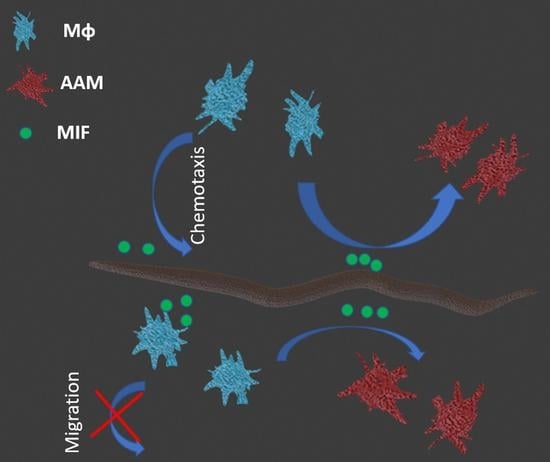
Nematode Orthologs of Macrophage Migration Inhibitory Factor (MIF) as Modulators of the Host Immune Response and Potential Therapeutic Targets
Abstract
1. Introduction
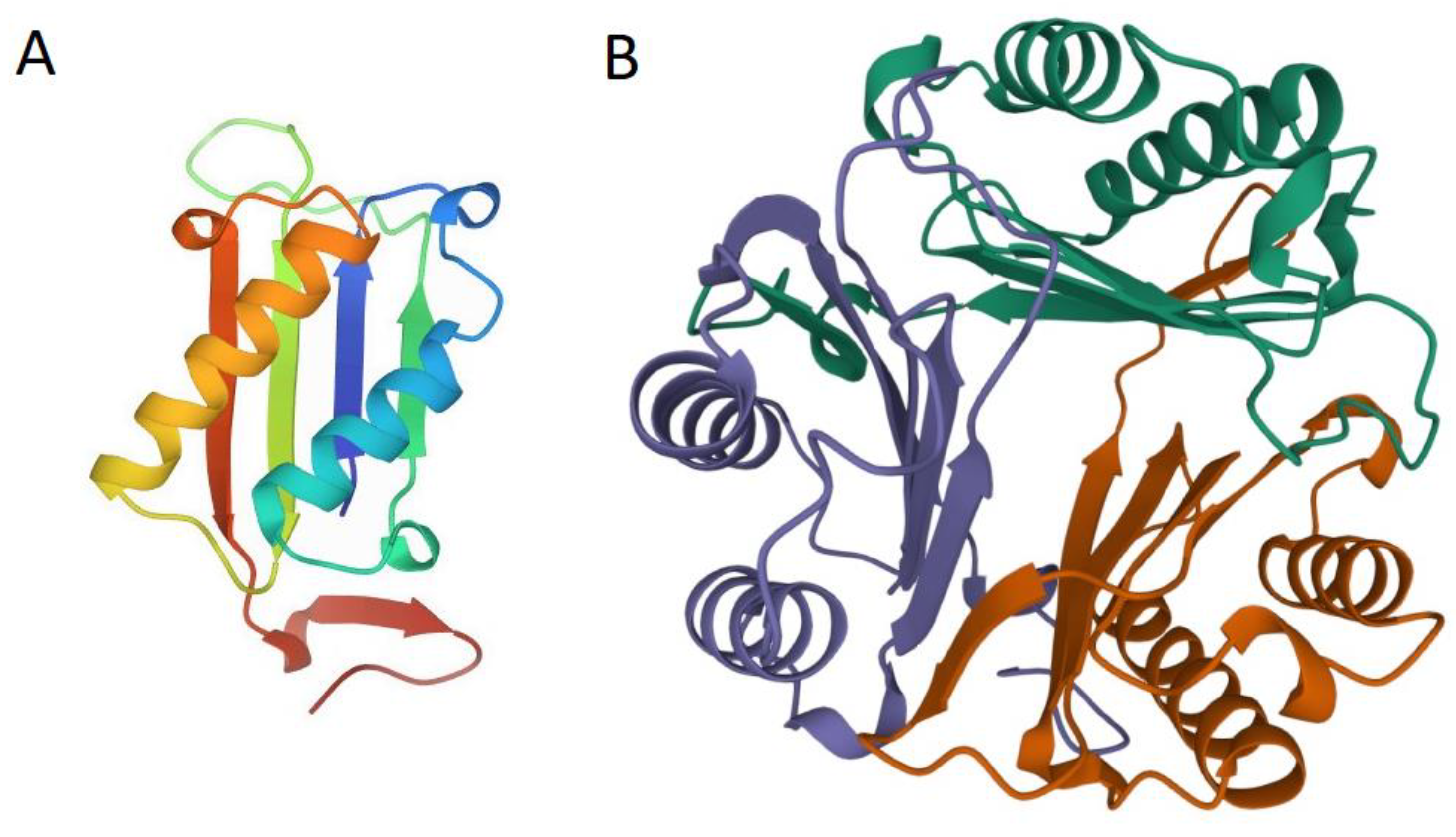
2. MIF Structure and Function
3. MIF Homologs (nMIFs) in Parasitic Nematodes
4. nMIFs: Structure, Function, Activity, and Expression
5. nMIFs and the Immune System of the Host
6. nMIFs in the Treatment of Autoimmune Diseases
7. nMIFs as a Potential Therapeutic Target
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Behnke, J.; Barnard, C.; Wakelin, D. Invited Review Article: Understanding Chronic Nematode Evolutionary Considerations, Current Infections: Hypotheses. Int. J. Parasitol. 1992, 22, 861–907. [Google Scholar] [CrossRef]
- Ghosh, S.; Jiang, N.; Farr, L.; Ngobeni, R.; Moonah, S. Parasite-Produced MIF Cytokine: Role in Immune Evasion, Invasion, and Pathogenesis. Front. Immunol. 2019, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Cho, M.K.; Park, H.-K.; Lee, K.H.; Lee, S.J.; Choi, S.H.; Ock, M.S.; Jeong, H.J.; Lee, M.H.; Yu, H.S. Macrophage Migration Inhibitory Factor Homologs of Anisakis simplex Suppress Th2 Response in Allergic Airway Inflammation Model via CD4 + CD25 + Foxp3 + T Cell Recruitment. J. Immunol. 2009, 182, 6907–6914. [Google Scholar] [CrossRef] [PubMed]
- Bungiro, R.; Cappello, M. Hookworm Infection: New Developments and Prospects for Control. Curr. Opin. Infect. Dis. 2004, 17, 421–426. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth Immunoregulation: The Role of Parasite Secreted Proteins in Modulating Host Immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Blaxter, M.L.; Scott, A.L. Immunological Genomics of Brugia Malayi: Filarial Genes Implicated in Immune Evasion and Protective Immunity. Parasite Immunol. 2001, 23, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T. Macrophage Migration Inhibitory Factor and Host Innate Immune Responses to Microbes. Scand. J. Infect. Dis. 2003, 35, 573–576. [Google Scholar] [CrossRef]
- Vermeire, J.J.; Cho, Y.; Lolis, E.; Bucala, R.; Cappello, M. Orthologs of Macrophage Migration Inhibitory Factor from Parasitic Nematodes. Trends Parasitol. 2008, 24, 355–363. [Google Scholar] [CrossRef]
- Bloom, B.R.; Bennett, B. Mechanism of a Reaction in Vitro Associated with Delayed-Type Hypersensitivity. Science 1966, 153, 80–82. [Google Scholar] [CrossRef]
- Kang, I.; Bucala, R. The Immunobiology of MIF: Function, Genetics and Prospects for Precision Medicine. Nat. Rev. Rheumatol. 2019, 15, 427–437. [Google Scholar] [CrossRef]
- Harris, J.; VanPatten, S.; Deen, N.S.; Al-Abed, Y.; Morand, E.F. Rediscovering MIF: New Tricks for an Old Cytokine. Trends Immunol. 2019, 40, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Mitchell, R.A.; Calandra, T.; Voelter, W.; Cerami, A.; Bucala, R. Purification, Bioactivity, and Secondary Structure Analysis of Mouse and Human Macrophage Migration Inhibitory Factor (MIF). Biochemistry 1994, 33, 14144–14155. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, H.R. Macrophage Migration Inhibitory Factor: A Potential Therapeutic Target for Rheumatoid Arthritis. Korean J. Intern. Med. 2016, 31, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Sun, S.; Al-Abed, Y. MIF, a Controversial Cytokine: A Review of Structural Features, Challenges, and Opportunities for Drug Development. Expert Opin. Ther. Targets 2016, 20, 1463–1475. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugimoto, H.; Nakagawa, A.; Tanaka, I.; Nishira, J.; Sakai, M. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat. Struct. Biol. 1996, 3, 259–266. [Google Scholar] [CrossRef]
