Abstract
Lyme disease (LD) is a common arthropod-borne inflammatory disorder prevalent in the northern hemisphere. LD is caused by a spirochete named Borrelia burgdorferi s.l., which is transmitted to humans by ticks. Climate, environment, and other factors affect land use; recreational-behavior changes affect human contact with infected ticks. Studies in Europe and North America have looked at these aspects, but studies in Asia have not. We searched databases to identify all relevant abstracts published until March 2021. A meta-analysis was undertaken using the standard methods and procedures established by the Cochrane Collaboration. Ninety-one articles were included in our meta-analysis. The literature search identified data from nine countries (China, Japan, Malaysia, Mongolia, Pakistan, Russia Siberia region, South Korea, Thailand and Turkey). Furthermore, 53,003 ticks from six genera (Amblyomma, Dermacentor, Haemaphysalis, Hyalomma, Ixodes and Rhipicephalus) were inspected for infection with B. burgdorferi. The pooled prevalence was 11.1% (95% CI = 8.3–14.2%). Among the nine countries, China had the most studies (56) and Malaysia had the highest infection rate (46.2%). Most infected ticks were from the genera Ixodes and Haemaphysalis. Ticks of the genus Ixodes had the highest infection rate (16.9%). Obvious heterogeneity was noted in our meta-analysis. We analyzed the heterogeneity with regard to countries, genera, time points, and detection methods. This study suggests that Ixodes, Haemaphysalis and Dermacentor may be the most common tike of B. burgdorferi-positive in Asia. The highest proportion of ticks infected by B. burgdorferi were from the genus Ixodes. This meta-analysis is the first attempt to explain the B. burgdorferi infection of hard-body ticks in Asia. The infection rate for each country and infection rate of different tick genera were analyzed: there were large differences between them. The literature is concentrates mainly on East Asia, and data are limited. Our study can provide a reference for a more comprehensive and in-depth investigation of ticks in Asia infected by B. burgdorferi spirochetes.
1. Introduction
Lyme disease (LD) is a tick-borne inflammatory disease caused by infection with Borrelia burgdorferi sensu lato (B. burgdorferi s.l.) complex. LD is of public-health importance in moderate-climate regions of the northern hemisphere, such as North America, Europe, North Africa, and Northern Asia. As landscapes have altered, the number of reported cases have revealed obvious differences in many regions such as Ixodes ricinus, Ixodes persulcatus, etc.
The clinical symptoms of LD can be divided into three stages. Erythema migrans (the most common clinical manifestation) is a typical sign of early acute infection [1]. It is an expanding skin redness that usually develops at the site of a tick bite. Often, several weeks to months after the tick bite, followed by early dissemination and development, B. burgdorferi s.l. can spread to other tissues and organs, and untreated infections can progress to neurologic abnormalities or heart dysfunction [2,3]. Usually, late LD manifests as arthritis or acrodermatitis chronica atrophicans, and is associated with spirochete invasion of joints [4,5,6]. A fatal outcome from LD is extremely rare.
There are several B. burgdorferi s.l. genospecies, and not all strains/genotypes cause LD in humans. B. burgdorferi sensu stricto, Borrelia afzelii, Borrelia garinii, and Borrelia bavariensis are considered to be of pathogenic relevance to humans. Despite cases of LB caused by Borrelia valaisiana, Borrelia lusitaniae, and Borrelia bissettiae have been described, their pathogenic ability has been questioned and their description is occasional. Borrelia mayonii has been recently incorporated in the Americas [7,8]. Globally, three genospecies of B. burgdorferi are principally pathogenic to humans. Borrelia burgdorferi sensu stricto (hereafter referred to as B. burgdorferi) is distributed mainly in the Americas. Borrelia afzelii and Borrelia garinii infections are predominant in LD cases in Europe. B. garinii is the primary cause of LD in Asia [9]. Other species, such as Borrelia bissettii, Borrelia lusitaniae, and Borrelia valaisiana, are also considered to cause human LD, but the prevalence of infections is low and so they are not considered to be major pathogens [10,11]. Interestingly, the genotype of pathogens seems to be the main factor causing the diversity of clinical symptoms of LD. For example, B. afzelii most frequently leads to skin lesions, B. burgdorferi is especially arthritogenic, and B. garinii is linked to neuroborreliosis [12,13].
