Peculiarities of Zika Immunity and Vaccine Development: Lessons from Dengue and the Contribution from Controlled Human Infection Model
Abstract
1. Introduction
2. Clinical Presentation
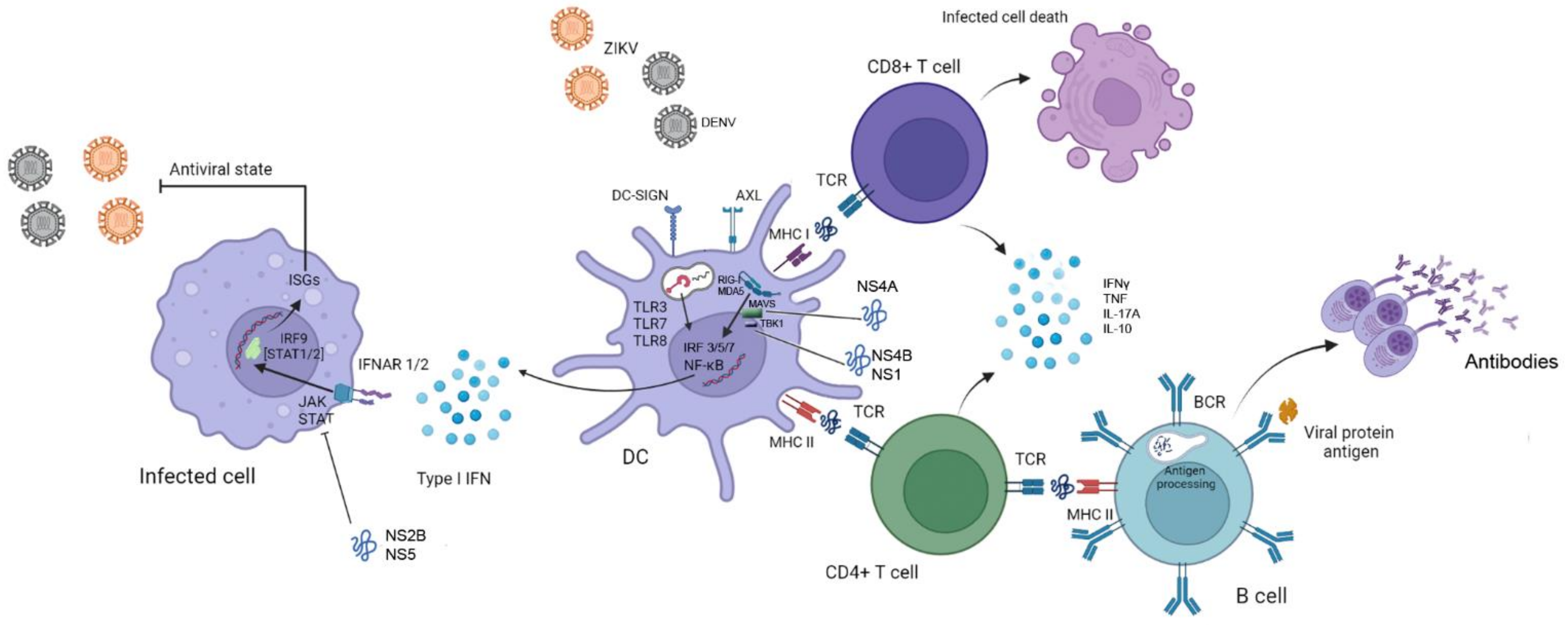
3. Immune Responses
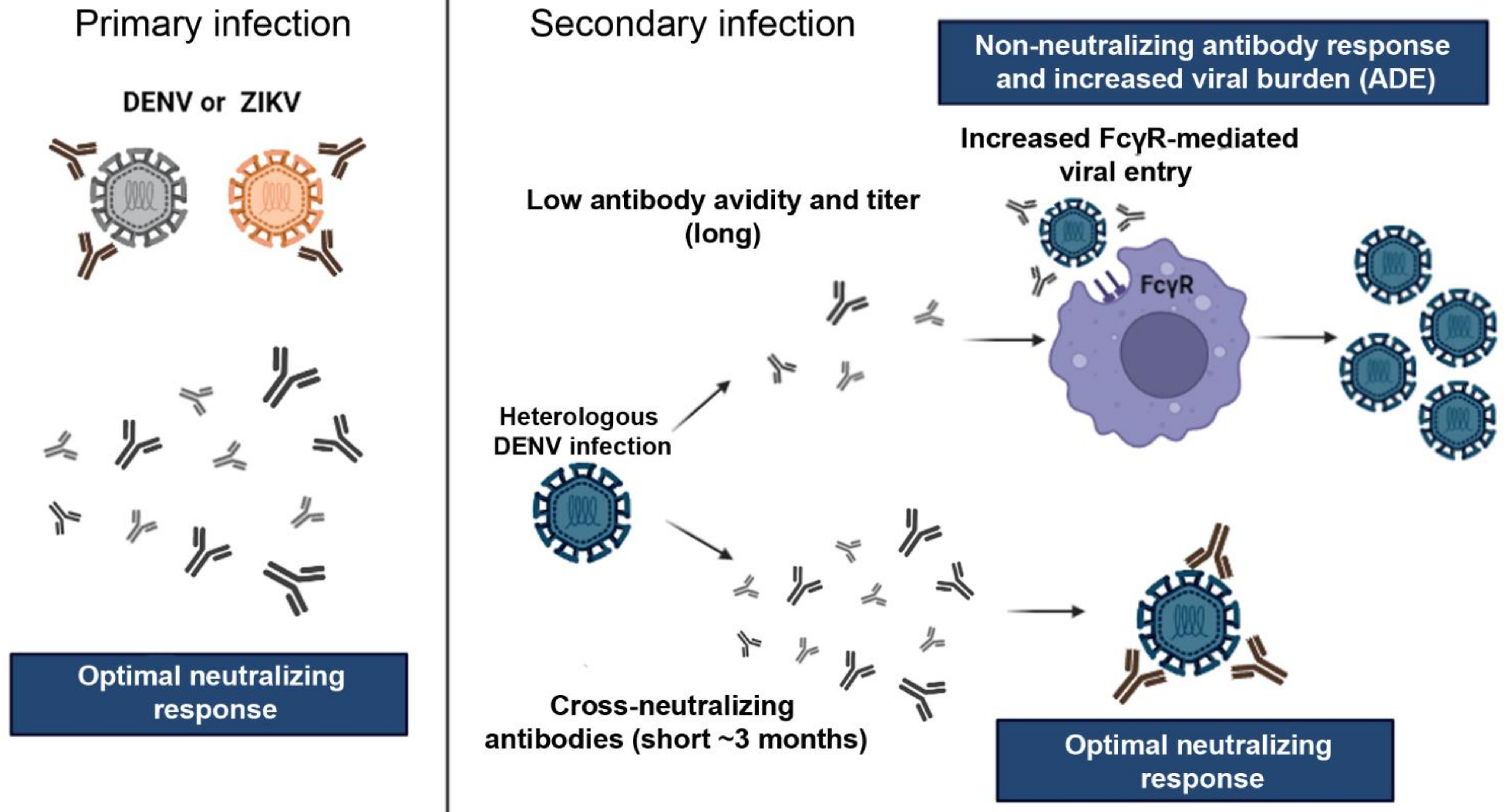
4. Implications of Flaviviruses Immunity on ZIKV Vaccine Development
5. Current Challenges and Solutions: A Role for Controlled Human Infection Model of ZIKV
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Desgraupes, S.; Hubert, M.; Gessain, A.; Ceccaldi, P.E.; Vidy, A. Mother-to-child transmission of Arboviruses during breastfeeding: From epidemiology to cellular mechanisms. Viruses 2021, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Richarte, A.; de Salazar, M.O.; Arbona, C.; Gimenez-Richarte, M.P.; Collado, M.; Fernandez, P.L.; Quiles, F.; Clavijo, C.; Marco, P.; Ramos-Rincon, J.M. Prevalence of chikungunya, dengue and Zika viruses in blood donors: A systematic literature review and meta-analysis. Blood Transfus. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Yuan, X.; Lou, Y.; He, D.; Wang, J.; Gao, D. A Zika endemic model for the contribution of multiple transmission routes. Bull. Math. Biol. 2021, 83, 111. [Google Scholar] [CrossRef]
- Hayes, E.B. Zika virus outside Africa. Emerg. Infect. Dis. 2009, 15, 1347–1350. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef]
- PAHO/WHO, PAHO. Timeline of Emergence of Zika Virus in the Americas. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=11959:timeline-of-emergence-of-zika-virus-in-the-americas&Itemid=41711&lang=en (accessed on 27 November 2021).
- Faria, N.R.; Azevedo, R.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Giovanetti, M.; Faria, N.R.; Nunes, M.R.T.; de Vasconcelos, J.M.; Lourenco, J.; Rodrigues, S.G.; Vianez, J.L., Jr.; da Silva, S.P.; Lemos, P.S.; Tavares, F.N.; et al. Zika virus complete genome from Salvador, Bahia, Brazil. Infect. Genet. Evol. 2016, 41, 142–145. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Epidemiological Alert: Neurological Syndrome, Congenital Malformations, and Zika Virus Infection. Implications for Public Health in the America. 2015. Available online: https://iris.paho.org/handle/10665.2/50697?show=full (accessed on 20 December 2021).
