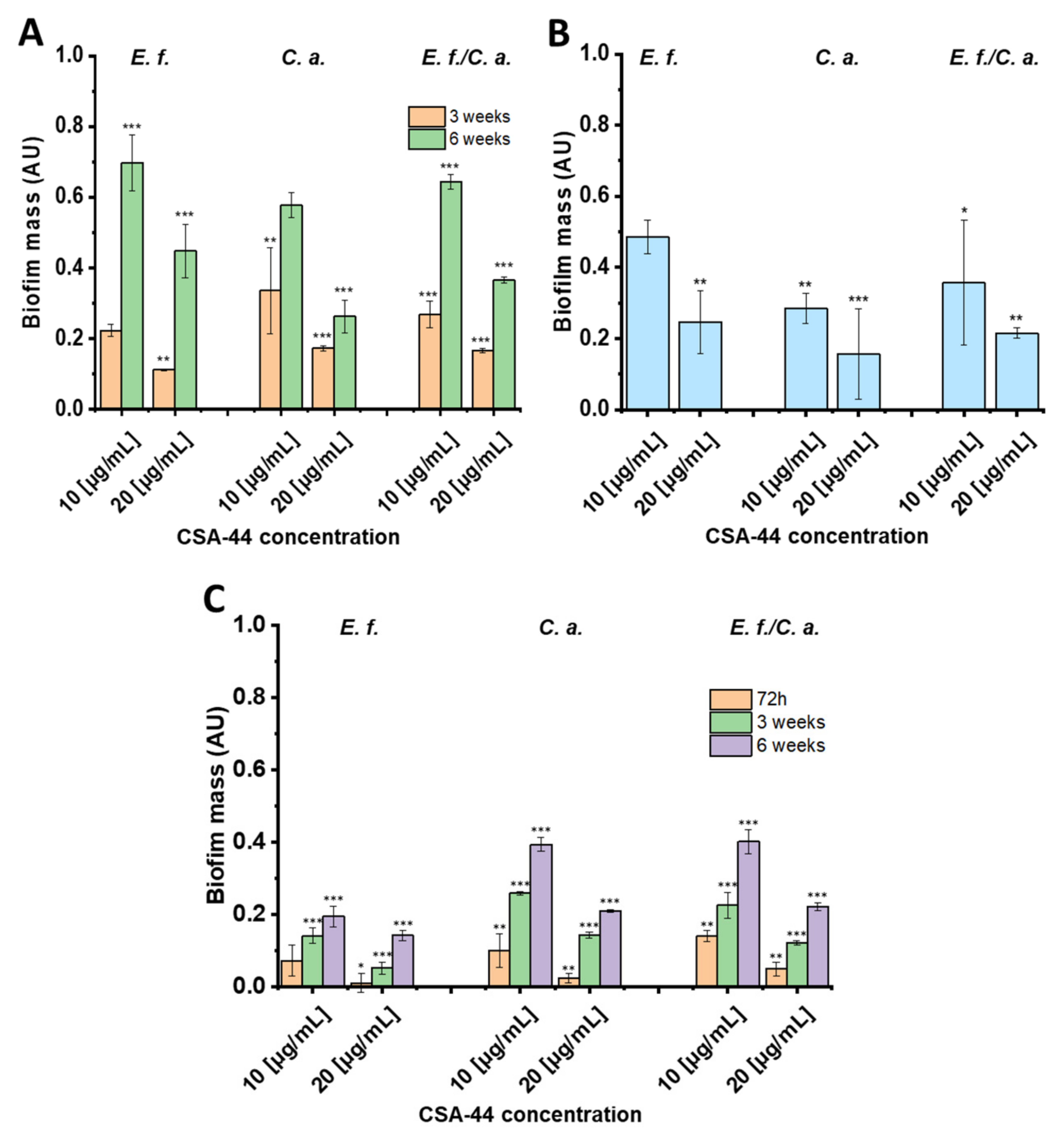
2.1. Biofilm Mass Studies
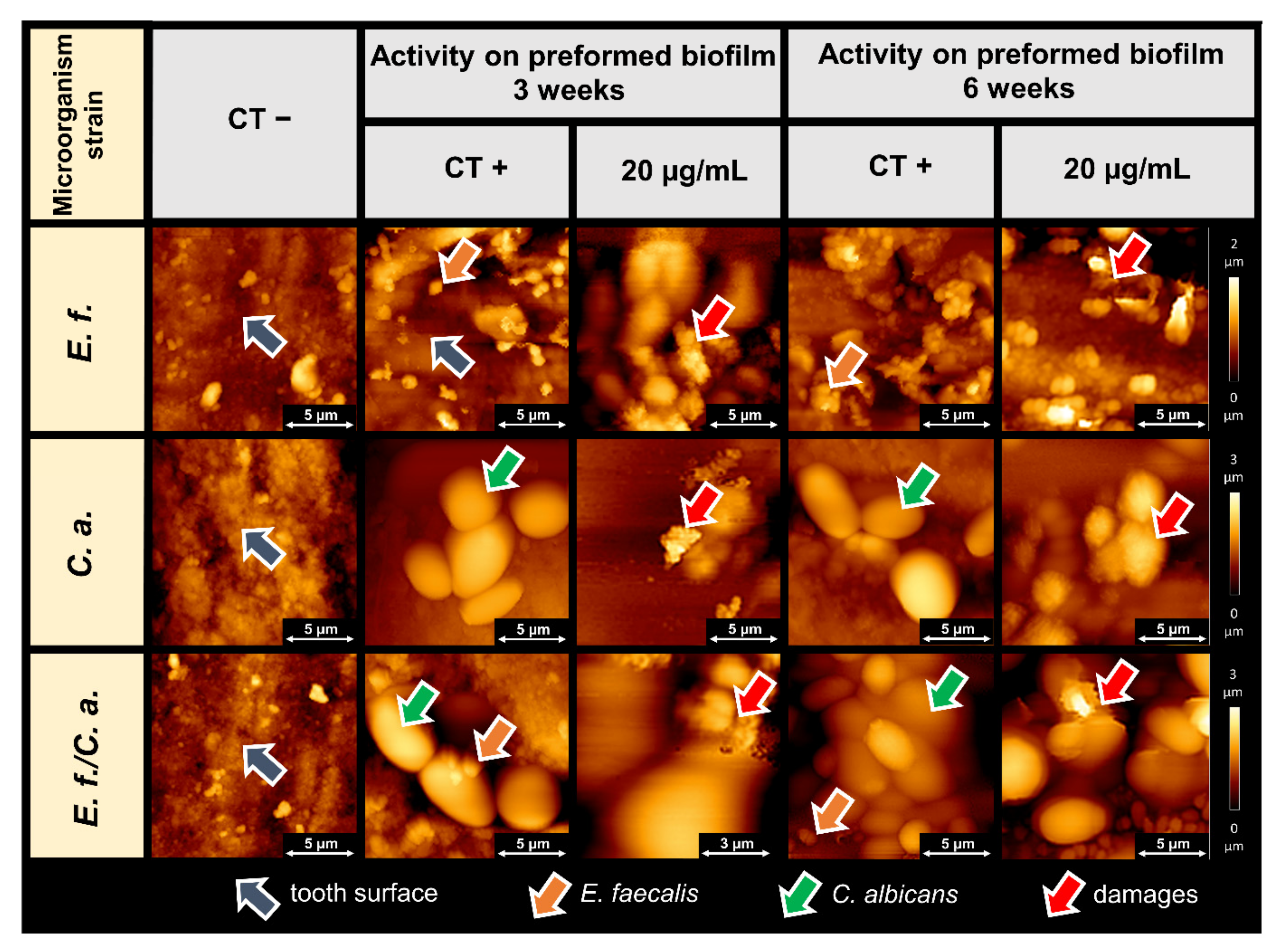
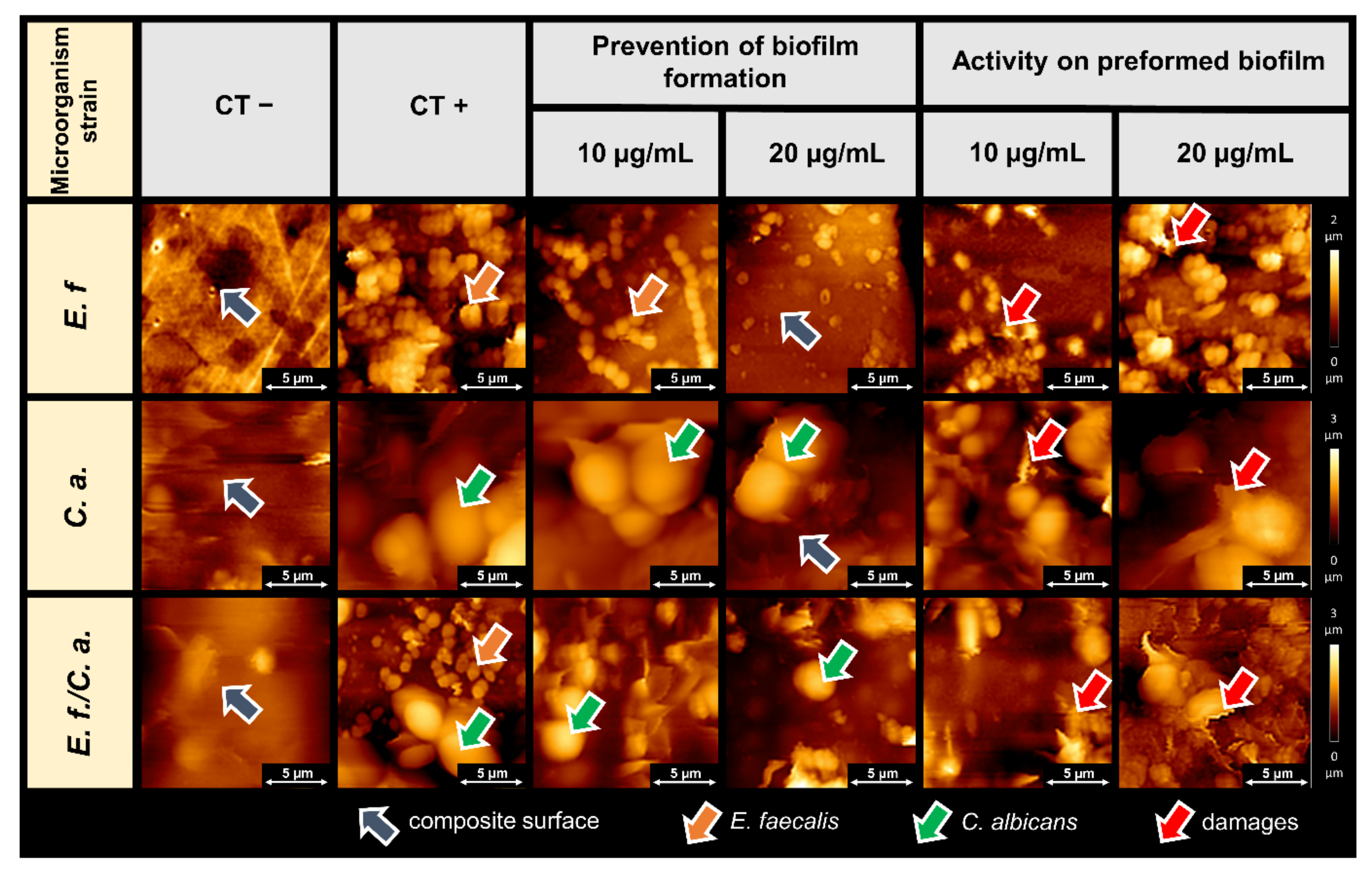
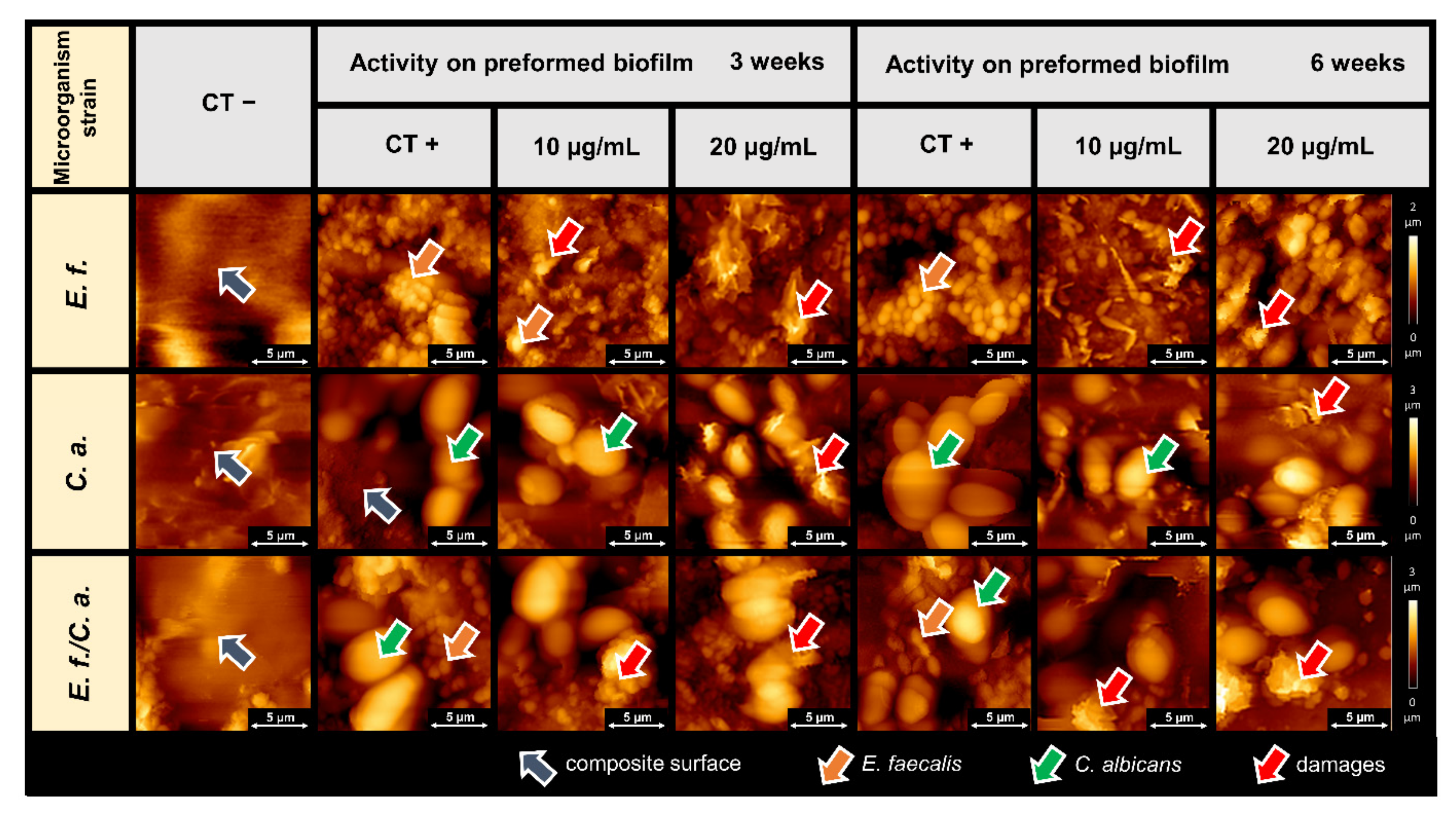
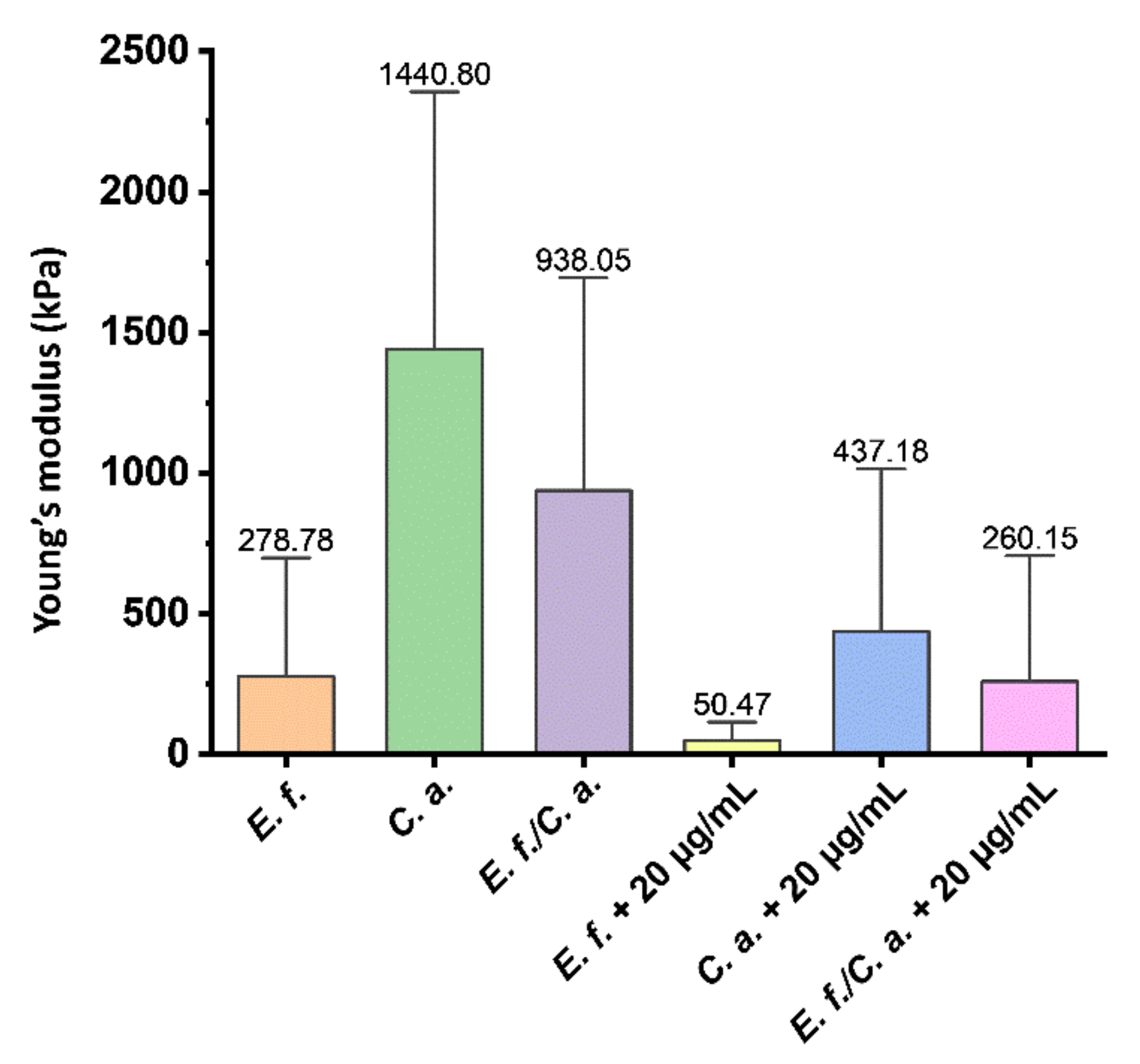
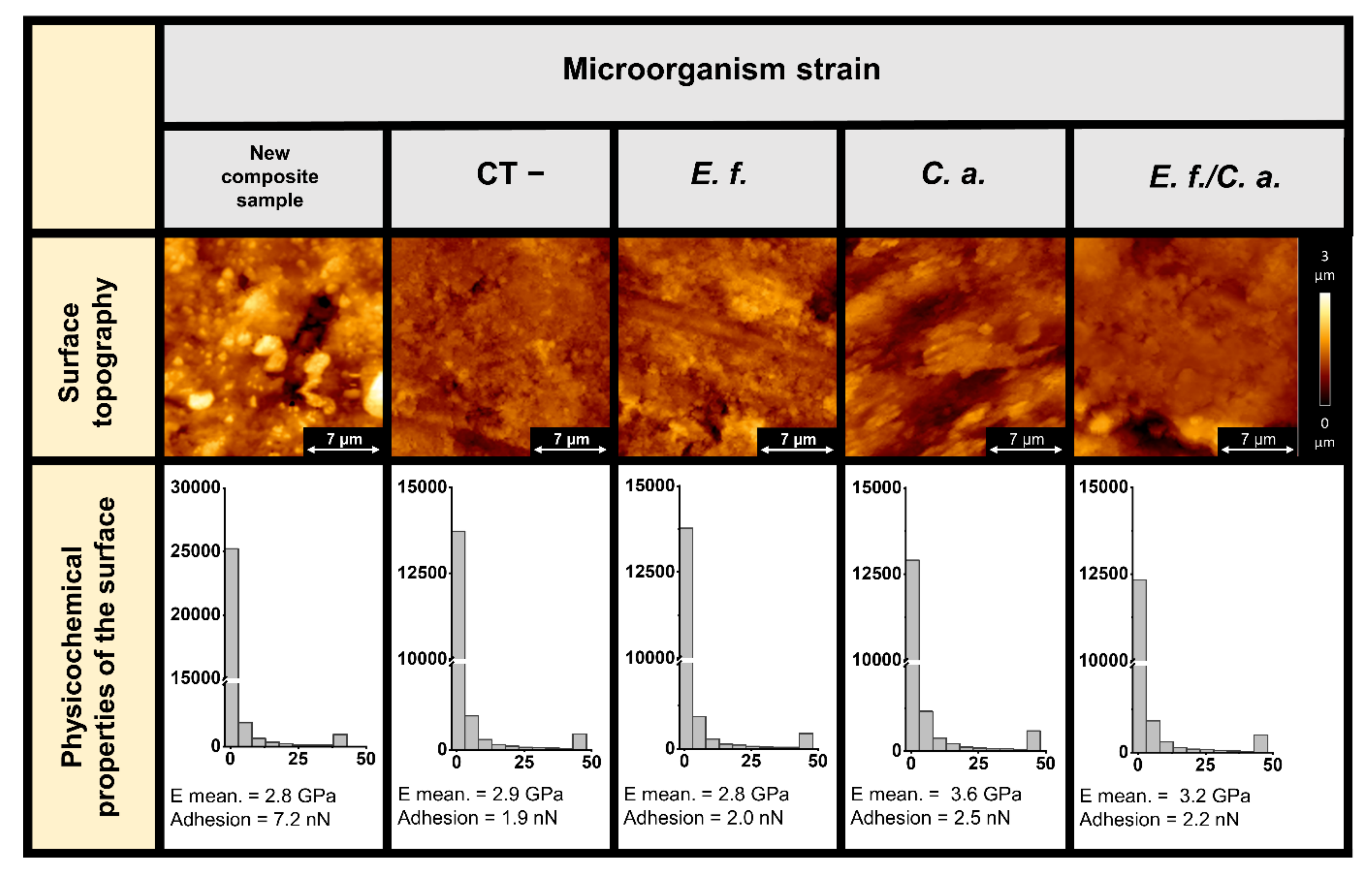
2.2. Microscopic Studies of the Surfaces of Teeth and Dental Composites
3. Discussion
4. Materials and Methods
4.1. Collection of Human Tooth and Fabrication of Composite Disc
4.2. Strains of Microorganism
4.3. Ceragenin
4.4. Prevention of Biofilm Formation
4.5. Disruption of Established Biofilms
4.6. Evaluation of Antibiofilm Activity-AFM Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: Virulence factors, host defense, escape strategies, and the microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections–An update. Apmis 2017, 125, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral biofilm architecture on natural teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Kim, A.R.; Perinpanayagam, H.; Han, S.H.; Kum, K.Y. Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms 2020, 8, 1300. [Google Scholar] [CrossRef]
- Ohshima, T.; Ikawa, S.; Kitano, K.; Maeda, N. A proposal of remedies for oral diseases caused by Candida: A mini review. Front. Microbiol. 2018, 9, 1522. [Google Scholar] [CrossRef]
- Zhang, K.; Baras, B.; Lynch, C.D.; Weir, M.D.; Melo, M.A.S.; Li, Y.; Reynolds, M.A.; Bai, Y.; Wang, L.; Wang, S.; et al. Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth. Materials 2018, 11, 1747. [Google Scholar] [CrossRef]