Different B. burgdorferi s.l. genospecies are transmitted by different genera of ticks, and some ticks can be infected with multiple genospecies of B. burgdorferi s.l. The main vectors transmitting LD-associated spirochetes to humans are Ixodes ricinus in Europe, Ixodes persulcatus in Asia, Ixodes scapularis in eastern North America, and Ixode pacificus in Western North America [14]. These vectors have four life stages (egg, larva, nymph, and adult). In the last three feeding stages, ticks require a blood meal from a variety of mammals, birds, and lizards [13]. The lifecycle of spirochetes in nature is dependent upon horizontal transmission between an infected tick and vertebrate host. Typically, tick larvae acquire spirochetes from infectious hosts via a blood meal. Spirochetes are carried in the midgut of ticks, and transmitted to susceptible host populations through injection of tick saliva during tick feeding. B. burgdorferi replicates in the mammalian dermis, and then disseminates to distant cutaneous sites and other organs, including joints [15].
The risk to humans of infection with Borrelia depends on outdoor recreational activity, on the density of tick populations, and on the infection of the ticks with Borrelia [16]. I.persulcatus is the prevalent vector in the southern forest zone on the Asian side of Eurasis, from the western border of Russia to its far eastern frontier bordering China, Korea, and Japan. However, on the Western sade of Eurasia, most European countries and North Africa harbor I. ricinus. I. ricinus, which is the most common tick species that bites humans in the study area and in most European countries. The jury is still out on the main spirochete transmission tick in Asia [17]. Identification of the genotypes of LD-associated spirochetes, geographic range, and understanding of the distribution of their vectors have essential epidemiologic and clinical importance. Meta-analysis of the prevalence and distribution of B. burgdorferi s.l. genospecies in ticks in Europe has been undertaken but, in Asia, such analyses are lacking [16,18,19]. A number of field studies have already pointed to increases in average densities and activities of questing ticks in parts of Europe with long-documented I. ricinus populations. Studies have identified a strong negative correlation between tick density and altitude, which is related to local climatic conditions [20].
In this study, we aimed to systematically analyze the existing literature on the prevalence of Borrelia burgdorferi in ticks in Asia. Tick prevalence was assessed by tick species, sampling area, and detection methods. The work made crude estimates of tick spirochete infection rates in Asia. It is hoped that this study can provide a more comprehensive and in-depth investigation of ticks infected with Borrelia burgdorferi in Asia.
2. Results
2.1. Search Results and Study Selection
A total of 2254 titles with abstracts were screened, 225 full-text articles were reviewed, and 91 articles were included in this study (Supplementary Materials 3 and 4). Initially, we identified 2254 records through four databases. After elimination of duplicates, 932 records remained. We screened the titles and abstracts and excluded 592 irrelevant records. We scrutinized the full text of the remaining 225 papers for eligibility, of which 130 were excluded. Through screening, we identified data from 91 articles that were suitable for our meta-analysis (56 in English and 35 in Chinese), which reported 91 studies from countries and 160 studies on species. Details of the article-screening procedure and reasons for exclusion are summarized in Figure 1.
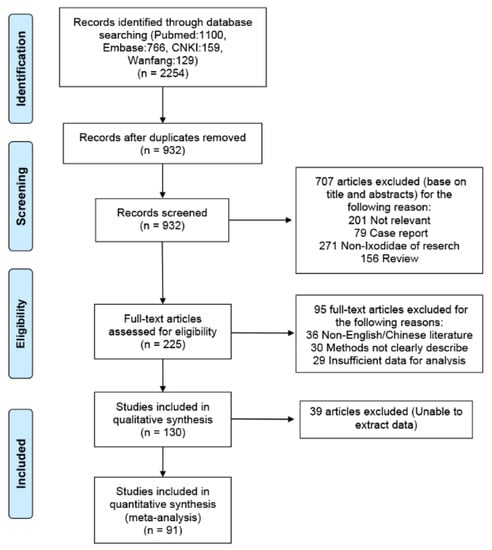
Figure 1.
