Human Polymorphonuclear Cells Support Zika Virus to Cross Endothelial Monolayer and Access Bloodstream
Abstract
:1. Introduction
2. Results
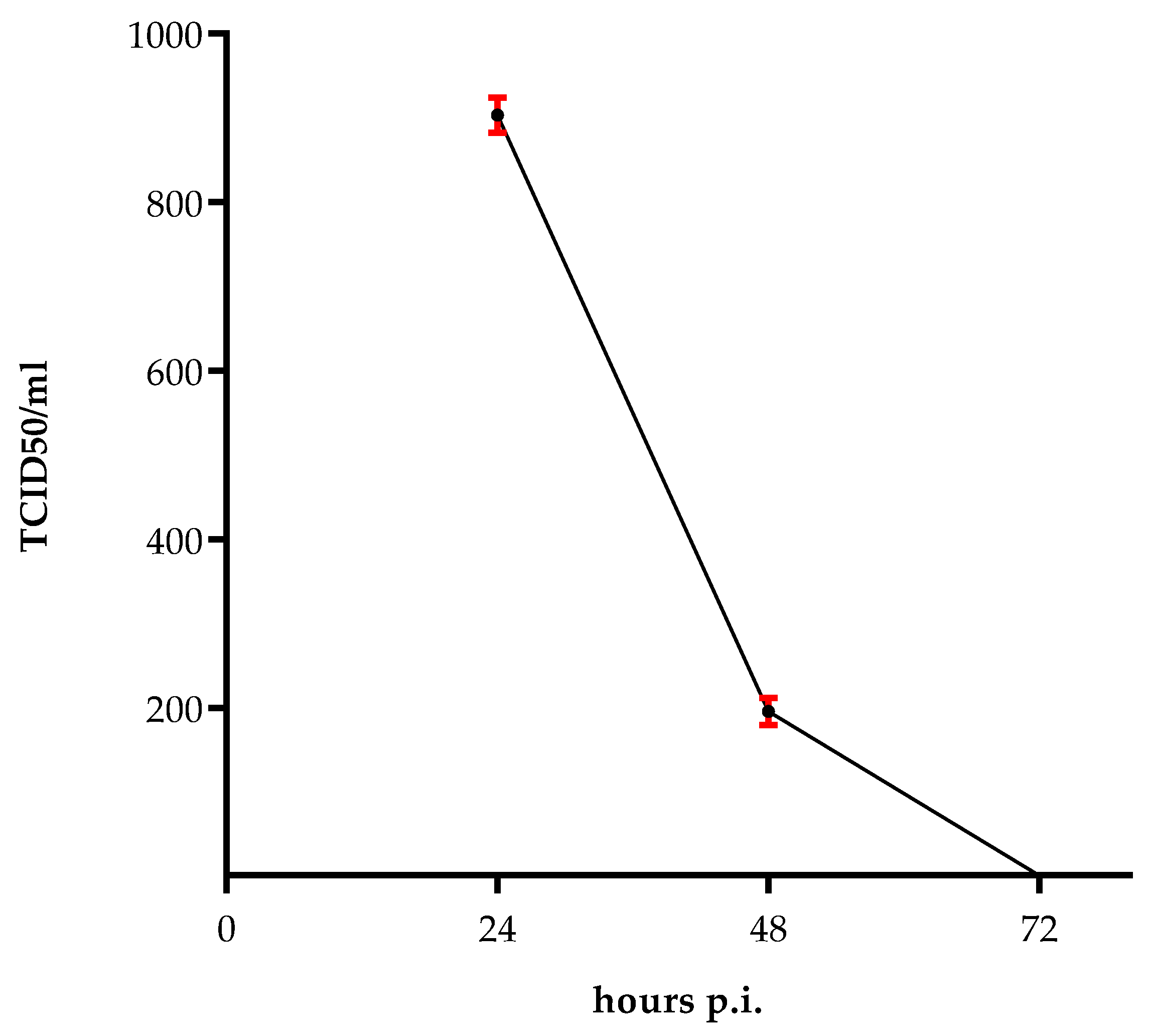
2.1. Survival of the Virus
2.2. Persisting ZIKV Production in HUVECs
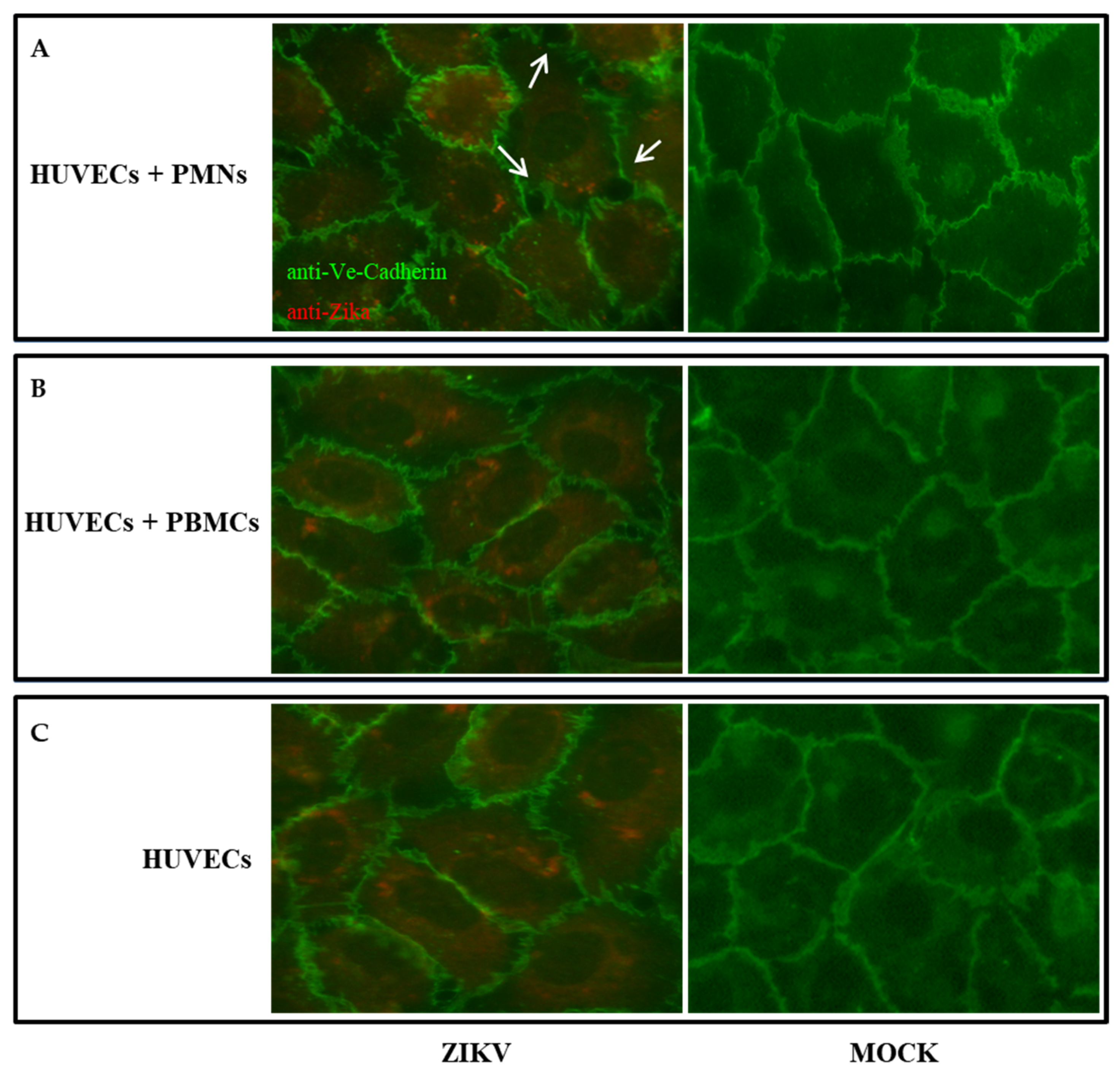
2.3. HUVEC Permeabilization after ZIKV Infection
2.4. The Presence of PMNs Influences the Permeability of the Endothelial Monolayer
2.5. Cytokine Profile
3. Discussion
4. Materials and Methods
4.1. Cells and Virus
4.2. ZIKV Replication in HUVECs along Time
4.3. Virus Titration
4.4. FITC Dextran Permeability Assay
4.5. Cell Viability Assay
4.6. Analysis of Endothelial Cell Adhesion Protein by Immunofluorescence
4.7. Cytokine Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayres, C.F.J.; Guedes, D.R.D.; Paiva, M.H.S.; Morais-Sobral, M.C.; Krokovsky, L.; Machado, L.C.; Melo-Santos, M.A.V.; Crespo, M.; Oliveira, C.M.F.; Ribeiro, R.S.; et al. Zika virus detection, isolation and genome sequencing through Culicidae sampling during the epidemic in Vitória, Espírito Santo, Brazil. Parasites Vectors 2019, 12, 220. [Google Scholar] [CrossRef] [Green Version]
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guirakhoo, F.; Bolin, R.A.; Roehrig, J. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology 1992, 191, 921–931. [Google Scholar] [CrossRef]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of Dengue Virus: Implications for flavivirus organization, maturation, and fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Cunha, M.S.; Esposito, D.; Rocco, I.M.; Maeda, A.Y.; Vasami, F.G.S.; Nogueira, J.S.; de Souza, R.P.; Suzuki, A.; Addas-Carvalho, M.; Barjas-Castro, M.D.L.; et al. First Complete Genome Sequence of Zika Virus (Flaviviridae, Flavivirus) from an Autochthonous Transmission in Brazil. Genome Announc. 2016, 4, e00032-16. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Wahid, B.; Rafique, S.; Idrees, M. Advances in research on Zika virus. Asian Pac. J. Trop. Med. 2017, 10, 321–331. [Google Scholar] [CrossRef]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic Characterization of Zika Virus Strains: Geographic Expansion of the Asian Lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [Green Version]
- Wikan, N.; Smith, D.R. Zika virus: History of a newly emerging arbovirus. Lancet Infect. Dis. 2016, 16, e119–e126. [Google Scholar] [CrossRef] [Green Version]