- Florez-Sampedro, L.; Soto-Gamez, A.; Poelarends, G.J.; Melgert, B.N. The Role of MIF in Chronic Lung Diseases: Looking beyond Inflammation. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2020, 318, L1183–L1197. [Google Scholar] [CrossRef]
- Sun, H.-W.; Bernhagentt, J.; Bucalat, R.; Lolis, E. Crystal Structure at 2.6-A Resolution of Human Macrophage Migration Inhibitory Factor. Proc. Natl. Acad. Sci. USA 1996, 93, 5191–5196. [Google Scholar] [CrossRef]
- Calandra, T.; Roger, T. Macrophage Migration Inhibitory Factor: A Regulator of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef]
- Sparkes, A.; de Baetselier, P.; Roelants, K.; de Trez, C.; Magez, S.; van Ginderachter, J.A.; Raes, G.; Bucala, R.; Stijlemans, B. The Non-Mammalian MIF Superfamily. Immunobiology 2017, 222, 473–482. [Google Scholar] [CrossRef]
- Bendrat, K.; Al-Abed, Y.; Callaway, D.J.E.; Peng, T.; Calandra, T.; Metz, C.N.; Bucala, R. Biochemical and Mutational Investigations of the Enzymatic Activity of Macrophage Migration Inhibitory Factor. Biochemistry 1997, 36, 15356–15362. [Google Scholar] [CrossRef]
- Stamps, S.L.; Fitzgerald, M.C.; Whitman, C.P. Characterization of the Role of the Amino-Terminal Proline in the Enzymatic Activity Catalyzed by Macrophage Migration Inhibitory Factor. Biochemistry 1998, 37, 10195–10202. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Fetterer, R.; Leng, L.; Du, X.; Zarlenga, D.; Shen, Z.; Han, W.; Bucala, R.; Tuo, W. Ostertagia Ostertagi Macrophage Migration Inhibitory Factor Is Present in All Developmental Stages and May Cross-Regulate Host Functions through Interaction with the Host Receptor. Int. J. Parasitol. 2014, 44, 355–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lubetsky, J.B.; Dios, A.; Han, J.; Aljabari, B.; Ruzsicska, B.; Mitchell, R.; Lolis, E.; Al-Abed, Y. The Tautomerase Active Site of Macrophage Migration Inhibitory Factor Is a Potential Target for Discovery of Novel Anti-Inflammatory Agents. J. Biol. Chem. 2002, 277, 24976–24982. [Google Scholar] [CrossRef] [PubMed]
- Cournia, Z.; Leng, L.; Gandavadi, S.; Du, X.; Bucala, R.; Jorgensen, W.L. Discovery of Human Macrophage Migration Inhibitory Factor (MIF)-CD74 Antagonists via Virtual Screening. J. Med. Chem. 2009, 52, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Merk, M.; Mitchell, R.A.; Endres, S.; Bucala, R. d-Dopachrome Tautomerase (d-DT or MIF-2): Doubling the MIF Cytokine Family. Cytokine 2012, 59, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hermanowski-Vosatka, A.; Mundt, S.S.; Ayala, J.M.; Goyal, S.; Hanlon, W.A.; Czerwinski, R.M.; Wright, S.D.; Whitman, C.P. Enzymatically Inactive Macrophage Migration Inhibitory Factor Inhibits Monocyte Chemotaxis and Random Migration. Biochemistry 1999, 38, 12841–12849. [Google Scholar] [CrossRef]
- Calandra, T.; Bernhagen, J.; Mitchell, R.A.; Bucala, R. The Macrophage Is an Important and Previously Unrecognized Source of Macrophage Migration Inhibitory Factor. J. Exp. Med. 1994, 179, 1895–1902. [Google Scholar] [CrossRef]
- Daryadel, A.; Grifone, R.F.; Simon, H.U.; Yousefi, S. Apoptotic Neutrophils Release Macrophage Migration Inhibitory Factor upon Stimulation with Tumor Necrosis Factor-α. J. Biol. Chem. 2006, 281, 27653–27661. [Google Scholar] [CrossRef]
- Rossi, A.G.; Haslett, C.; Hirani, N.; Greening, A.P.; Rahman, I.; Metz, C.N.; Bucala, R.; Donnelly, S.C. Human Circulating Eosinophils Secrete Macrophage Migration Inhibitory Factor (MIF): Potential Role in Asthma. J. Clin. Investig. 1998, 101, 2869–2874. [Google Scholar] [CrossRef]