Flowchart of our study.
2.2. Study Characteristics
Reports identified by the database search were first assessed for eligibility by their titles and abstracts, followed by an in-depth analysis for relevant data regarding B. burgdorferi prevalence in the Ixodid tick family Ixodidae. Of the 91 included studies, 61 (67.00%) had a “low” risk, 30 (33.00%) had a “moderate” risk, and 0 (0.00%) had a “high” risk of bias. The studies were cross-sectional, and 56 studies were reported from China, 12 from Japan, six from Turkey, five from South Korea, four from Mongolia, four from Russia (Siberia), two from Thailand, one from Malaysia, and one from Pakistan. Nine countries reported the rates of ticks infected with B. burgdorferi in Asia, with China and Japan accounting for 74.73% of the total number of studies. Therefore, most data were from East Asia. A total of 53,003 ticks were involved in this meta-analysis, and the number of B. burgdorferi-positive ticks was 7777. Among these ticks, most were from the genera Ixodes and Haemaphysalis, followed by Rhipicephalus, Amblyomma, and Hyalomma. The collected literature was published between 1990 and 2010. Most of the ticks checked were caught between April and July, and most of them were detected by polymerase chain reaction (PCR). The detailed characteristics of each study are provided in Figure 2.
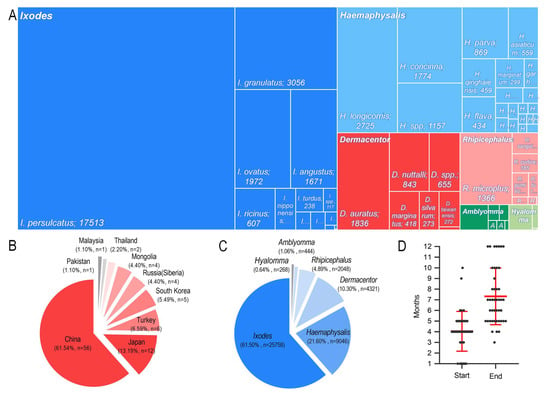
Figure 2.
Study characteristics. (A) Rectangular dendrogram of Ixodid species. (B) Proportion of studies from each country. (C) Proportion of each genus. (D) Tick capture time.
2.3. Pooling and Heterogeneity of Selected Studies
The pooled prevalence was calculated based on a random-effects model, with all studies being included in our meta-analysis. The pooled prevalence of infection by B. burgdorferi was 11.1% (95% confidence interval (CI) = 8.3–14.2%), and significant heterogeneity was found regarding the pooled prevalence (I2 = 0.99; p < 0.001). The rate of infection by B. burgdorferi in ticks among the included studies varied between 0% and 55% (Figure 3).

Figure 3.
Forest plot showing the prevalence of B. burgdorferi s.l. in Ixodidae. Events: Number of Borrelia-positive ticks; Total: Number of ticks detected. Please refer to Supplementary Material 3, 4 for details.
2.4. Country
The estimates of prevalence for different countries and genera, and heterogeneities are presented in Figure 4. Estimates of infection rates for different subgroups and heterogeneities are presented in Table 1. Pooled infection rates for each subgroup were calculated using a random-effects model because of the observed high heterogeneity among studies within subgroups.
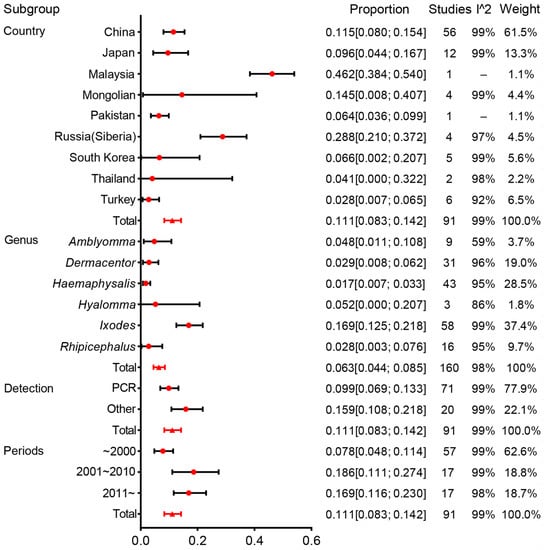
Figure 4.