- Waggoner, J.J.; Pinsky, B.A. Zika Virus: Diagnostics for an Emerging Pandemic Threat. J. Clin. Microbiol. 2016, 54, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [Green Version]
- Surasombatpattana, P.; Hamel, R.; Patramool, S.; Luplertlop, N.; Thomas, F.; Desprès, P.; Briant, L.; Yssel, H.; Missé, D. Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses. Infect. Genet. Evol. 2011, 11, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.-Y.; Burleigh, L.; Staropoli, I.; Navarro-Sanchez, E.; Harriague, J.; Virelizier, J.-L.; Rey, F.; Desprès, P.; Arenzana-Seisdedos, F.; Amara, A. Dendritic Cell-specific Intercellular Adhesion Molecule 3-grabbing Non-integrin (DC-SIGN)-mediated Enhancement of Dengue Virus Infection Is Independent of DC-SIGN Internalization Signals. J. Biol. Chem. 2005, 280, 23698–23708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, M.I.; Pérez-García, M.; Terreros-Tinoco, M.; Castro-Mussot, M.E.; Diegopérez-Ramírez, J.; Ramírez-Reyes, A.G.; Aguilera, P.; Cedillo-Barrón, L.; García-Flores, M.M. Dengue Virus Type 2: Protein Binding and Active Replication in Human Central Nervous System Cells. Sci. World J. 2013, 2013, 904067. [Google Scholar] [CrossRef] [PubMed]
- Mladinich, M.C.; Schwedes, J.; Mackow, E.R. Zika Virus Persistently Infects and Is Basolaterally Released from Primary Human Brain Microvascular Endothelial Cells. mBio 2017, 8, e00952-17. [Google Scholar] [CrossRef] [Green Version]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.-Y.; Zuo, G.-L.; Li, X.-F.; Ye, Q.; Deng, Y.-Q.; Huang, X.-Y.; Cao, W.-C.; Qin, C.-F.; Luo, Z.-G. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016, 26, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Sips, G.J.; Wilschut, J.; Smit, J.M. Neuroinvasive flavivirus infections. Rev. Med. Virol. 2012, 22, 69–87. [Google Scholar] [CrossRef]
- Greenwood, J.; Heasman, S.J.; Alvarez, J.I.; Prat, A.; Lyck, R.; Engelhardt, B. Review: Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 2011, 37, 24–39. [Google Scholar] [CrossRef]
- Liu, S.; DeLalio, L.; Isakson, B.E.; Wang, T.T. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ. Res. 2016, 119, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Richard, A.S.; Shim, B.-S.; Kwon, Y.-C.; Zhang, R.; Otsuka, Y.; Schmitt, K.; Berri, F.; Diamond, M.S.; Choe, H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2024–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ortenzio, E.; Matheron, S.; De Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Yazdanpanah, Y.