- Sousa, R.P.; Zanin, I.C.; Lima, J.P.; Vasconcelos, S.M.; Melo, M.A.; Beltrao, H.C.; Rodrigues, L.K. In situ effects of restorative materials on dental biofilm and enamel demineralisation. J. Dent. 2009, 37, 44–51. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Pashley, D.H. Clinical considerations of microleakage. J. Endod. 1990, 16, 70–77. [Google Scholar] [CrossRef]
- Suprewicz, L.; Tokajuk, G.; Ciesluk, M.; Deptula, P.; Sierpinska, T.; Wolak, P.; Wollny, T.; Tokajuk, J.; Gluszek, S.; Piktel, E.; et al. Bacteria Residing at Root Canals can Induce Cell Proliferation and Alter the Mechanical Properties of Gingival and Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7914. [Google Scholar] [CrossRef]
- Do, T.; Devine, D.; Marsh, P.D. Oral biofilms: Molecular analysis, challenges, and future prospects in dental diagnostics. Clin. Cosmet. Investig. Dent. 2013, 5, 11–19. [Google Scholar] [CrossRef]
- Wright, C.J.; Burns, L.H.; Jack, A.A.; Back, C.R.; Dutton, L.C.; Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Microbial interactions in building of communities. Mol. Oral Microbiol. 2013, 28, 83–101. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Zawrotniak, M.; Gogol, M.; Bartnicka, D.; Satala, D.; Smolarz, M.; Karkowska- Kuleta, J.; Kozik, A. Interactions of Candida albicans Cells with Aerobic and Anaerobic Bacteria during Formation of Mixed Biofilms in the Oral Cavity. IntechOpen 2019, 119. [Google Scholar] [CrossRef]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F.; Savage, P.B. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 2008, 21, 307–311. [Google Scholar] [CrossRef]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnol. 2020, 18, 1–18. [Google Scholar] [CrossRef]
- Chmielewska, S.J.; Sklodowski, K.; Piktel, E.; Suprewicz, L.; Fiedoruk, K.; Daniluk, T.; Wolak, P.; Savage, P.B.; Bucki, R. NDM-1 Carbapenemase-Producing Enterobacteriaceae are Highly Susceptible to Ceragenins CSA-13, CSA-44, and CSA-131. Infect. Drug Resist. 2020, 13, 3277–3294. [Google Scholar] [CrossRef]
- Majima, T.; Ito-Kuwa, S.; Nagatomi, R.; Nakamura, K. Study of the oral carriage of Candida sp. in dental students and staff—Identification of Candida sp. and background survey. Oral Sci. Int. 2014, 11, 30–34. [Google Scholar] [CrossRef]
- Camarillo-Marquez, O.; Cordova-Alcantara, I.M.; Hernandez-Rodriguez, C.H.; Garcia-Perez, B.E.; Martinez-Rivera, M.A.; Rodriguez-Tovar, A.V. Antagonistic Interaction of Staphylococcus aureus toward Candida glabrata during in vitro Biofilm Formation Is Caused by an Apoptotic Mechanism. Front. Microbiol. 2018, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; Fernandes, F.S.F.; Skupien, J.A.; Mesko, M.E.; Straioto, F.G.; Del Bel Cury, A.A. Can new dentures decrease Candida levels? Int. J. Prosthodont. 2013, 26, 470–477. [Google Scholar] [CrossRef]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and penetration of denture soft lining materials by Candida albicans. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Eguia, A.; Arakistain, A.; De-la-Pinta, I.; López-Vicente, J.; Sevillano, E.; Quindós, G.; Eraso, E. Candida albicans biofilms on different materials for manufacturing implant abutments and prostheses. Med. Oral Patol. Oral y Cirugía Bucal 2020, 25, e13. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Weir, M.D.; Xu, H.H. Denture Acrylic Resin Material with Antibacterial and Protein-Repelling Properties for the Prevention of Denture Stomatitis. Polymers 2022, 14, 230. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Cieśluk, M.; Chmielewska, S.; Durnaś, B.; Król, G.; Wollny, T.; Deptuła, P.; Kochanowicz, J. Rod-shaped gold nanoparticles exert potent candidacidal activity and decrease the adhesion of fungal cells. Nanomedicine 2020, 15, 2733–2752. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Niemirowicz-Laskowska, K.; Deptuła, P.; Wilczewska, A.Z.; Misiak, P.; Durnaś, B.; Fiedoruk, K.; Piktel, E.; Mystkowska, J.; Janmey, P.A. Susceptibility of microbial cells to the modified PIP 2-binding sequence of gelsolin anchored on the surface of magnetic nanoparticles. J. Nanobiotechnol. 2019, 17, 81. [Google Scholar] [CrossRef]
- Durnas, B.; Wnorowska, U.; Pogoda, K.; Deptula, P.; Watek, M.; Piktel, E.; Gluszek, S.; Gu, X.; Savage, P.B.; Niemirowicz, K.; et al. Candidacidal Activity of Selected Ceragenins and Human Cathelicidin LL-37 in Experimental Settings Mimicking Infection Sites. PLoS ONE 2016, 11, e0157242. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, Y.S.; Han, I.; Kim, M.H.; Jung, M.H.; Park, H.K. Quantitative and qualitative analyses of the cell death process in Candida albicans treated by antifungal agents. PLoS ONE 2011, 6, e28176. [Google Scholar] [CrossRef][Green Version]
- Spałek, J.; Deptuła, P.; Cieśluk, M.; Strzelecka, A.; Łysik, D.; Mystkowska, J.; Daniluk, T.; Król, G.; Góźdź, S.; Bucki, R. Biofilm Growth Causes Damage to Silicone Voice Prostheses in Patients after Surgical Treatment of Locally Advanced Laryngeal Cancer. Pathogens 2020, 9, 793. [Google Scholar] [CrossRef]
- Wnorowska, U.; Piktel, E.; Durnaś, B.; Fiedoruk, K.; Savage, P.B.; Bucki, R. Use of ceragenins as a potential treatment for urinary tract infections. BMC Infect. Dis. 2019, 19, 369. [Google Scholar] [CrossRef]
- Wnorowska, U.; Niemirowicz, K.; Myint, M.; Diamond, S.L.; Wroblewska, M.; Savage, P.B.; Janmey, P.A.; Bucki, R. Bactericidal activities of cathelicidin LL-37 and select cationic lipids against the hypervirulent Pseudomonas aeruginosa strain LESB58. Antimicrob. Agents Chemother. 2015, 59, 3808–3815. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal activity of ceragenin CSA-13 in cell culture and in an animal model of peritoneal infection. Antimicrob. Agents Chemother. 2015, 59, 6274–6282. [Google Scholar] [CrossRef]
- Pogoda, K.; Piktel, E.; Deptula, P.; Savage, P.B.; Lekka, M.; Bucki, R. Stiffening of bacteria cells as a first manifestation of bactericidal attack. Micron 2017, 101, 95–102. [Google Scholar] [CrossRef]