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 47, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.; de Sequeira, P.C.; de Mendonca, M.C.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Cardenas, D.M.; Jaimes, M.A.; Vega, L.D.; Oliveros, N.L.; Soto, J.A.; Chia, C.R.; Osorio, J.E.; Ciuoderis, K.A. Immunological memory to Zika virus in a University Community in Colombia, South America. Acad. Bras. Cienc. 2020, 92, e20190883. [Google Scholar] [CrossRef]
- Netto, E.M.; Moreira-Soto, A.; Pedroso, C.; Hoser, C.; Funk, S.; Kucharski, A.J.; Rockstroh, A.; Kummerer, B.M.; Sampaio, G.S.; Luz, E.; et al. High Zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. mBio 2017, 8, e01390-17. [Google Scholar] [CrossRef]
- Barreto, F.K.A.; Alencar, C.H.; Araujo, F.M.C.; Oliveira, R.; Cavalcante, J.W.; Lemos, D.R.Q.; Farias, L.; Boriz, I.L.F.; Medeiros, L.Q.; Melo, M.N.P.; et al. Seroprevalence, spatial dispersion and factors associated with flavivirus and chikungunha infection in a risk area: A population-based seroprevalence study in Brazil. BMC Infect. Dis. 2020, 20, 881. [Google Scholar] [CrossRef]
- WHO. Zika Epidemiology Update. July 2019. Available online: https://www.who.int/emergencies/diseases/zika/zika-epidemiology-update-july-2019.pdf?ua=1 (accessed on 20 December 2021).
- PAHO/WHO. Cases of Zika Virus Disease by Country or Territory. Weekly Report. 2021. Available online: https://www3.paho.org/data/index.php/en/?option=com_content&view=article&id=524:zika-weekly-en&Itemid=352 (accessed on 20 December 2021).
- Jacques, I.; Katz, L.; Sena, M.A.; Guimaraes, A.B.G.; Silva, Y.L.; Albuquerque, G.D.M.; Pereira, R.O.; de Albuquerque, C.; Silva, M.A.L.; Oliveira, P.A.S.; et al. High Incidence of Zika or Chikungunya Infection among pregnant women hospitalized due to obstetrical complications in Northeastern Brazil-Implications for laboratory screening in Arbovirus endemic area. Viruses 2021, 13, 744. [Google Scholar] [CrossRef]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, O595–O596. [Google Scholar] [CrossRef]
- Bautista, L.E.; Sethi, A.K. Association between Guillain-Barre syndrome and Zika virus infection. Lancet 2016, 387, 2599–2600. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. Eur. Commun. Dis. Bull. 2014, 19, 20720–20722. [Google Scholar] [CrossRef]
- Mier, Y.T.-R.L.; Delorey, M.J.; Sejvar, J.J.; Johansson, M.A. Guillain-Barre syndrome risk among individuals infected with Zika virus: A multi-country assessment. BMC Med. 2018, 16, 67. [Google Scholar] [CrossRef]
- Dirlikov, E.; Major, C.G.; Medina, N.A.; Lugo-Robles, R.; Matos, D.; Munoz-Jordan, J.L.; Colon-Sanchez, C.; Garcia, M.; Olivero-Segarra, M.; Malave, G.; et al. Clinical features of Guillain-Barre Syndrome with vs without Zika virus infection, Puerto Rico, 2016. JAMA Neurol. 2018, 75, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; De Oliveira, W.T.; do Carmo, G.M.; Henriques, C.M.; Coelho, G.E.; Araujo de Franca, G.V. Increase in Reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy-Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Freitas, D.A.; Souza-Santos, R.; Carvalho, L.M.A.; Barros, W.B.; Neves, L.M.; Brasil, P.; Wakimoto, M.D. Congenital Zika syndrome: A systematic review. PLoS ONE 2020, 15, e0242367. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, X.C.; Jia, R.Y. Toll-Like Receptors and RIG-I-Like Receptors play important roles in resisting Flavivirus. J. Immunol. Res. 2018, 2018, 6106582. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika virus infection in human skin cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef]
- Hertzog, J.; Dias Junior, A.G.; Rigby, R.E.; Donald, C.L.; Mayer, A.; Sezgin, E.; Song, C.; Jin, B.; Hublitz, P.; Eggeling, C.; et al. Infection with a Brazilian isolate of Zika virus generates RIG-I stimulatory RNA and the viral NS5 protein blocks type I IFN induction and signaling. Eur. J. Immunol. 2018, 48, 1120–1136. [Google Scholar] [CrossRef]
