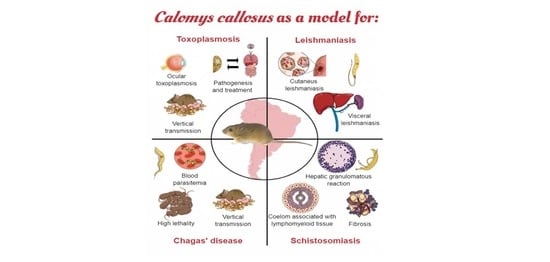
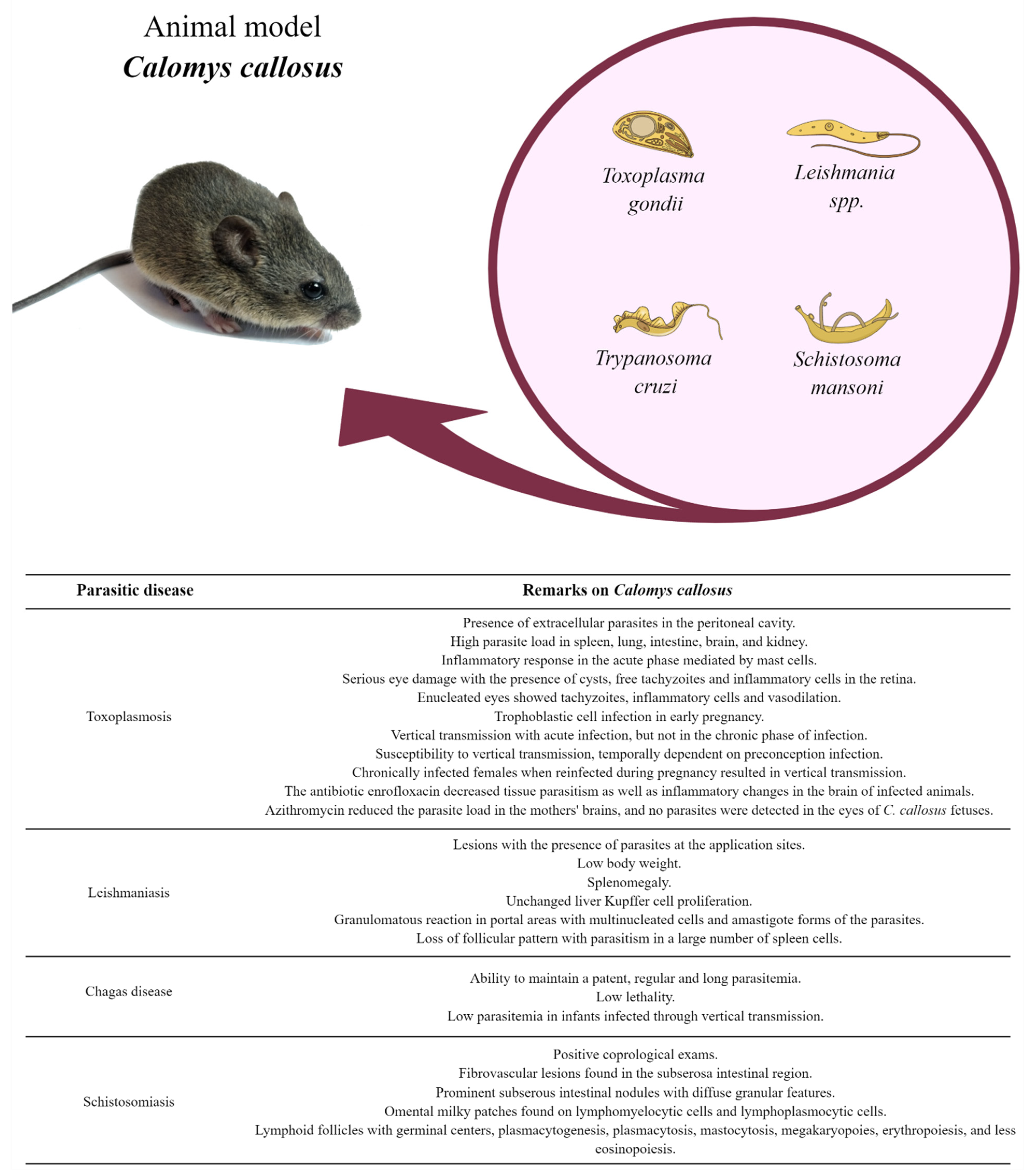
Calomys callosus: An Experimental Animal Model Applied to Parasitic Diseases Investigations of Public Health Concern
Abstract
1. Introduction
2. Toxoplasmosis
2.1. Acute Phase of Toxoplasmosis
2.2. Acquired and Congenital Ocular Toxoplasmosis
2.3. Congenital Toxoplasmosis
3. Leishmaniasis
3.1. Visceral Leishmaniasis (VL)
3.2. Cutaneous Leishmaniasis (CL)
4. Chagas Disease
4.1. Parasitemia and Lethality
4.2. Congenital Transmission
5. Schistosomiasis
6. Challenges in Maintaining C. callosus as an Experimental Model
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moojen, J. Os Roedores do Brasil; Instituto Nacional do Livro: Rio de Janeiro, Brazil, 1952. [Google Scholar]
- Hershkovitz, P. Evolution of Neotropical Cricetine Rodents (Muridae) with Special Reference to the Phyllotine Group; Chicago Natural History Museum: Chicago, IL, USA, 1962. [Google Scholar]
- Massoia, E.; Fornes, A. Nuevos dados sobre la morfologia distribucion geografica y etoecologia de Calomys callosus (Rengger) (Rodentia Cricetidae). Physis 1965, 25, 325. [Google Scholar]
- Mello, D.A.; Moojen, L.E. Nota sobre uma coleção de roedores e marsupiais superiores de algumas regiões do cerrado do Brasil central. Rev. Bras. Pesqui Med. Biol. 1979, 12, 287–291. [Google Scholar] [PubMed]
- Petter, F.; de Karimi Almeida, C.R. A new laboratory rodent, Cricetida Calomys callosus. Comptes Rendus Hebd. Séances L’académie Sci. 1967, 265, 1974–1976. [Google Scholar]
- Karimi, Y.; Almeida, C.R.; Petter, F. Note sur Les Rongeurs du Nord.-Est du Brésil. Mammalia 1976, 40, 257–266. [Google Scholar] [CrossRef]
- Mello, D.A. Calomys Callosus Rengger, 1930, (Rodentia-Cricetidae): Sua caracterização, distribuição, biologia, criação e manenjo de uma cepa em laboratório. Mem. Inst. Oswaldo Cruz 1984, 79, 37–44. [Google Scholar] [CrossRef][Green Version]
- Mello, D.A. Studies on reproduction and logevity of Calomys callosus (Rosentia, Cricetidae) under laboratory conditions. Rev. Bras. Biol. 1981, 41, 841–843. [Google Scholar]
- Justines, G.; Johnson, K.M. Observations on the laboratory breeding of the cricetine rodent Calomys callosus. Lab. Anim. Care 1970, 20, 57–60. [Google Scholar]
- Vaz-de-Lima, L.R.; Kipnis, A.; Kipnis, T.L.; Dias-da-Silva, W. The complement system of Calomys callosus, Rengger, 1830 (Rodentia, Cricetidae). Braz. J. Med. Biol. Res. 1992, 25, 161–166. [Google Scholar]
- Ferro, E.A.V.; Silva, D.A.O.; Bevilacqua, E.; Mineo, J.R. Effect of Toxoplasma gondii infection kinetics on trophoblast cell population in Calomys callosus, a model of congenital toxoplasmosis. Infect. Immun. 2002, 70, 7089–7094. [Google Scholar] [CrossRef]
- Andrade, S.G.; Kloetzel, J.K.; Borges, M.M.; Ferrans, V.J. Morphological aspects of the myocarditis and myositis in Calomys callosus experimentally infected with Trypanosoma cruzi: Fibrogenesis and spontaneous regression of fibrosis. Mem. Inst. Oswaldo Cruz 1994, 89, 379–393. [Google Scholar] [CrossRef]