- Bacher, M.; Metz, C.N.; Calandra, T.; Mayert, K.; Chesney, J.; Lohofft, M.; Gemsat, D.; Donnelly, T.; Bucala, R. An Essential Regulatory Role for Macrophage Migration Inhibitory Factor in T-Cell Activation (Cytokines/Glucocorticoid/Steroid/Interleukin 2/Interferon-y). Proc. Natl. Acad. Sci. USA 1996, 93, 7849–7854. [Google Scholar] [CrossRef]
- Nishihira, J.; Koyama, Y.; Mizue, Y. Identification of Macrophage Migration Inhibitory Factor (MIF) in Human Vascular Endothelial Cells and Its Induction by Lipopolysaccharide. Cytokine 1998, 10, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Role of Macrophage Migration Inhibitory Factor (MIF) in the Skin. J. Dermatol. Sci. 2005, 37, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, L.; Lindeman, J.H.N.; van Hajo Bockel, J.; Abdul-Hussien, H.; Kooistra, T.; Kleemann, R. Up-Regulation and Coexpression of MIF and Matrix Metalloproteinases in Human Abdominal Aortic Aneurysms. Antioxid. Redox Signal. 2005, 7, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Foote, A.; Lee, J.P.W.; Morand, E.F.; Harris, J. MIF: Implications in the Pathoetiology of Systemic Lupus Erythematosus. Front. Immunol. 2015, 6, 577. [Google Scholar] [CrossRef] [PubMed]
- Flieger, O.; Engling, A.; Bucala, R.; Lue, H.; Nickel, W.; Bernhagen, J. Regulated Secretion of Macrophage Migration Inhibitory Factor Is Mediated by a Non-Classical Pathway Involving an ABC Transporter. FEBS Lett. 2003, 551, 78–86. [Google Scholar] [CrossRef]
- Baugh, J.A.; Chitnis, S.; Donnelly, S.C.; Monteiro, J.; Lin, X.; Plant, B.J.; Wolfe, F.; Gregersen, P.K.; Bucala, R. A Functional Promoter Polymorphism in the Macrophage Migration Inhibitory Factor (MIF) Gene Associated with Disease Severity in Rheumatoid Arthritis. Genes Immun. 2002, 3, 170–176. [Google Scholar] [CrossRef]
- Bai, F.; Asojo, O.A.; Cirillo, P.; Ciustea, M.; Ledizet, M.; Aristoff, P.A.; Leng, L.; Koski, R.A.; Powell, T.J.; Bucala, R.; et al. A Novel Allosteric Inhibitor of Macrophage Migration Inhibitory Factor (MIF). J. Biol. Chem. 2012, 287, 30653–30663. [Google Scholar] [CrossRef]
- Gore, Y.; Starlets, D.; Maharshak, N.; Becker-Herman, S.; Kaneyuki, U.; Leng, L.; Bucala, R.; Shachar, I. Macrophage Migration Inhibitory Factor Induces B Cell Survival by Activation of a CD74-CD44 Receptor Complex. J. Biol. Chem. 2008, 283, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.D.; Shoaibi, M.A.; Maestro, R.; Carnero, A.; Hannon, G.J.; Beach, D.H. A Proinflammatory Cytokine Inhibits P53 Tumor Suppressor Activity. J. Exp. Med. 1999, 190, 1375–1382. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving Complexity of MIF Signaling. Cell. Signal. 2019, 57, 76–88. [Google Scholar] [CrossRef]
- Bilsborrow, J.B.; Doherty, E.; Tilstam, P.V.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF) as a Therapeutic Target for Rheumatoid Arthritis and Systemic Lupus Erythematosus. Expert Opin. Ther. Targets 2019, 23, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A Nuclease That Mediates Cell Death Induced by DNA Damage and Poly(ADP-Ribose) Polymerase-1. Science 2016, 354, aad6872. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shu, T.; Zhao, H.; Sun, Y.; Xu, W.; Tu, G. Knockdown of Macrophage Migration Inhibitory Factor (MIF), a Novel Target to Protect Neurons from Parthanatos Induced by Simulated Post-Spinal Cord Injury Oxidative Stress. Biochem. Biophys. Res. Commun. 2020, 523, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, C.; Mcdaniel, K.; Leng, L.; Bucala, R. Tumor Growth-Promoting Properties of Macrophage Migration Inhibitory Factor. Curr. Pharm. Des. 2008, 14, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Bozza, M.T.; Martins, Y.C.; Carneiro, L.A.M.; Paiva, C.N. Macrophage Migration Inhibitory Factor in Protozoan Infections. J. Parasitol. Res. 2012, 2012, 413052. [Google Scholar] [CrossRef]