- Chazal, M.; Beauclair, G.; Gracias, S.; Najburg, V.; Simon-Loriere, E.; Tangy, F.; Komarova, A.V.; Jouvenet, N. RIG-I Recognizes the 5′ region of dengue and Zika virus genomes. Cell Rep. 2018, 24, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.; Bridgeman, A.; Gray, N.; Hertzog, J.; Hublitz, P.; Kohl, A.; Rehwinkel, J. RIG-I plays a dominant role in the induction of transcriptional changes in Zika virus-infected cells, which protect from virus-induced cell death. Cells 2020, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika virus pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dong, X.; He, Z.; Wu, Y.; Zhang, S.; Lin, J.; Yang, Y.; Chen, J.; An, S.; Yin, Y.; et al. Zika virus antagonizes interferon response in patients and disrupts RIG-I-MAVS interaction through its CARD-TM domains. Cell Biosci. 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hur, S. How RIG-I like receptors activate MAVS. Curr. Opin. Virol. 2015, 12, 91–98. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Zhou, J.; Xie, W.; Chen, C.; Wang, Z.; Yang, H.; Cui, J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017, 3, 17006. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sanchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Kumar, A.; Hou, S.; Airo, A.M.; Limonta, D.; Mancinelli, V.; Branton, W.; Power, C.; Hobman, T.C. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016, 17, 1766–1775. [Google Scholar] [CrossRef]
- Ashour, J.; Morrison, J.; Laurent-Rolle, M.; Belicha-Villanueva, A.; Plumlee, C.R.; Bernal-Rubio, D.; Williams, K.L.; Harris, E.; Fernandez-Sesma, A.; Schindler, C.; et al. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe 2010, 8, 410–421. [Google Scholar] [CrossRef]
- Perry, S.T.; Buck, M.D.; Lada, S.M.; Schindler, C.; Shresta, S. STAT2 mediates innate immunity to Dengue virus in the absence of STAT1 via the type I interferon receptor. PLoS Pathog. 2011, 7, e1001297. [Google Scholar] [CrossRef]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef]
- Dowd, K.A.; Pierson, T.C. Antibody-mediated neutralization of flaviviruses: A reductionist view. Virology 2011, 411, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Pierson, T.C. Molecular insight into Dengue Virus pathogenesis and its implications for disease control. Cell 2015, 162, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue Antibody-dependent enhancement: Knowns and unknowns. Microbiol. Spectr. 2014, 2, 249–271. [Google Scholar] [CrossRef]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef]
- Ayala-Nunez, N.V.; Hoornweg, T.E.; van de Pol, D.P.; Sjollema, K.A.; Flipse, J.; van der Schaar, H.M.; Smit, J.M. How antibodies alter the cell entry pathway of dengue virus particles in macrophages. Sci. Rep. 2016, 6, 28768. [Google Scholar] [CrossRef]
- Goncalves Pereira, M.H.; Figueiredo, M.M.; Queiroz, C.P.; Magalhaes, T.V.B.; Mafra, A.; Diniz, L.M.O.; da Costa, U.L.; Gollob, K.J.; Antonelli, L.; Santiago, H.D.C. T-cells producing multiple combinations of IFNgamma, TNF and IL10 are associated with mild forms of dengue infection. Immunology 2020, 160, 90–102. [Google Scholar] [CrossRef]
- Quintino-de-Carvalho, I.L.; Goncalves-Pereira, M.H.; Faria Ramos, M.; de Aguiar Milhim, B.H.G.; Da Costa, U.L.; Santos, E.G.; Nogueira, M.L.; Da Costa Santiago, H. Type 1 Innate Lymphoid Cell and Natural Killer Cells Are Sources of Interferon-gamma and Other Inflammatory Cytokines Associated With Distinct Clinical Presentation in Early Dengue Infection. J. Infect. Dis. 2022, 225, 84–93. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Nascimento, E.J.M.; Braga, C.; Cordeiro, M.T.; de Carvalho, O.V.; de Mendonca, L.R.; Azevedo, E.A.N.; Franca, R.F.O.; Dhalia, R.; Marques, E.T.A. Dengue Virus-Specific Antibodies Enhance Brazilian Zika Virus Infection. J. Infect. Dis. 2017, 215, 781–785. [Google Scholar] [CrossRef]
- Chareonsirisuthigul, T.; Kalayanarooj, S.; Ubol, S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J. Gen. Virol. 2007, 88, 365–375. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P. Dengue antibody and Zika: Friend or foe? Trends Immunol. 2016, 37, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Young, M.P.; Bunz, M.; Xu, Z.; Hattakam, S.; Vizcarra, E.; Regla-Nava, J.A.; Tang, W.W.; Yamabhai, M.; Wen, J.; et al. CD4+ T cells promote humoral immunity and viral control during Zika virus infection. PLoS Pathog. 2019, 15, e1007474. [Google Scholar] [CrossRef]
- Elong Ngono, A.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and role of the CD8+ T cell response during primary Zika virus infection in mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef]