- Borges, M.M.; De Andrade, S.G.; Pilatti, C.G.; do Prado Júnior, J.C.; Kloetzel, J.K. Macrophage activation and histopathological findings in Calomys callosus and Swiss mice infected with several strains of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 1992, 87, 493–502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliveira, L.; Borges, M.M.; Leal, R.C.; Assreuy, J.; Kloetzel, J.K. Nitric oxide involvement in experimental Trypanosoma cruzi infection in Calomys callosus and Swiss mice. Parasitol. Res. 1997, 83, 762–770. [Google Scholar] [PubMed]
- Prado, J.C.J.; Levy, A.M.; Leal, M.P.; Bernard, E.; Kloetzel, J.K. Influence of male gonadal hormones on the parasitemia and humoral response of male Calomys callosus infected with the Y strain of Trypanosoma cruzi. Parasitol. Res. 1999, 85, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.A. Infecção Experimental de Calomys Callosus (Rengger, 1830), (Cricetidae-Rodentia) a quatro espécies de parasitos. Rev. Bras. Med. Trop. 1978, 13, 5–9. [Google Scholar] [CrossRef]
- Mello, D.A.; Teixeira, M.L. Infecção Experimental de Calomys Callosus (Rodentia-Cricetidae) com Leishmania Donovani Chagasi (Laison, 1982). Rev. Saúde Pública 1984, 18, 4–8. [Google Scholar] [CrossRef][Green Version]
- Coelho, P.M.; Dias, M.; Mayrink, W.; Magalhães, P.; Mello, M.N.; Costa, C.A. Wild reservoirs of Schistosoma mansoni from Caratinga, an endemic schistosomiasis area of Minas Gerais State, Brazil. Am. J. Trop. Med. Hyg. 1979, 28, 163–164. [Google Scholar] [CrossRef]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 145–158. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. EcoHealth 2019, 16, 378–390. [Google Scholar] [CrossRef]
- Harker, K.S.; Ueno, N.; Lodoen, M.B. Toxoplasma gondii Dissemination: A Parasite’s Journey through the Infected Host. Parasite Immunol. 2015, 37, 141–149. [Google Scholar] [CrossRef]
- Favoreto, S.; Ferro, E.A.V.; Clemente, D.; Silva, D.A.O.; Mineo, J.R. Experimental Infection of Calomys callosus (Rodentia, Cricetidae) by Toxoplasma gondii. Mem. Inst. Oswaldo Cruz 1998, 93, 103–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferreira, G.L.S.; Mineo, J.R.; Oliveira, J.G.; Ferro, E.A.V.; Souza, M.A.; Santos, A.A.D. Toxoplasma gondii and mast cell interactions in vivo and in vitro: Experimental infection approaches in Calomys callosus (Rodentia, Cricetidae). Microbes Infect. 2004, 6, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.S.; Ribeiro, M.; Lopes-Maria, J.B.; Costa, L.F.; Silva, D.A.O.; De Freitas Barbosa, B.; de Oliveira Gomes, A.; Mineo, J.R.; Ferro, E.A.V. Experimental infection of Calomys callosus with atypical strains of Toxoplasma gondii shows gender differences in severity of infection. Parasitol. Res. 2014, 113, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Nam, H.W. Clinical features and treatment of ocular toxoplasmosis. Korean J. Parasitol. 2013, 51, 393–399. [Google Scholar] [CrossRef]