- Bucala, R. MIF Rediscovered: Cytokine, Pituitary Hormone, and Glucocorticoid-induced Regulator of the Immune Response. FASEB J. 1996, 10, 1607–1613. [Google Scholar] [CrossRef]
- Nobre, C.C.G.; de Araújo, J.M.G.; de Medeiros Fernandes, T.A.A.; Cobucci, R.N.O.; Lanza, D.C.F.; Andrade, V.S.; Fernandes, J.V. Macrophage Migration Inhibitory Factor (MIF): Biological Activities and Relation with Cancer. Pathol. Oncol. Res. 2017, 23, 235–244. [Google Scholar] [CrossRef]
- Stojanovic, I.; Saksida, T.; Stosic-Grujicic, S. Beta Cell Function: The Role of Macrophage Migration Inhibitory Factor. Immunol. Res. 2012, 52, 81–88. [Google Scholar] [CrossRef]
- Basile, M.S.; Battaglia, G.; Bruno, V.; Mangano, K.; Fagone, P.; Petralia, M.C.; Nicoletti, F.; Cavalli, E. The Dichotomic Role of Macrophage Migration Inhibitory Factor in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3023. [Google Scholar] [CrossRef]
- Greven, D.; Leng, L.; Bucala, R. Autoimmune Diseases: MIF as a Therapeutic Target. Expert Opin. Ther. Targets 2010, 14, 253–264. [Google Scholar] [CrossRef]
- Sauler, M.; Bucala, R.; Lee, P.J. Role of Macrophage Migration Inhibitory Factor in Age-Related Lung Disease. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2015, 309, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sinitski, D.; Kontos, C.; Krammer, C.; Asare, Y.; Kapurniotu, A.; Bernhagen, J. Macrophage Migration Inhibitory Factor (MIF)-Based Therapeutic Concepts in Atherosclerosis and Inflammation. Thromb. Haemost. 2019, 119, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; He, J.; Liu, S.; Peng, S.; Yan, Z.; Zhang, Y.; Fan, H. Macrophage Migration Inhibitory Factor -173G/C Gene Polymorphism Increases the Risk of Renal Disease: A Meta-Analysis. Nephrology 2015, 20, 68–76. [Google Scholar] [CrossRef]
- Holowka, T.; Bucala, R. Role of Host and Parasite MIF Cytokines during Leishmania Infection. Trop. Med. Infect. Dis. 2020, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Michelet, C.; Danchin, E.G.J.; Jaouannet, M.; Bernhagen, J.; Panstruga, R.; Kogel, K.H.; Keller, H.; Coustau, C. Cross-Kingdom Analysis of Diversity, Evolutionary History, and Site Selection within the Eukaryotic Macrophage Migration Inhibitory Factor Superfamily. Genes 2019, 10, 740. [Google Scholar] [CrossRef]
- Marson, A.L.; Ellen, D.; Tarr, K.; Scott, A.L. Macrophage Migration Inhibitory Factor (Mif ) Transcription Is Significantly Elevated in Caenorhabditis elegans Dauer Larvae. Gene 2001, 278, 53–62. [Google Scholar] [CrossRef]
- Younis, A.E.; Soblik, H.; Ajonina-Ekoti, I.; Erttmann, K.D.; Luersen, K.; Liebau, E.; Brattig, N.W. Characterization of a Secreted Macrophage Migration Inhibitory Factor Homologue of the Parasitic Nematode Strongyloides Acting at the Parasite-Host Cell Interface. Microbes Infect. 2012, 14, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Miska, K.B.; Fetterer, R.H.; Lillehoj, H.S.; Jenkins, M.C.; Allen, P.C.; Harper, S.B. Characterisation of Macrophage Migration Inhibitory Factor from Eimeria Species Infectious to Chickens. Mol. Biochem. Parasitol. 2007, 151, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Uinuk-Ool, T.S.; Kuroda, N.; Mayer, W.E.; Takezaki, N.; Dongak, R.; Figueroa, F.; Cooper, M.D.; Klein, J. Macrophage Migration Inhibitory Factor (MIF) of Jawed and Jawless Fishes: Implications for Its Evolutionary Origin. Dev. Comp. Immunol. 2003, 27, 401–412. [Google Scholar] [CrossRef]