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, D.; Andrade, P.; Gonzalez, K.; Balmaseda, A.; Harris, E. CD14+CD16+ monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat. Microbiol. 2017, 2, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Papa, M.P.; Meuren, L.M.; Coelho, S.V.A.; Lucas, C.G.D.O.; Mustafá, Y.M.; Matassoli, F.L.; Silveira, P.P.; Frost, P.S.; Pezzuto, P.; Ribeiro, M.R.; et al. Zika Virus Infects, Activates, and Crosses Brain Microvascular Endothelial Cells, without Barrier Disruption. Front. Microbiol. 2017, 8, 2557. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.-C.; Li, X.-F.; Deng, Y.-Q.; Tong, Y.-G.; Qin, C.-F. Excretion of infectious Zika virus in urine. Lancet Infect. Dis. 2016, 16, 641–642. [Google Scholar] [CrossRef] [Green Version]
- Bonaldo, M.C.; Ribeiro, I.P.; Lima, N.S.; dos Santos, A.A.C.; Menezes, L.S.R.; da Cruz, S.O.D.; de Mello, I.S.; Furtado, N.D.; de Moura, E.E.; Damasceno, L.; et al. Isolation of Infective Zika Virus from Urine and Saliva of Patients in Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004816. [Google Scholar] [CrossRef] [Green Version]
- Dupont-Rouzeyrol, M.; Biron, A.; O’Connor, O.; Huguon, E.; Descloux, E. Infectious Zika viral particles in breastmilk. Lancet 2016, 387, 1051, Erratum in Lancet 2016, 387, 1056. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, K.; Drebot, M.; MacDonald, J.; Lindsay, R.; Pabbaraju, K.; Tellier, R.; Wong, S.; Meatherall, B.; Webster, P.; Zarra, D. First Case of Zika Virus Infection in a Returning Canadian Traveler. Am. J. Trop. Med. Hyg. 2014, 91, 1035–1038. [Google Scholar] [CrossRef]
- De Souza, A.S.; Dias, C.M.; Braga, F.D.C.B.; Terzian, A.C.B.; Estofolete, C.F.; Oliani, A.H.; Oliveira, G.H.; de Mattos, C.C.B.; de Mattos, L.C.; Nogueira, M.L.; et al. Fetal Infection by Zika Virus in the Third Trimester: Report of 2 Cases. Clin. Infect. Dis. 2016, 63, 1622–1625. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, S.; Schlaberg, R.; Lewis, J.; Hanson, K.E.; Couturier, M.R. Fatal Zika Virus Infection with Secondary Nonsexual Transmission. N. Engl. J. Med. 2016, 375, 1907–1909. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Lim, E.X.Y.; Zhang, S.; Fibriansah, G.; Ng, T.-S.; Ooi, J.; Shi, J.; Lok, S.-M. Structure of the thermally stable Zika virus. Nature 2016, 533, 425–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Adams, L.E.; et al. Persistence of Zika Virus in Body Fluids—Final Report. N. Engl. J. Med. 2018, 379, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Mustafá, Y.M.; Meuren, L.M.; Coelho, S.; De Arruda, L.B. Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System. Front. Microbiol. 2019, 10, 525. [Google Scholar] [CrossRef]