- Montoya, J.G.; Remington, J.S. Toxoplasmic chorioretinitis in the setting of acute acquired toxoplasmosis. Clin. Infect. Dis. 1996, 23, 277–282. [Google Scholar] [CrossRef]
- Butler, N.J.; Furtado, J.M.; Winthrop, K.L.; Smith, J.R. Ocular toxoplasmosis II: Clinical features, pathology and management. Clin. Exp. Ophthalmol. 2013, 41, 95–108. [Google Scholar] [CrossRef]
- Burnett, A.J.; Shortt, S.G.; Isaac-Renton, J.; King, A.; Werker, D.; Bowie, W.R. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology 1998, 105, 1032–1037. [Google Scholar] [CrossRef]
- Balasundaram, M.B.; Andavar, R.; Palaniswamy, M.; Venkatapathy, N. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch. Ophthalmol. 2010, 128, 206–207. [Google Scholar] [CrossRef]
- Smith, J.R.; Ashander, L.M.; Arruda, S.L.; Cordeiro, C.A.; Lie, S.; Rochet, E.; Belfort, R., Jr.; Furtado, J.M. Pathogenesis of ocular toxoplasmosis. Prog. Retin. Eye Res. 2020, 81, 100882. [Google Scholar] [CrossRef]
- Pereira, M.D.F.; Silva, D.A.O.; Ferro, E.A.V.; Mineo, J.R. Acquired and Congenital Ocular Toxoplasmosis Experimentally Induced in Calomys callosus (Rodentia, Cricetidae). Mem. Inst. Oswaldo Cruz 1999, 94, 103–114. [Google Scholar] [CrossRef][Green Version]
- Gil, C.D.; Mineo, J.R.; Smith, R.L.; Oliani, S.M. Mast cells in the eyes of Calomys callosus (Rodentia: Cricetidae) infected by Toxoplasma gondii. Parasitol. Res. 2002, 88, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Joulia, R.; Gaudenzio, N.; Rodrigues, M.; Lopez, J.; Blanchard, N.; Valitutti, S.; Espinosa, E. Mast cells form antibody-dependent degranulatory synapse for dedicated secretion and defence. Nat. Commun. 2015, 6, 6174. [Google Scholar] [CrossRef] [PubMed]
- Cowen, D.; Wolf, A. Experimental Congenital Toxoplasmosis. Toxoplasmosis in the offspring of mice infected by the vaginal route. Incid. Manif. Dis. 1950, 92, 417–429. [Google Scholar]
- Hutchison, W.M.; Hay, J.; Siim, J.C. A study of cataract in murine congenital toxoplasmosis. Ann. Trop. Med. Parasitol. 1982, 76, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.M. Congenital toxoplasmosis: A review. Neonatal Netw. 2015, 34, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Ferro, E.A.V.; Bevilacqua, E.; Favoreto-Junior, S.; Silva, D.A.O.; Mortara, R.A.; Mineo, J.R. Calomys callosus (Rodentia: Cricetidae) trophoblast cells as host cells to Toxoplasma gondii in early pregnancy. Parasitol. Res. 1999, 85, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, B.F.; Silva, D.A.O.; Costa, I.N.; Pena, J.D.O.; Mineo, J.R.; Ferro, E.A.V. Susceptibility to Vertical Transmission of Toxoplasma gondii is Temporally Dependent on the Preconceptional Infection in Calomys callosus. Placenta 2007, 28, 624–630. [Google Scholar] [CrossRef]