- Pastrana, D.V.; Raghavan, N.; Fitzgerald, P.; Eisinger, S.W.; Metz, C.; Bucala, R.; Schleimer, R.P.; Bickel, C.; Scott, A.L. Filarial Nematode Parasites Secrete a Homologue of the Human Cytokine Macrophage Migration Inhibitory Factor. Infect. Immun. 1998, 66, 5955–5963. [Google Scholar] [CrossRef]
- Falcone, F.H.; Loke, P.; Zang, X.; MacDonald, A.S.; Maizels, R.M.; Allen, J.E. A Brugia Malayi Homolog of Macrophage Migration Inhibitory Factor Reveals an Important Link Between Macrophages and Eosinophil Recruitment During Nematode Infection. J. Immunol. 2001, 167, 5348–5354. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Taylor, P.; Wang, J.M.; Meyer, D.J.; Scott, A.L.; Walkinshaw, M.D.; Maizels, R.M. Homologues of Human Macrophage Migration Inhibitory Factor from a Parasitic Nematode: Gene Cloning, Protein Activity, and Crystal Structure. J. Biol. Chem. 2002, 277, 44261–44267. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Lafuente, L.; Gregory, W.F.; Allen, J.E.; Maizels, R.M. MIF Homologues from a Filarial Nematode Parasite Synergize with IL-4 to Induce Alternative Activation of Host Macrophages. J. Leukoc. Biol. 2009, 85, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Ajonina-Ekoti, I.; Kurosinski, M.A.; Younis, A.E.; Ndjonka, D.; Tanyi, M.K.; Achukwi, M.; Eisenbarth, A.; Ajonina, C.; Lüersen, K.; Breloer, M.; et al. Comparative Analysis of Macrophage Migration Inhibitory Factors (MIFs) from the Parasitic Nematode Onchocerca volvulus and the Free-Living Nematode Caenorhabditis elegans. Parasitol. Res. 2013, 112, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Hoti, S.L.; Meena, R.L.; Vasuki, V.; Sankari, T.; Kaliraj, P. Molecular and Functional Characterization of Macrophage Migration Inhibitory Factor (MIF) Homolog of Human from Lymphatic Filarial Parasite Wuchereria bancrofti. Parasitol. Res. 2012, 111, 2035–2047. [Google Scholar] [CrossRef]
- Chauhan, N.; Sharma, R.; Hoti, S.L. Identification and Biochemical Characterization of Macrophage Migration Inhibitory Factor-2 (MIF-2) Homologue of Human Lymphatic Filarial Parasite, Wuchereria bancrofti. Acta Trop. 2015, 142, 71–78. [Google Scholar] [CrossRef]
- Cai, J.; Huang, L.; Tang, H.; Xu, H.; Wang, L.; Zheng, M.; Yu, H.; Liu, H. Macrophage Migration Inhibitory Factor of Thelazia callipaeda Induces M2-like Macrophage Polarization through TLR4-Mediated Activation of the PI3K-Akt Pathway. FASEB J. 2021, 35, e21866. [Google Scholar] [CrossRef]
- Cho, M.K.; Lee, C.H.; Yu, H.S. Amelioration of Intestinal Colitis by Macrophage Migration Inhibitory Factor Isolated from Intestinal Parasites through Toll-like Receptor 2. Parasite Immunol. 2011, 33, 265–275. [Google Scholar] [CrossRef]
- Park, Y.H.; Jeong, M.S.; Ha, K.T.; Yu, H.S.; Jang, S.B. Structural Characterization of As-MIF and HJAB1 during the Inhibition of Cell-Cycle Regulation. BMB Rep. 2017, 50, 269–274. [Google Scholar] [CrossRef]
- Tan, T.H.P.; Edgerton, S.A.V.; Kumari, R.; McAlister, M.S.B.; Rowe, S.M.; Nagl, S.; Pearl, L.H.; Selkirk, M.E.; Bianco, A.E.; Totty, N.F.; et al. Macrophage Migration Inhibitory Factor of the Parasitic Nematode Trichinella spiralis. Biochem. J. 2001, 357, 373–383. [Google Scholar] [CrossRef]