- Alimonti, J.B.; Ribecco-Lutkiewicz, M.; Sodja, C.; Jezierski, A.; Stanimirovic, D.B.; Liu, Q.; Haqqani, A.S.; Conlan, W.; Bani-Yaghoub, M. Zika virus crosses an in vitro human blood brain barrier model. Fluids Barriers CNS 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Ubogu, E.E.; Williams, K.A.; Tucky, B.; Callahan, M.K.; Ransohoff, R.M. Human Brain Microvascular Endothelial Cells and Umbilical Vein Endothelial Cells Differentially Facilitate Leukocyte Recruitment and Utilize Chemokines for T Cell Migration. Clin. Dev. Immunol. 2008, 2008, 384982. [Google Scholar] [CrossRef] [Green Version]
- Stins, M.F.; Gilles, F.; Kim, K.S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 1997, 76, 81–90. [Google Scholar] [CrossRef]
- Blazquez, A.; Escribano-Romero, E.; Merino-Ramos, T.; Saiz, J.-C.; Martã n-Acebes, M.A. Stress responses in flavivirus-infected cells: Activation of unfolded protein response and autophagy. Front. Microbiol. 2014, 5, 266. [Google Scholar] [CrossRef]
- Peng, H.; Liu, B.; Yves, T.D.; He, Y.; Wang, S.; Tang, H.; Ren, H.; Zhao, P.; Qi, Z.; Qin, Z. Zika Virus Induces Autophagy in Human Umbilical Vein Endothelial Cells. Viruses 2018, 10, 259. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kline, B.A.; Kenny, T.A.; Smith, D.R.; Soloveva, V.; Beitzel, B.; Pang, S.; Lockett, S.; Hess, H.F.; Palacios, G.; et al. A novel sheet-like virus particle array is a hallmark of Zika virus infection. Emerg. Microbes Infect. 2018, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Sager, G.; Gabaglio, S.; Sztul, E.; Belov, G.A. Role of Host Cell Secretory Machinery in Zika Virus Life Cycle. Viruses 2018, 10, 559. [Google Scholar] [CrossRef] [Green Version]
- Cosgriff, T.M. Viruses and Hemostasis. Rev. Infect. Dis. 1989, 11 (Suppl. 4), S672–S688. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of Human Endothelial Cells Derived from Umbilical Veins. Identification by Morphologic and Immunologic Criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Authorisation, no. 9/2014—General Authorisation to Process Personal Data for Scientific Research Purposes. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPbblicazioneGazzetta=20141230&atto.codiceRedazionale=14A09916&elenco30giorni=true (accessed on 30 December 2014).