- Abou-Bacar, A.; Pfaff, A.W.; Letscher-Bru, V.; Filisetti, D.; Rajapakse, R.; Antoni, E.; Villard, O.; Klein, J.-P.; Candolfi, E. Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol. 2004, 26, 315–318. [Google Scholar] [CrossRef]
- Pfaff, A.W.; Abou-Bacar, A.; Letscher-Bru, V.; Villard, O.; Senegas, A.; Mousli, M.; Candolfi, E. Cellular and molecular physiopathology of congenital toxoplasmosis: The dual role of IFN-γ. Parasitology 2007, 134, 1895–1902. [Google Scholar] [CrossRef]
- Dao, A.; Fortier, B.; Soete, M.; Plenat, F.; Dubremetz, J.F. Successful reinfection of chronically infected mice by a different Toxoplasma gondii genotype. Int. J. Parasitol. 2001, 31, 63–65. [Google Scholar] [CrossRef]
- Freyre, A.; Falcón, J.; Méndez, J.; Rodriguez, A.; Correa, L.; Gonzalez, M. Toxoplasma gondii: Partial cross-protection among several strains of the parasite against congenital transmission in a rat model. Exp. Parasitol. 2006, 112, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Elbez-Rubinstein, A.; Ajzenberg, D.; Dardé, M.L.; Cohen, R.; Dumètre, A.; Yera, H.; Gondon, E.; Janaud, J.-C.; Thulliez, P. Congenital toxoplasmosis and reinfection during pregnancy: Case report, strain characterization, experimental model of reinfection, and review. J. Infect. Dis. 2009, 199, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Pezerico, S.B.; Langoni, H.; Da Silva, A.V.; Da Silva, R.C. Evaluation of Toxoplasma gondii placental transmission in BALB/c mice model. Exp. Parasitol. 2009, 123, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.S.; da Silva, N.M.; de Barbosa, B.F.; de Gomes, A.O.; Ietta, F.; Shwab, E.K.; Su, C.; Mineo, J.R.; Ferro, E.A.V. Calomys callosus chronically infected by Toxoplasma gondii clonal type II strain and reinfected by Brazilian strains is not able to prevent vertical transmission. Front. Microbiol. 2015, 6, 181. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S. Toxoplasmosis complications and novel therapeutic synergism combination of diclazuril plus atovaquone. Front. Microbiol. 2014, 5, 484. [Google Scholar] [CrossRef] [PubMed]
- Black, M.W.; Boothroyd, J.C. Lytic Cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 2000, 64, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, J.C.; Dubremetz, J.F. Kiss and spit: The dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 2008, 6, 79–88. [Google Scholar] [CrossRef]
- Bigna, J.J.; Tochie, J.N.; Tounouga, D.N.; Bekolo, A.O.; Ymele, N.S.; Simé, P.S.; Nansseu, J.R. Global, regional and national estimates of Toxoplasma gondii seroprevalence in pregnant women: A protocol for a systematic review and modelling analysis. BMJ Open 2019, 9, e030472. [Google Scholar] [CrossRef]
- Benoit-Vical, F.; Santillana-Hayat, M.; Kone-Bamba, D.; Mallie, M.; Derouin, F. Anti-Toxoplasma activity of vegetal extracts used in west african traditional medicine. Parasite 2000, 7, 3–7. [Google Scholar] [CrossRef]