- Cho, Y.; Jones, B.F.; Vermeire, J.J.; Leng, L.; DiFedele, L.; Harrison, L.M.; Xiong, H.; Kwong, Y.K.A.; Chen, Y.; Bucala, R.; et al. Structural and Functional Characterization of a Secreted Hookworm Macrophage Migration Inhibitory Factor (MIF) That Interacts with the Human MIF Receptor CD74. J. Biol. Chem. 2007, 282, 23447–23456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, M.; Wang, S.; Ehsan, M.; Yan, R.; Song, X.; Xu, L.; Li, X. Characterization of a Secreted Macrophage Migration Inhibitory Factor Homologue of the Parasitic Nematode Haemonchus contortus Acting at the Parasite-Host Cell Interface. Oncotarget 2017, 8, 40052–40064. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, A.J.; Bell, N.E.V.; McNeilly, T.N.; Knox, D.P.; Maizels, R.M.; Meikle, L.I.; Wildblood, L.A.; Matthews, J.B. A Macrophage Migration Inhibitory Factor-like Tautomerase from Teladorsagia circumcincta (Nematoda: Strongylida). Parasite Immunol. 2010, 32, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Corpet, F. Nucleic Acids Research Multiple Sequence Alignment with Hierarchical Clustering. Nucl. Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Thiele, M.; Bernhagen, J. Link between Macrophage Migration Inhibitory Factor and Cellular Redox Regulation. Antioxid. Redox Signal. 2005, 7, 1234–1248. [Google Scholar] [CrossRef]
- Taylor, M.J.; Bilo, K.; Cross, H.F.; Archer, J.P.; Underwood, A.P. 168 RDNA Phylogeny and Ultrastructural Characterization of Wolbachia Intracellular Bacteria of the Filarial Nematodes Brugia malayi, B. pahangi, and Wuchereria bancrofti. Exp. Parasitol. 1999, 91, 356–361. [Google Scholar] [CrossRef]
- Lubetsky, J.B.; Swope, M.; Dealwis, C.; Blake, P.; Lolis, E. Pro-1 of Macrophage Migration Inhibitory Factor Functions as a Catalytic Base in the Phenylpyruvate Tautomerase Activity. Biochemistry 1999, 38, 7346–7354. [Google Scholar] [CrossRef]
- Chauhan, N.; Hoti, S.L. Role of Cysteine-58 and Cysteine-95 Residues in the Thiol Di-Sulfide Oxidoreductase Activity of Macrophage Migration Inhibitory Factor-2 of Wuchereria bancrofti. Acta Trop. 2016, 153, 14–20. [Google Scholar] [CrossRef]
- Pennock, J.L.; Behnke, J.M.; Bickle, Q.D.; Devaney, E.; Grencis, R.K.; Isaac, R.E.; Joshua, G.W.P.; Selkirk, M.E.; Zhang, Y.; Meyer, D.J. Rapid Purification and Characterization of l-Dopachrome-Methyl Ester Tautomerase (Macrophage-Migration-Inhibitory Factor) from Trichinella spiralis, Trichuris muris and Brugia pahangi. Biochem. J. 1998, 335, 495–498. [Google Scholar] [CrossRef]
- Leng, L.; Metz, C.N.; Fang, Y.; Xu, J.; Donnelly, S.; Baugh, J.; Delohery, T.; Chen, Y.; Mitchell, R.A.; Bucala, R. MIF Signal Transduction Initiated by Binding to CD74. J. Exp. Med. 2003, 197, 1467–1476. [Google Scholar] [CrossRef]
- Kleemann, R.; Hausser, A.; Geiger, G.; Mischke, R.; Burger-Kentischer, A.; Flieger, O.; Johannes, F.J.; Roger, T.; Calandra, T.; Kapurniotu, A.; et al. Intracellular Action of the Cytokine MIF to Modulate AP-1 Activity and the Cell Cycle through Jab1. Nature 2000, 408, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Coakley, G.; Harris, N.L. Interactions between Macrophages and Helminths. Parasite Immunol. 2020, 42, e12717. [Google Scholar] [CrossRef] [PubMed]
- Filbey, K.J.; Varyani, F.; Harcus, Y.; Hewitson, J.P.; Smyth, D.J.; McSorley, H.J.; Ivens, A.; Nylén, S.; Rottenberg, M.; Löser, S.; et al. Macrophage Migration Inhibitory Factor (MIF) Is Essential for Type 2 Effector Cell Immunity to an Intestinal Helminth Parasite. Front. Immunol. 2019, 10, 2375. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in Infection, Inflammation and Immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Schaible, U.E. Antigen Presentation and Recognition in Bacterial Infections. Curr. Opin. Immunol. 2005, 17, 79–87. [Google Scholar] [CrossRef]
- Grant, E.N.; Wagner, R.; Weiss, K.B. Observations on Emerging Patterns of Asthma in Our Society. J. Allergy Clin. Immunol. 1999, 104, 1–9. [Google Scholar] [CrossRef]