- Law Decree 22 December 2017, No. 219, Published in the Official Gazette No. 12 of 16 January 2018. Available online: https://www.gazzettaufficiale.it/eli/gu/2018/01/16/12/sg/pdf (accessed on 16 January 2016).
- Sroka, J.; Kordecka, A.; Włosiak, P.; Madeja, Z.; Korohoda, W. Separation methods for isolation of human polymorphonuclear leukocytes affect their motile activity. Eur. J. Cell Biol. 2009, 88, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]

| Permeability Assay | |
|---|---|
| Samples | upper/lower |
| HUVECs | 7.147 ± 0.53 |
| HUVECs + ZIKV | 5.75 ± 0.46 |
| PBMCs | 9.35 ± 0.54 |
| PBMCs + ZIKV | 8.036 ± 0.67 |
| PMNs | 8.69 ± 0.54 |
| PMNs + ZIKV | 3.97 ± 0.71 |
| Samples | ANALYTES (pg/mL) | ||||
|---|---|---|---|---|---|
| GM-CSF | IL-1b | IL-6 | INF-γ | TNF-α | |
| HUVECs | - | <0.13 * | 28.08 | - | <1.14 * |
| HUVECs + ZIKV | - | <0.13 * | 24.93 | - | <1.14 * |
| PMNs | - | 0.97 | 47.99 | - | <1.14 * |
| PMNs + ZIKV | 4.51 | 3.26 | 162.92 | - | 3.08 |
| PBMCs | - | 0.57 | 41.9 | - | <1.14 * |
| PBMCs + ZIKV | - | <0.13 * | 34.82 | - | <1.14 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandolfo, C.; Terrosi, C.; Prathyumnan, S.; Anichini, G.; Savellini, G.G.; Morgante, G.; Cusi, M.G. Human Polymorphonuclear Cells Support Zika Virus to Cross Endothelial Monolayer and Access Bloodstream. Pathogens 2022, 11, 321. https://doi.org/10.3390/pathogens11030321
Gandolfo C, Terrosi C, Prathyumnan S, Anichini G, Savellini GG, Morgante G, Cusi MG. Human Polymorphonuclear Cells Support Zika Virus to Cross Endothelial Monolayer and Access Bloodstream. Pathogens. 2022; 11(3):321. https://doi.org/10.3390/pathogens11030321
Chicago/Turabian StyleGandolfo, Claudia, Chiara Terrosi, Shibily Prathyumnan, Gabriele Anichini, Gianni Gori Savellini, Giuseppe Morgante, and Maria Grazia Cusi. 2022. "Human Polymorphonuclear Cells Support Zika Virus to Cross Endothelial Monolayer and Access Bloodstream" Pathogens 11, no. 3: 321. https://doi.org/10.3390/pathogens11030321
APA StyleGandolfo, C., Terrosi, C., Prathyumnan, S., Anichini, G., Savellini, G. G., Morgante, G., & Cusi, M. G. (2022). Human Polymorphonuclear Cells Support Zika Virus to Cross Endothelial Monolayer and Access Bloodstream. Pathogens, 11(3), 321. https://doi.org/10.3390/pathogens11030321