- Le, P.H.; Nguyen, D.H.; Medina, A.A.; Linklater, D.P.; Loebbe, C.; Crawford, R.J.; MacLaughlin, S.; Ivanova, E.P. Surface Architecture Influences the Rigidity of Candida albicans Cells. Nanomaterials 2022, 12, 567. [Google Scholar] [CrossRef]
- Kreis, C.T.; Sullan, R.M.A. Interfacial nanomechanical heterogeneity of the E. coli biofilm matrix. Nanoscale 2020, 12, 16819–16830. [Google Scholar] [CrossRef]
- Pillet, F.; Lemonier, S.; Schiavone, M.; Formosa, C.; Martin-Yken, H.; Francois, J.M.; Dague, E. Uncovering by atomic force microscopy of an original circular structure at the yeast cell surface in response to heat shock. BMC Biol. 2014, 12, 6. [Google Scholar] [CrossRef]
- Elbourne, A.; Chapman, J.; Gelmi, A.; Cozzolino, D.; Crawford, R.J.; Truong, V.K. Bacterial-nanostructure interactions: The role of cell elasticity and adhesion forces. J. Colloid Interface Sci. 2019, 546, 192–210. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterial surface destruction–corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Yulianto, H.D.K.; Rinastiti, M.; Cune, M.S.; de Haan-Visser, W.; Atema-Smit, J.; Busscher, H.J.; van der Mei, H.C. Biofilm composition and composite degradation during intra-oral wear. Dent. Mater. 2019, 35, 740–750. [Google Scholar] [CrossRef]