- Vercelli, D. Mechanisms of the Hygiene Hypothesis—Molecular and Otherwise. Curr. Opin. Immunol. 2006, 18, 733–737. [Google Scholar] [CrossRef]
- De Ruiter, K.; Tahapary, D.L.; Sartono, E.; Soewondo, P.; Supali, T.; Smit, J.W.A.; Yazdanbakhsh, M. Helminths, Hygiene Hypothesis and Type 2 Diabetes. Parasite Immunol. 2017, 39, e12404. [Google Scholar] [CrossRef]
- Loke, P.; Lim, Y.A.L. Helminths and the Microbiota: Parts of the Hygiene Hypothesis. Parasite Immunol. 2015, 37, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Zaccone, P.; Raine, T.; Phillips, J.M.; Dunne, D.W. Infection and Autoimmunity: Are We Winning the War, Only to Lose the Peace? Trends Parasitol. 2004, 20, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Feillet, H.; Bach, J.F. Increased Incidence of Inflammatory Bowel Disease: The Price of the Decline of Infectious Burden? Curr. Opin. Gastroenterol. 2004, 20, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.; Fabry, Z. The Hygiene Hypothesis and Multiple Sclerosis. Ann. Neurol. 2007, 61, 85–89. [Google Scholar] [CrossRef]
- Magen, E.; Borkow, G.; Bentwich, Z.; Mishal, J.; Scharf, S. Can Worms Defend Our Hearts? Chronic Helminthic Infections May Attenuate the Development of Cardiovascular Diseases. Med. Hypotheses 2005, 64, 904–909. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.; Yang, Y.; Zhang, L.; Yang, X.; Zhu, X.; Ji, M.; Sun, N.; Su, C. Schistosoma japonicum Egg Antigens Stimulate CD4+ CD25+ T Cells and Modulate Airway Inflammation in a Murine Model of Asthma. Immunology 2007, 120, 8–18. [Google Scholar] [CrossRef]
- Van Die, I.; Cummings, R.D. Glycan Gimmickry by Parasitic Helminths: A Strategy for Modulating the Host Immune Response? Glycobiology 2010, 20, 2–12. [Google Scholar] [CrossRef]
- Khan, W.I.; Blennerhasset, P.A.; Varghese, A.K.; Chowdhury, S.K.; Omsted, P.; Deng, Y.; Collins, S.M. Intestinal Nematode Infection Ameliorates Experimental Colitis in Mice. Infect. Immun. 2002, 70, 5931–5937. [Google Scholar] [CrossRef]
- Ramani, S.; Chauhan, N.; Khatri, V.; Vitali, C.; Kalyanasundaram, R. Wuchereria bancrofti Macrophage Migration Inhibitory Factor-2 (RWbaMIF-2) Ameliorates Experimental Colitis. Parasite Immunol. 2020, 42, e12698. [Google Scholar] [CrossRef]
- Reardon, C.; Sanchez, A.; Hogaboam, C.M.; McKay, D.M. Tapeworm Infection Reduces Epithelial Ion Transport Abnormalities in Murine Dextran Sulfate Sodium-Induced Colitis. Infect. Immun. 2001, 69, 4417–4423. [Google Scholar] [CrossRef]
- Pascoal, V.F.; da Cunha, A.A.; Morassutti, A.L.; Antunes, G.L.; da Silveira, K.A.; Silveira, J.S.; Nuñez, N.K.; de Souza, R.G.; Graeff-Teixeira, C.; Pitrez, P.M. Immunomodulatory Effect of Different Extracts from Angiostrongylus Cantonensis on Airway Inflammation in an Allergic Asthma Model. Parasitol. Res. 2020, 119, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Schabussova, I.; Ul-Haq, O.; Hoflehner, E.; Akgün, J.; Wagner, A.; Loupal, G.; Joachim, A.; Ruttkowski, B.; Maizels, R.M.; Wiedermann, U. Oesophagostomum dentatum Extract Modulates T Cell-Dependent Immune Responses to Bystander Antigens and Prevents the Development of Allergy in Mice. PLoS ONE 2013, 8, e67544. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, H.; Yuan, Y.; Wang, L.; He, W.; Xie, H.; Gao, S.; Cheng, R.; Qian, H.; Jiang, H.; et al. Preventive and Therapeutic Effects of Trichinella spiralis Adult Extracts on Allergic Inflammation in an Experimental Asthma Mouse Model. Parasites Vectors 2019, 12, 326. [Google Scholar] [CrossRef]