- Hassert, M.; Harris, M.G.; Brien, J.D.; Pinto, A.K. Identification of protective CD8 T Cell responses in a mouse model of Zika virus infection. Front. Immunol. 2019, 10, 1678. [Google Scholar] [CrossRef]
- Regla-Nava, J.A.; Elong Ngono, A.; Viramontes, K.M.; Huynh, A.T.; Wang, Y.T.; Nguyen, A.T.; Salgado, R.; Mamidi, A.; Kim, K.; Diamond, M.S.; et al. Cross-reactive Dengue virus-specific CD8(+) T cells protect against Zika virus during pregnancy. Nat. Commun. 2018, 9, 3042. [Google Scholar] [CrossRef]
- Naveca, F.G.; Pontes, G.S.; Chang, A.Y.; Silva, G.; Nascimento, V.A.D.; Monteiro, D.; Silva, M.S.D.; Abdalla, L.F.; Santos, J.H.A.; Almeida, T.A.P.; et al. Analysis of the immunological biomarker profile during acute Zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Do Inst. Oswaldo Cruz 2018, 113, e170542. [Google Scholar] [CrossRef]
- Hassert, M.; Wolf, K.J.; Schwetye, K.E.; DiPaolo, R.J.; Brien, J.D.; Pinto, A.K. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018, 14, e1007237. [Google Scholar] [CrossRef]
- Pereira Neto, T.A.; Goncalves-Pereira, M.H.; de Queiroz, C.P.; Ramos, M.F.; de Oliveira, F.F.S.; Oliveira-Prado, R.; do Nascimento, V.A.; Abdalla, L.F.; Santos, J.H.A.; Martins-Filho, O.A.; et al. Multifunctional T cell response in convalescent patients two years after ZIKV infection. J. Leukoc. Biol. 2020, 108, 1265–1277. [Google Scholar] [CrossRef]
- Hatch, S.; Endy, T.P.; Thomas, S.; Mathew, A.; Potts, J.; Pazoles, P.; Libraty, D.H.; Gibbons, R.; Rothman, A.L. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J. Infect. Dis. 2011, 203, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; Del Rio, C.; et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009, 183, 7919–7930. [Google Scholar] [CrossRef]
- Blom, K.; Braun, M.; Ivarsson, M.A.; Gonzalez, V.D.; Falconer, K.; Moll, M.; Ljunggren, H.G.; Michaelsson, J.; Sandberg, J.K. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J. Immunol. 2013, 190, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, D.; Therrien, R.; Kettaf, N.; Angermann, B.R.; Boucher, G.; Filali-Mouhim, A.; Moser, J.M.; Mehta, R.S.; Drake, D.R., 3rd; Castro, E.; et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008, 205, 3119–3131. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Twiddy, S.S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003, 3, 19–28. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; Barton, J.P.; McKay, M.R. Cross-serotypically conserved epitope recommendations for a universal T cell-based dengue vaccine. PLoS Negl. Trop. Dis. 2020, 14, e0008676. [Google Scholar] [CrossRef] [PubMed]
- Durham, N.D.; Agrawal, A.; Waltari, E.; Croote, D.; Zanini, F.; Fouch, M.; Davidson, E.; Smith, O.; Carabajal, E.; Pak, J.E.; et al. Broadly neutralizing human antibodies against dengue virus identified by single B cell transcriptomics. eLife 2019, 8, e52384. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhu, Z.; Li, S.; Deng, Y.; Wu, Y.; Zhang, N.; Puri, V.; Wang, C.; Zou, P.; Lei, C.; et al. A broadly neutralizing germline-like human monoclonal antibody against dengue virus envelope domain III. PLoS Pathog. 2019, 15, e1007836. [Google Scholar] [CrossRef]
- Keasey, S.L.; Pugh, C.L.; Jensen, S.M.; Smith, J.L.; Hontz, R.D.; Durbin, A.P.; Dudley, D.M.; O’Connor, D.H.; Ulrich, R.G. Antibody responses to Zika virus infections in environments of Flavivirus endemicity. Clin. Vaccine Immunol. CVI 2017, 24, e00036-17. [Google Scholar] [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef]
- Delgado, F.G.; Torres, K.I.; Castellanos, J.E.; Romero-Sanchez, C.; Simon-Loriere, E.; Sakuntabhai, A.; Roth, C. Improved immune responses against Zika virus after sequential dengue and Zika virus infection in humans. Viruses 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior dengue virus exposure shapes T cell immunity to Zika virus in humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.B.; Tsai, W.Y.; Brites, C.; Luz, E.; Pedroso, C.; Drexler, J.F.; Wang, W.K.; Kanki, P.J. T cell responses to nonstructural protein 3 distinguish infections by dengue and Zika viruses. mBio 2018, 9, e00755-18. [Google Scholar] [CrossRef]