- Richert, A.; Dąbrowska, G.B. Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone polymers in various environments. Int. J. Biol. Macromol. 2021, 176, 226–232. [Google Scholar] [CrossRef]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of dental resins resistant to enzymatic and hydrolytic degradation in oral environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, Y.; Weir, M.D.; Xu, H.H.; Bai, Y.; Melo, M.A.S. Current insights into the modulation of oral bacterial degradation of dental polymeric restorative materials. Materials 2017, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, microcracks and fracture in polymer structures: Formation, detection, autonomic repair. Prog. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Botsis, J.; Drummond, J.L. Fracture studies of selected dental restorative composites. Dent. Mater. 1997, 13, 198–207. [Google Scholar] [CrossRef]
- Bjerke, T.W. Thermomechanical Behavior of Amorphous Polymers during High-Speed Crack Propagation; U.S. Army Research, Laboratory Technical Report ARL-TR-2793; Army Research Lab Aberdeen Proving Ground: Adelphi, MD, USA, 2002. [Google Scholar]
- Madeira, P.L.; Carvalho, L.T.; Paschoal, M.A.; De Sousa, E.M.; Moffa, E.B.; da Silva, M.A.; Tavarez, R.d.J.R.; Gonçalves, L.M. In vitro effects of lemongrass extract on Candida albicans biofilms, human cells viability, and denture surface. Front. Cell. Infect. Microbiol. 2016, 6, 71. [Google Scholar] [CrossRef]