- Youn, H.J.; Lakritz, J.; Kim, D.Y.; Rottinghaus, G.E.; Marsh, A.E. Anti-protozoal efficacy of medicinal herb extracts against Toxoplasma gondii and Neospora caninum. Vet. Parasitol. 2003, 116, 7–14. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.C.; de Souza, G.; Borges, B.C.; Araujo, T.E.; de Rosini, A.M.; Aguila, F.A.; Ambrósio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Silva, M.J.B.; et al. Copaifera spp. oleoresins impair Toxoplasma gondii infection in both human trophoblastic cells and human placental explants. Sci. Rep. 2020, 10, 15158. [Google Scholar] [PubMed]
- Costa, I.N.; Angeloni, M.B.; Santana, L.A.; Barbosa, B.F.; Silva, M.C.P.; Rodrigues, A.A.; Rostkowsa, C.; Magalhães, P.M.; Pena, J.D.O.; Silva, D.A.O.; et al. Azithromycin Inhibits Vertical Transmission of Toxoplasma gondii in Calomys callosus (Rodentia: Cricetidae). Placenta 2009, 30, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.D.; Silva, N.M.; Ferro, E.A.V.; Sousa, R.A.; Firmino, M.L.; Bernardes, E.S.; Roque-Barreira, M.C.; Pena, J.D.O. Azithromycin reduces ocular infection during congenital transmission of toxoplasmosis in the Calomys callosus model. J. Parasitol. 2009, 95, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, B.F.; Gomes, A.O.; Ferro, E.A.V.; Napolitano, D.R.; Mineo, J.R.; Silva, N.M. Enrofloxacin is able to control Toxoplasma gondii infection in both in vitro and in vivo experimental models. Vet. Parasitol. 2012, 187, 44–52. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Control of the Leishmaniases; WHO Technical Report Series 949; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Casey, D.C.; Charlson, F.J.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global leishmaniasis surveillance, 2017–2018, and first report on 5 additional indicators. Wkly. Epidemiol. Rec. 2020, 95, 265–280. [Google Scholar]
- Mcgwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. Q. J. Med. 2014, 107, 7–14. [Google Scholar] [CrossRef]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian Hamster as an Animal Model for the Study on Infectious Diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Saini, S.; Rai, A.K. Hamster, a close model for visceral leishmaniasis: Opportunities and challenges. Parasite Immunol. 2020, 42, e12768. [Google Scholar] [CrossRef]
- Prianti, M.G.; Yokoo, M.; Saldanha, L.C.B.; Costa, F.A.L.; Goto, H. Leishmania (Leishmania) chagasi-infected mice as a model for the study of glomerular lesions in visceral leishmaniasis. Braz. J. Med. Biol. Res. 2007, 40, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Loeuillet, C.; Bañuls, A.L.; Hide, M. Study of Leishmania pathogenesis in mice: Experimental considerations. Parasites Vectors 2016, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C. Nova tripanozomiaze humana. Estudos sobre a morfologia e o ciclo evolutivo do Shizotrypanum cruzi n. gen., n. sp., agente etiologico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Chagas Disease (American Trypanosomiasis). 2021. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 4 November 2021).