Forest plot of the prevalence of B. burgdorferi s.l. in Ixodidae by subgroup. Red circles denote the infection rate estimated by random effects meta-analysis and whisker bars denote 95%CI. Subgroups according to country, genus, detection, and periods. Results (bottom line, n = 95) are shown for all included studies. Please refer to Supplementary Material 5 for details.

Table 1.
Influence analysis in meta-analysis.
In the survey on Asian prevalence, 91 studies were conducted from nine countries, and 53,003 ticks were checked. The prevalence results were 11.5% (95%CI, 8.0–15.4%) for China, 9.6% (4.4–16.7%) for Japan, 46.2% (38.4–54.0%) for Malaysia, 14.5% (8.0–40.7%) for Mongolia, 6.4% (3.6–9.9%) for Pakistan, 28.8% (21.0–37.2%) for Russia (Siberia), 6.6% (0.2–20.7%) for South Korea, 4.1% (0.0–32.2%) for Thailand, and 2.8% (0.7–6.5%) for Turkey.
Hence, big differences in the species and genera of Ixodidae in different countries were documented. Ixodes and Haemaphysalis were the main genera in China, Japan, South Korea, Turkey, and Siberia. Dermacentor was the genus with the largest proportion in Mongolia. Rhipicephalus was distributed in China, South Korea, Turkey, and Pakistan, but the Rhipicephalus tested number was relatively small. Amblyomma was distributed mainly in Thailand, South Korea, and Malaysia. Hyalomma was found only in Turkey and Pakistan. In all studies, China accounted for 61.5% of the weight and contributed significantly to the results of the study. Only one study was done in Malaysia, so we had doubts about the high rate of infection documented in that study (Figure 5).
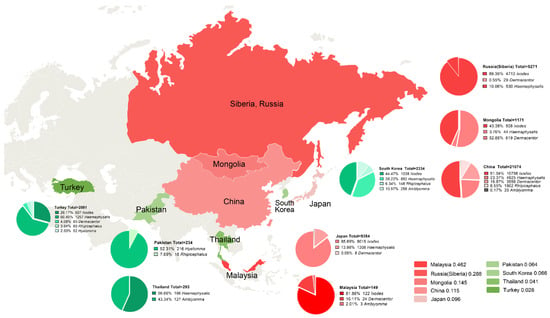
Figure 5.
Distribution of included studies. Red: Top five countries with tick infection; Green: Tick infection rate after four countries; Pie chart: Percentage of tick species per country.
2.5. Genus
In our analyses, 41,885 ticks were identified to genera, and the prevalence at the genus level could be calculated. Ixodes had an infection rate of 16.9% (95% CI, 12.5–21.8%), whereas it was 1.7% (0.7–3.3%) for Haemaphysalis, 2.9% (0.8–6.2%) for Dermacentor, 2.8% (0.3–7.6%) for Rhipicephalus, 4.8% (1.1–10.8%) for Amblyomma, and 5.2% (0.0–20.7%) for Hyalomma. Of tick genera infected with B. burgdorferi (which explained 36.1% of the heterogeneity), an infection rate of 16.9% from the Ixodes genus was higher than that of other genera. Among the genus were classified further, and I. persulcatus and I. granulatus were the most numerous and had a higher infection rate. The tick species with the most frequently identified in Amblyomma, Dermacentor, Rhipicephalus, and Hyalomma were Amblyomma variegatum, Dermacentor auratus, Rhipicephalus microplus, and Hyalomma anatolicum, and the infection rate was 8.2%, 2.7%, 2.1%, and 6.6%, respectively (Figure 6).