- Nisbet, A.J.; McNeilly, T.N.; Wildblood, L.A.; Morrison, A.A.; Bartley, D.J.; Bartley, Y.; Longhi, C.; McKendrick, I.J.; Palarea-Albaladejo, J.; Matthews, J.B. Successful Immunization against a Parasitic Nematode by Vaccination with Recombinant Proteins. Vaccine 2013, 31, 4017–4023. [Google Scholar] [CrossRef]
- Cho, Y.; Vermeire, J.J.; Merkel, J.S.; Leng, L.; Du, X.; Bucala, R.; Cappello, M.; Lolis, E. Drug repositioning and pharmacophore identification in the discovery of hookworm MIF inhibitors. Chem Biol. 2011, 18, 1089–1101. [Google Scholar] [CrossRef]
- Swope, M.; Sun, H.W.; Blake, P.R.; Lolis, E. Direct Link between Cytokine Activity and a Catalytic Site for Macrophage Migration Inhibitory Factor. EMBO J. 1998, 17, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Crichlow, G.V.; Kai, F.C.; Dabideen, D.; Ochani, M.; Aljabari, B.; Pavlov, V.A.; Miller, E.J.; Lolis, E.; Al-Abed, Y. Alternative Chemical Modifications Reverse the Binding Orientation of a Pharmacophore Scaffold in the Active Site of Macrophage Migration Inhibitory Factor. J. Biol. Chem. 2007, 282, 23089–23095. [Google Scholar] [CrossRef]
- Dabideen, D.R.; Cheng, K.F.; Aljabari, B.; Miller, E.J.; Pavlov, V.A.; Al-Abed, Y. Phenolic Hydrazones Are Potent Inhibitors of Macrophage Migration Inhibitory Factor Proinflammatory Activity and Survival Improving Agents in Sepsis 1. J. Med. Chem. 2007, 50, 1993–1997. [Google Scholar] [CrossRef]

| Order | Species | Accession Number | Acronym | References |
|---|---|---|---|---|
| Rhabditida: Onchocercidae | Brugia malayi | U88035.1 | Bm-MIF Bm-MIF-1 | [60,61,62,63] |
| Brugia malayi | AY004865.1 | Bm-MIF-2 | [62,63] | |
| Onchocerca volvulus | AF384027.1 | OvMIF-1 | [56,64] | |
| Onchocerca volvulus | AF384028.1 | OvMIF-2 | [56,64] | |
| Wuchereria bancrofti | AF040629.1 | Wb-MIF Wb-MIF-1 | [60,65] | |
| Wuchereria bancrofti | KJ939449.1 | Wba-MIF-2 | [66] | |
| Rhabditida: Thelaziidae | Thelazia callipaeda | No data | T.ca-MIF | [67] |
| Rhabditida: Anisakidae | Anisakis simplex | EF165010.1 | As-MIF | [3,68,69] |
| Rhabditida: Stronyloididae | Strongyloides ratti | FJ026392.1 | Sra-MIF | [57] |
| Trichinellida: Trichinellidae | Trichinella spiralis | AJ012740.1 | TsMIF | [70] |
| Strongylida: Ancylostomatidae | Ancylostoma ceylanicum | EF410151.1 | AceMIF | [71] |
| Strongylida: Haemonchidae | Haemonchus contortus | CB012470.1 | HCMIF-1 | [72] |
| Ostertagia ostertagii | BQ457911 | Oos-MIF-1.1 | [22] | |
| Teladorsagia circumcincta | FN599526.1 | Tci-MIF-1 | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabowicz, J.; Długosz, E.; Bąska, P.; Wiśniewski, M. Nematode Orthologs of Macrophage Migration Inhibitory Factor (MIF) as Modulators of the Host Immune Response and Potential Therapeutic Targets. Pathogens 2022, 11, 258. https://doi.org/10.3390/pathogens11020258
Karabowicz J, Długosz E, Bąska P, Wiśniewski M. Nematode Orthologs of Macrophage Migration Inhibitory Factor (MIF) as Modulators of the Host Immune Response and Potential Therapeutic Targets. Pathogens. 2022; 11(2):258. https://doi.org/10.3390/pathogens11020258
Chicago/Turabian StyleKarabowicz, Justyna, Ewa Długosz, Piotr Bąska, and Marcin Wiśniewski. 2022. "Nematode Orthologs of Macrophage Migration Inhibitory Factor (MIF) as Modulators of the Host Immune Response and Potential Therapeutic Targets" Pathogens 11, no. 2: 258. https://doi.org/10.3390/pathogens11020258
APA StyleKarabowicz, J., Długosz, E., Bąska, P., & Wiśniewski, M. (2022). Nematode Orthologs of Macrophage Migration Inhibitory Factor (MIF) as Modulators of the Host Immune Response and Potential Therapeutic Targets. Pathogens, 11(2), 258. https://doi.org/10.3390/pathogens11020258