- Lana, M.; Tafuri, W.L. Trypanosoma cruzi e Doença de Chagas. In Parasitology Humana; Neves, D.P., de Melo, A.L., Linardi, P.M., Vitor, R.w.A.V., Eds.; Atheneu: São Paulo, Brazil, 2012. [Google Scholar]
- Ministério da Saúde. Informações Técnicas sobre Doença de Chagas; Portal do Ministério da Saúde: Online, 2019.
- Kawaguchi, W.H.; Leonart, L.P.; Fachi, M.M.; Böger, B.; Pontarolo, R. Doença de Chagas: Do surgimento ao tratamento. J. Health Sci. Inst. Rev. Inst. Ciências Saúde 2019, 37, 182–189. [Google Scholar]
- Nguyen, T.; Waseem, M. Chagas Disease (American Trypanosomiasis). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Vieira, J.L.; Távora, F.; Sobral, M.; Vasconcelos, G.G.; Almeida, G.; Fernandes, J.R.; Da Escóssia Marinho, L.L.; De Mendonça Trompieri, D.F.; De Souza Neto, J.D.; Mejia, J. Chagas Cardiomyopathy in Latin America Review. Curr. Cardiol. Rep. 2019, 21, 8. [Google Scholar] [CrossRef]
- Santos, C.D.; Toldo, M.P.A.; Levy, A.M.A.; Prado, J.C. Trypanosoma cruzi: Effects of social stress in Calomys callosus a natural reservoir of infection. Exp. Parasitol. 2008, 119, 197–201. [Google Scholar] [CrossRef]
- Mello, D.A.; Valin, E.; Teixeira, M.L. Alguns aspectos do comportamento de cepas silvestres de Trypanosoma cruzi em camundongos e Calomys callosus (Rodentia). Rev. Saude Publica 1979, 13, 314–325. [Google Scholar] [CrossRef][Green Version]
- Borges, M.A.; Mello, D. Infectividade de cepas silvestres de Trypanosoma cruzi, mantidas em cultura, para Calomys callosus (Rodentia) e camundongos albinos. J. Trop. Pathol. 1980, 9, 2–5. [Google Scholar]
- Galvão, C. Vetores da Doença de Chagas No Brasil; Sociedade Brasileira de Zoologia: Curitiba, Brazil, 2014; ISBN 9788598203096. [Google Scholar]
- Basso, B.; Eraso, A.J.; Moretti, E.R.; Albesa, I.; Kravetz, F.O. Natural infection of Calomys musculinus (Rodentia, Cricetidae) by Trypanosoma cruzi. Rev. Argent. Microbiol. 1977, 9, 11–16. [Google Scholar]
- Mello, D.A.; Teixeira, M.L. Nota sobre a infecção natural de Calomys expulsus, Lund, 1841 (Cricetidae-Rodentia) pelo Trypanosoma cruzi. Rev. Saude Publica 1977, 11, 561–564. [Google Scholar] [CrossRef][Green Version]
- Mello, D.A. Trypanosoma (Herpetosoma) mariae n. sp., isolated from Calomys callosus Rengger, 1830 (Rodentia-Cricetinae). Ann. Parasitol. Hum. Comparée 1978, 53, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Dobigny, G.; Gauthier, P.; Houéménou, G.; Dossou, H.J.; Badou, S.; Etougbétché, J.; Tartard, C.; Truc, P. Spatio-temporal survey of small mammal-borne Trypanosoma lewisi in Cotonou, Benin, and the potential risk of human infection. Infect. Genet. Evol. 2019, 75, 103967. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.M.; Marcili, A.; Ortiz, P.A.; Epifânio, S.; Campaner, M.; Shaw, J.J.; de Camargo, E.P.; Maria, M.; Teixeira, G. Análises filogenéticas, morfológicas e comportamentais apoiam a troca de hospedeiros de Trypanosoma (Herpetosoma) lewisi de ratos domésticos para primatas. Infect. Genet. Evol. 2010, 10, 522–529. [Google Scholar]