- Herrera, B.B.; Tsai, W.Y.; Chang, C.A.; Hamel, D.J.; Wang, W.K.; Lu, Y.; Mboup, S.; Kanki, P.J. Sustained specific and cross-reactive T cell responses to Zika and dengue virus NS3 in West Africa. J. Virol. 2018, 92, e01992-17. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Suleyman, O.M.; Ortega-Prieto, A.M.; Skelton, J.K.; Bonnesoeur, P.; Blohm, A.; Carregaro, V.; Silva, J.S.; James, E.A.; Maillere, B.; et al. T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci. Rep. 2018, 8, 672. [Google Scholar] [CrossRef]
- Ngono, A.E.; Shresta, S. Immune response to dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Gimblet-Ochieng, C.; Modirian, F.; Collins, M.; Cardenas, M.; Katzelnick, L.C.; Montoya, M.; Michlmayr, D.; Kuan, G.; Balmaseda, A.; et al. Impact of pre-existing dengue immunity on human antibody and memory B cell responses to Zika. Nat. Commun. 2019, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; Porterfield, J.S. Antibody-mediated enhancement of Flavivirus replication in macrophage-like cell lines. Nature 1979, 282, 509–511. [Google Scholar] [CrossRef]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Zhang, C.; Wang, X.; Hong, W.; Sun, J.; Liu, R.; Yu, L.; Wang, J.; Zhang, F.; et al. Dengue immune sera enhance Zika virus infection in human peripheral blood monocytes through Fc gamma receptors. PLoS ONE 2018, 13, e0200478. [Google Scholar] [CrossRef]
- Paul, L.M.; Carlin, E.R.; Jenkins, M.M.; Tan, A.L.; Barcellona, C.M.; Nicholson, C.O.; Michael, S.F.; Isern, S. Dengue virus antibodies enhance Zika virus infection. Clin. Transl. Immunol. 2016, 5, e117. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; Saron, W.A.A.; Lim, T.; Jahan, N.; St John, A.L. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci. Adv. 2019, 5, eaav3208. [Google Scholar] [CrossRef] [PubMed]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.C.; Medits, I.; Sharma, A.; Simon-Loriere, E.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Haouz, A.; et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536, 7614. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.W.; Lee, C.Y.; Teo, T.H.; Howland, S.W.; Amrun, S.N.; Lum, F.M.; See, P.; Kng, N.Q.; Huber, R.G.; Xu, M.H.; et al. Cross-reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections. JCI Insight 2017, 2, e92428. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.J.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Elong Ngono, A.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017, 8, 1459. [Google Scholar] [CrossRef]
- De Goes Cavalcanti, L.P.; Tauil, P.L.; Alencar, C.H.; Oliveira, W.; Teixeira, M.M.; Heukelbach, J. Zika virus infection, associated microcephaly, and low yellow fever vaccination coverage in Brazil: Is there any causal link? J. Infect. Dev. Ctries. 2016, 10, 563–566. [Google Scholar] [CrossRef][Green Version]
- Vicente Santos, A.C.; Guedes-da-Silva, F.H.; Dumard, C.H.; Ferreira, V.N.S.; da Costa, I.P.S.; Machado, R.A.; Barros-Aragao, F.G.Q.; Neris, R.L.S.; Dos-Santos, J.S.; Assuncao-Miranda, I.; et al. Yellow fever vaccine protects mice against Zika virus infection. PLoS Negl. Trop. Dis. 2021, 15, e0009907. [Google Scholar] [CrossRef]
- Blom, K.; Sandberg, J.T.; Lore, K.; Ljunggren, H.G. Prospects for induction of CD8 T cell-mediated immunity to Zika virus infection by yellow fever virus vaccination. J. Intern. Med. 2017, 282, 206–208. [Google Scholar] [CrossRef]
- Pantoja, P.; Perez-Guzman, E.X.; Rodriguez, I.V.; White, L.J.; Gonzalez, O.; Serrano, C.; Giavedoni, L.; Hodara, V.; Cruz, L.; Arana, T.; et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun. 2017, 8, 15674. [Google Scholar] [CrossRef]
- McCracken, M.K.; Gromowski, G.D.; Friberg, H.L.; Lin, X.; Abbink, P.; De La Barrera, R.; Eckles, K.H.; Garver, L.S.; Boyd, M.; Jetton, D.; et al. Impact of prior Flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog. 2017, 13, e1006487. [Google Scholar] [CrossRef] [PubMed]
- Terzian, A.C.B.; Schanoski, A.S.; Oliveira Mota, M.T.; Silva, R.A.; Estofolete, C.F.; Colombo, T.E.; Rahal, P.; Hanley, K.A.; Vasilakis, N.; Kalil, J.; et al. Viral load and cytokine response profile does not support antibody-dependent enhancement in dengue-primed zika virus–infected patients. Clin. Infect. Dis. 2017, 65, 1260. [Google Scholar] [CrossRef] [PubMed]