- Ding, B.; Guan, Q.; Walsh, J.P.; Boswell, J.S.; Winter, T.W.; Winter, E.S.; Boyd, S.S.; Li, C.; Savage, P.B. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 2002, 45, 663–669. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokajuk, J.; Deptuła, P.; Chmielewska, S.J.; Skłodowski, K.; Mierzejewska, Ż.A.; Grądzka-Dahlke, M.; Tołstoj, A.; Daniluk, T.; Paprocka, P.; Savage, P.B.; et al. Ceragenin CSA-44 as a Means to Control the Formation of the Biofilm on the Surface of Tooth and Composite Fillings. Pathogens 2022, 11, 491. https://doi.org/10.3390/pathogens11050491
Tokajuk J, Deptuła P, Chmielewska SJ, Skłodowski K, Mierzejewska ŻA, Grądzka-Dahlke M, Tołstoj A, Daniluk T, Paprocka P, Savage PB, et al. Ceragenin CSA-44 as a Means to Control the Formation of the Biofilm on the Surface of Tooth and Composite Fillings. Pathogens. 2022; 11(5):491. https://doi.org/10.3390/pathogens11050491
Chicago/Turabian StyleTokajuk, Joanna, Piotr Deptuła, Sylwia J Chmielewska, Karol Skłodowski, Żaneta A Mierzejewska, Małgorzata Grądzka-Dahlke, Adam Tołstoj, Tamara Daniluk, Paulina Paprocka, Paul B Savage, and et al. 2022. "Ceragenin CSA-44 as a Means to Control the Formation of the Biofilm on the Surface of Tooth and Composite Fillings" Pathogens 11, no. 5: 491. https://doi.org/10.3390/pathogens11050491
APA StyleTokajuk, J., Deptuła, P., Chmielewska, S. J., Skłodowski, K., Mierzejewska, Ż. A., Grądzka-Dahlke, M., Tołstoj, A., Daniluk, T., Paprocka, P., Savage, P. B., & Bucki, R. (2022). Ceragenin CSA-44 as a Means to Control the Formation of the Biofilm on the Surface of Tooth and Composite Fillings. Pathogens, 11(5), 491. https://doi.org/10.3390/pathogens11050491