- Lun, Z.R.; Wen, Y.Z.; Uzureau, P.; Lecordier, L.; Lai, D.H.; Lan, Y.G.; Desquesnes, M.; Geng, G.-Q.; Yang, T.-B.; Zhou, W.-L.; et al. Resistance to normal human serum reveals Trypanosoma lewisi as an underestimated human pathogen. Mol. Biochem. Parasitol. 2015, 199, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.A.; Borges, M.M. Neonatal transmission of T. cruzi in Calomys callosus (rodentia). Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 754–755. [Google Scholar] [CrossRef]
- Carvalho, O.S.; Coelho, P.M.Z.; Lenzi, H.L. Schistosoma Mansoni e Esquistossomose: Uma visão Multidisciplinar; Editora FIOCRUZ: Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Andrade, Z.A. A esquistossomose no Brasil após quase um século de pesquisas. Rev. Soc. Bras. Med. Trop. 2002, 35, 509–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chabasse, D.; Bertrand, G.; Leroux, J.P.; Gauthey, N.; Hocquet, P. Developmental bilharziasis caused by Schistosoma mansoni discovered 37 years after infestation. Bull. Soc. Pathol. Exot. Fil. 1985, 78, 643–647. [Google Scholar]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef]
- Andrade, Z.A.; Ramos, E.G.; Dos Reis, M.G. Pathology of Schistosomiasis mansoni in rabbits. Mem. Inst. Oswaldo Cruz 1988, 83, 323–333. [Google Scholar] [CrossRef]
- Cheever, A.W.; Lenzi, J.A.; Lenzi, H.L.; Andrade, Z.A. Experimental models of Schistosoma mansoni infection. Mem. Inst. Oswaldo Cruz 2002, 97, 917–940. [Google Scholar] [CrossRef] [PubMed]
- Farah, I.O.; Kariuki, T.M.; King, C.L.; Hau, J. An overview of animal models in experimental schistosomiasis and refinements in the use of non-human primates. Lab. Anim. 2001, 35, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Santana, L.; Jesus, A. A resposta imune na forma aguda da esquistossomose mansoni. In Schistosoma Mansoni & Esquistossomose. Uma Visão Multidisciplinar, 1st ed.; Carvalho, O.S., Coelho, P.M.Z., Lenzi, H.L., Eds.; Fiocruz: Rio de Janeiro, Brazil, 2008; pp. 546–568. ISBN 9788575413708. [Google Scholar]
- Lenzi, J.A.; Mota, E.M.; Pelajo-Machado, M.; Vale, L.S.; Vale, B.S.; Andrade, Z.A.; Lenzi, H.L. Intestinal fibrovascular nodules caused by Schistosoma mansoni infection in Calomys callosus Rengger, 1830 (Rodentia: Cricetidae): A model of concomitant fibrosis and angiogenesis. Mem. Inst. Oswaldo Cruz 2002, 97, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.A.; Mota, E.M.; Pelajo-Machado, M.; Paiva, R.A.; Lenzi, H.L. Calomys callosus: An alternative model to study fibrosis in Schistosomiasis mansoni. The pathology of the acute phase. Mem. Inst. Oswaldo Cruz 1995, 90, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, H.L.; Oliveira, D.N.; Borojevic, R.; Lenzi, J.A. Milky spots reaction to Schistosomal mansoni infection. Mem. Inst. Oswaldo Cruz 1992, 87 (Suppl. S5), 111–116. [Google Scholar] [CrossRef]
- Lenzi, H.L.; Oliveira, D.N.; Pelajo-Machado, M.; Borojevic, R.; Lenzi, J.A. Coelom-associated lymphomyeloid tissue (milky spots): Site of lymphoid and myelomonocytic cell generation. Braz. J. Med. Biol. Res. 1996, 29, 19–24. [Google Scholar]
- Lenzi, J.A.; Pelajo-Machado, M.; Mota, E.M.; Oliveira, D.N.; Panasco, M.S.; Andrade, Z.A.; Lenzi, H.L. Effects of Schistosoma mansoni Infection on Calomys callosus Coelom-associated Lymphomyeloid Tissue (Milky Spots). Mem. Inst. Oswaldo Cruz 1998, 93, 13–23. [Google Scholar] [CrossRef][Green Version]