- Henein, S.; Swanstrom, J.; Byers, A.M.; Moser, J.M.; Shaik, S.F.; Bonaparte, M.; Jackson, N.; Guy, B.; Baric, R.; de Silva, A.M. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J. Infect. Dis. 2017, 215, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Galan-Herrera, J.F.; Forrat, R.; Zambrano, B.; Bouckenooghe, A.; Harenberg, A.; Guy, B.; Lang, J. Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naive adults in Mexico. Hum. Vaccines Immunother. 2014, 10, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; Jiwariyavej, V.; Dulyachai, W.; Pengsaa, K.; Wartel, T.A.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef]
- Torresi, J.; Richmond, P.C.; Heron, L.G.; Qiao, M.; Marjason, J.; Starr-Spires, L.; van der Vliet, D.; Jin, J.; Wartel, T.A.; Bouckenooghe, A. Replication and excretion of the live attenuated tetravalent dengue vaccine CYD-TDV in a Flavivirus-naive adult population: Assessment of vaccine viremia and virus shedding. J. Infect. Dis. 2017, 216, 834–841. [Google Scholar] [CrossRef][Green Version]
- Harenberg, A.; Begue, S.; Mamessier, A.; Gimenez-Fourage, S.; Ching Seah, C.; Wei Liang, A.; Li Ng, J.; Yun Toh, X.; Archuleta, S.; Wilder-Smith, A.; et al. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum. Vaccines Immunother. 2013, 9, 2317–2325. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Pugachev, K.; Zhang, Z.; Myers, G.; Levenbook, I.; Draper, K.; Lang, J.; Ocran, S.; Mitchell, F.; Parsons, M.; et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 2004, 78, 4761–4775. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Weltzin, R.; Chambers, T.J.; Zhang, Z.X.; Soike, K.; Ratterree, M.; Arroyo, J.; Georgakopoulos, K.; Catalan, J.; Monath, T.P. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J. Virol. 2000, 74, 5477–5485. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-Garcia, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramirez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-Garcia, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Moodie, Z.; Juraska, M.; Huang, Y.; Zhuang, Y.; Fong, Y.; Carpp, L.N.; Self, S.G.; Chambonneau, L.; Small, R.; Jackson, N.; et al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J. Infect. Dis. 2018, 217, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Nougarede, N.; Begue, S.; Sanchez, V.; Souag, N.; Carre, M.; Chambonneau, L.; Morrisson, D.N.; Shaw, D.; Qiao, M.; et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008, 26, 5712–5721. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Erdos, G.; Watkins, S.C.; Falo, L.D., Jr.; Marques, E.T.A.; Barratt-Boyes, S.M. Reciprocal immune enhancement of dengue and Zika virus infection in human skin. JCI Insight 2020, 5, e133653. [Google Scholar] [CrossRef]
- Fagbami, A.H.; Halstead, S.B.; Marchette, N.J.; Larsen, K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios 1987, 49, 49–55. [Google Scholar]
- Castanha, P.M.S.; Marques, E.T.A. Zika vaccines: Can we solve one problem without creating another one? Lancet Infect. Dis. 2021, 21, 1198–1200. [Google Scholar] [CrossRef]
- Skibinski, D.A.; Baudner, B.C.; Singh, M.; O’Hagan, D.T. Combination vaccines. J. Glob. Infect. Dis. 2011, 3, 63–72. [Google Scholar] [CrossRef]
- Nivarthi, U.K.; Swanstrom, J.; Delacruz, M.J.; Patel, B.; Durbin, A.P.; Whitehead, S.S.; Kirkpatrick, B.D.; Pierce, K.K.; Diehl, S.A.; Katzelnick, L.; et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat. Commun. 2021, 12, 1102. [Google Scholar] [CrossRef]
- Weiskopf, D.; Cerpas, C.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; Sanches, F.P.; Silvera, C.G.; Costa, P.R.; et al. Human CD8+ T-cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J. Infect. Dis. 2015, 212, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Castanha, P.M.S.; Souza, W.V.; Braga, C.; Araujo, T.V.B.; Ximenes, R.A.A.; Albuquerque, M.; Montarroyos, U.R.; Miranda-Filho, D.B.; Cordeiro, M.T.; Dhalia, R.; et al. Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: A microcephaly case-control study in an area of high dengue endemicity in Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007246. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, R.A.A.; Miranda-Filho, D.B.; Montarroyos, U.R.; Martelli, C.M.T.; Araujo, T.V.B.; Brickley, E.; Albuquerque, M.; Souza, W.V.; Ventura, L.O.; Ventura, C.V.; et al. Zika-related adverse outcomes in a cohort of pregnant women with rash in Pernambuco, Brazil. PLoS Negl. Trop. Dis. 2021, 15, e0009216. [Google Scholar] [CrossRef] [PubMed]