- Yong, L.C.; Watkins, S.; Wilhelm, D.L. The mast cell: Distribution and maturation in the peritoneal cavity of the adult rat. Pathology 1975, 7, 307–318. [Google Scholar] [CrossRef]
- Yong, L.C.; Watkins, S.G.; Boland, J.E. The mast cell: III. Distribution and maturation in various organs of the young rat. Pathology 1977, 11, 221–232. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; Gray, H.E.; Smith, L.M.; Braithwaite, V.; Cotter, S.C.; Elwood, R.W.; Wilkinson, A.; Collins, L.M. The welfare and ethics of research involving wild animals: A primer. Methods Ecol. Evol. 2020, 11, 1164–1181. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente. Instrução Normativa nº 03, de 01 de setembro de 2014. Instituto Chico Mendes De Conservação Da Biodiversidade. (ONLINE). 2014. Available online: https://www.icmbio.gov.br/flonatapajos/images/stories/INSTRU%C3%87%C3%83O_NORMATIVA_ICMBio_N%C2%BA_3_DE_2014__com_retifica%C3%A7%C3%A3o_do_DOU18062015.pdf (accessed on 25 February 2022).
- Chen, P.H.; Trundy, R. Biosegurity in laboratory animal. Research facilities. In Biosecurity in Animal Pruction and Veterinary Medicine; CABI Publishing: São Paulo, Brazil, 2019. [Google Scholar]
- Guillen, J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar] [PubMed]
- Hutchinson, E.; Avery, A.; VandeWoude, S. Environmental enrichment for laboratory rodents. ILAR J. 2005, 46, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.L.; West, R.S. Taming anxiety in laboratory mice. Nat. Methods 2010, 7, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, K.; Hurst, J.L. Reducing Mouse Anxiety during Handling: Effect of Experience with Handling Tunnels. PLoS ONE 2013, 8, e66401. [Google Scholar] [CrossRef] [PubMed]
- Poiley, S.M. A systematic method of breeder rotation for non-inbred laboratory animal colonies. Proc. Anim. Care Panel 1960, 10, 159–166. [Google Scholar]
- Falconer, D.S.; MCKAY, T.F.C. Introduction to quantitative genetics. In Quantitative Genetics; Pearson Prentice Hall: Harlow, UK, 1996; Volume 4, p. 480. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, R.B.; Costa, M.S.d.; Teixeira, S.C.; Castro, E.F.d.; Dantas, W.M.; Ferro, E.A.V.; Silva, M.V.d. Calomys callosus: An Experimental Animal Model Applied to Parasitic Diseases Investigations of Public Health Concern. Pathogens 2022, 11, 369. https://doi.org/10.3390/pathogens11030369
Rosa RB, Costa MSd, Teixeira SC, Castro EFd, Dantas WM, Ferro EAV, Silva MVd. Calomys callosus: An Experimental Animal Model Applied to Parasitic Diseases Investigations of Public Health Concern. Pathogens. 2022; 11(3):369. https://doi.org/10.3390/pathogens11030369
Chicago/Turabian StyleRosa, Rafael Borges, Mylla Spirandelli da Costa, Samuel Cota Teixeira, Emilene Ferreira de Castro, Willyenne Marília Dantas, Eloisa Amália Vieira Ferro, and Murilo Vieira da Silva. 2022. "Calomys callosus: An Experimental Animal Model Applied to Parasitic Diseases Investigations of Public Health Concern" Pathogens 11, no. 3: 369. https://doi.org/10.3390/pathogens11030369
APA StyleRosa, R. B., Costa, M. S. d., Teixeira, S. C., Castro, E. F. d., Dantas, W. M., Ferro, E. A. V., & Silva, M. V. d. (2022). Calomys callosus: An Experimental Animal Model Applied to Parasitic Diseases Investigations of Public Health Concern. Pathogens, 11(3), 369. https://doi.org/10.3390/pathogens11030369