- Castanha, P.M.S.; Marques, E.T.A. A glimmer of hope: Recent updates and future challenges in zika vaccine development. Viruses 2020, 12, 1371. [Google Scholar] [CrossRef]
- Cohen, J. As massive Zika vaccine trial struggles, researchers revive plan to intentionally infect humans. Science 2018, 361, 1055–1056. [Google Scholar] [CrossRef]
- Jamrozik, E.; Selgelid, M.J. Ethical issues surrounding controlled human infection challenge studies in endemic low-and middle-income countries. Bioethics 2020, 34, 797–808. [Google Scholar] [CrossRef]
- Gordon, S.B.; Rylance, J.; Luck, A.; Jambo, K.; Ferreira, D.M.; Manda-Taylor, L.; Bejon, P.; Ngwira, B.; Littler, K.; Seager, Z.; et al. A framework for Controlled Human Infection Model (CHIM) studies in Malawi: Report of a Wellcome Trust workshop on CHIM in Low Income Countries held in Blantyre, Malawi. Wellcome Open Res. 2017, 2, 70. [Google Scholar] [CrossRef]
- Casares, S.; Brumeanu, T.D.; Richie, T.L. The RTS,S malaria vaccine. Vaccine 2010, 28, 4880–4894. [Google Scholar] [CrossRef]
- Stanisic, D.I.; McCarthy, J.S.; Good, M.F. Controlled Human Malaria Infection: Applications, advances, and challenges. Infect. Immun. 2018, 86, e00479-17. [Google Scholar] [CrossRef]
- Losonsky, G.A.; Tacket, C.O.; Wasserman, S.S.; Kaper, J.B.; Levine, M.M. Secondary Vibrio cholerae-specific cellular antibody responses following wild-type homologous challenge in people vaccinated with CVD 103-HgR live oral cholera vaccine: Changes with time and lack of correlation with protection. Infect. Immuninty 1993, 61, 729–733. [Google Scholar] [CrossRef]
- Shirley, D.A.; McArthur, M.A. The utility of human challenge studies in vaccine development: Lessons learned from cholera. Vaccine 2011, 2011, 3–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tacket, C.O.; Cohen, M.B.; Wasserman, S.S.; Losonsky, G.; Livio, S.; Kotloff, K.; Edelman, R.; Kaper, J.B.; Cryz, S.J.; Giannella, R.A.; et al. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect. Immun. 1999, 67, 6341–6345. [Google Scholar] [CrossRef] [PubMed]
- Tacket, C.O.; Losonsky, G.; Nataro, J.P.; Cryz, S.J.; Edelman, R.; Kaper, J.B.; Levine, M.M. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 1992, 166, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Richie, E.E.; Punjabi, N.H.; Sidharta, Y.Y.; Peetosutan, K.K.; Sukandar, M.M.; Wasserman, S.S.; Lesmana, M.M.; Wangsasaputra, F.F.; Pandam, S.S.; Levine, M.M.; et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 2000, 18, 2399–2410. [Google Scholar] [CrossRef]
- Vannice, K.S.; Giersing, B.K.; Kaslow, D.C.; Griffiths, E.; Meyer, H.; Barrett, A.; Durbin, A.P.; Wood, D.; Hombach, J. Meeting Report: WHO consultation on considerations for regulatory expectations of Zika virus vaccines for use during an emergency. Vaccine 2019, 37, 7443–7450. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, H.C.; Pereira-Neto, T.A.; Gonçalves-Pereira, M.H.; Terzian, A.C.B.; Durbin, A.P. Peculiarities of Zika Immunity and Vaccine Development: Lessons from Dengue and the Contribution from Controlled Human Infection Model. Pathogens 2022, 11, 294. https://doi.org/10.3390/pathogens11030294
Santiago HC, Pereira-Neto TA, Gonçalves-Pereira MH, Terzian ACB, Durbin AP. Peculiarities of Zika Immunity and Vaccine Development: Lessons from Dengue and the Contribution from Controlled Human Infection Model. Pathogens. 2022; 11(3):294. https://doi.org/10.3390/pathogens11030294
Chicago/Turabian StyleSantiago, Helton C., Tertuliano A. Pereira-Neto, Marcela H. Gonçalves-Pereira, Ana C. B. Terzian, and Anna P. Durbin. 2022. "Peculiarities of Zika Immunity and Vaccine Development: Lessons from Dengue and the Contribution from Controlled Human Infection Model" Pathogens 11, no. 3: 294. https://doi.org/10.3390/pathogens11030294
APA StyleSantiago, H. C., Pereira-Neto, T. A., Gonçalves-Pereira, M. H., Terzian, A. C. B., & Durbin, A. P. (2022). Peculiarities of Zika Immunity and Vaccine Development: Lessons from Dengue and the Contribution from Controlled Human Infection Model. Pathogens, 11(3), 294. https://doi.org/10.3390/pathogens11030294