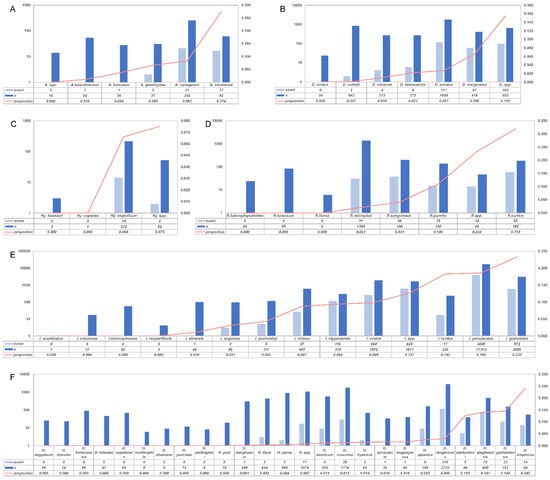
Figure 6.
Infection rate according to species. (A): Amblyomma infection rates; (B): Dermacentor infection rates; (C): Hyalomma infection rates; (D): Rhipicephalus infection rates; (E): Ixodes infection rates; (F): Haemaphysalis infection rates; event: ticks that test positive for spirochetes were shown as light blue; n: total number of ticks detected were shown as blue; proportion: Borrelia-positive rate.
The meta-regression analysis revealed that the country, genus, period of publication, and detection methods were the source of heterogeneity (Table 1).
2.6. Publication Bias
Egger’s linear regression test was undertaken and a funnel plot was constructed to examine the publication bias (Supplementary Information). They showed that the studies had a symmetrical distribution. Egger’s test (t = −0.181, p = 0.857) did not show a significant value.
3. Discussion
LD occurs most frequently in the Northern Hemisphere, where some ticks of the Ixodidae family are present. Each year, ~300,000 people in the USA and ≤85,000 people in Europe are infected with B. burgdorferi s.l. and suffer LD [21,22]. Although the true incidence of LD in Asian populations is not known, its distribution appears to be widening.
Ticks are ectoparasites that carry multiple pathogens. They transmit these pathogens to humans and animals. Persistent and relapsing infection as well as long-term sequelae caused by tick-borne pathogens worsen human health further. As the infection rate is very high, animal husbandry is a global economic burden. [23,24].
One of the most notable functions of ticks is that they serve as vectors of LD. In LD, there is a dynamic interplay between spirochetes, vectors, and reservoir hosts. The spirochetes involved in LD hold a wide range of reservoir hosts, so clarifying the distribution of infected ticks in Asia could help for estimating the prevalence of B. burgdorferi s.l. and improve the prevention and control of LD.
Ticks transmit a wide range of pathogens into humans and animals. In North America, I. scapularis and I. pacificus have been shown to be vectors of the major LD-causing spirochete B. burgdorferi s.l. I. ricinus and I. persulcatus have been confirmed experimentally to be the carriers of LD-causing spirochetes in Eurasia [25,26]. Due to the genetic diversity of ticks, the relative abundance of certain pathogens is quite different across different tick genera [27]. None of the eight tick species from three genera (one species from the genus Amblyomma, five from Dermacentor, and two from Haemaphysalis) evaluated to date have been unequivocally and experimentally confirmed to be vectors of B. burgdorferi s.l. spirochetes [25,28,29]. The host specialization and/or vector compatibility of LD spirochetes may affect the distribution of spirochetes of different genospecies.
The genetic structure and pathogen composition of different tick genera are affected mainly by ecologic and geographic factors. For example, H. longicornis is a widely distributed tick species indigenous to eastern Asia, whereas Hyalomma asiaticum prefers to live in desert or semi-desert environments, I. ricinus is distributed widely at high altitudes [19,30,31]. Within an endemic area, the risk of infection by B. burgdorferi s.l. in humans is determined by the local abundance and infection rate of vector ticks, and by human behavior that affects the likelihood of being bitten. Research on tick genera infected with spirochetes helps public-health agencies make strategies to prevent LD.
With a total area of land and population, China is the largest country in Asia. China has a total area of ~9.6 million km2, which is almost the size of Europe. Due to influencing factors such as the size of geographic area and number of reports on infected ticks, our included data were concentrated mainly in East Asia (especially China). With tick activity, LD shows relatively constant regional characteristics and seasonal peaks. The habitat types of ticks and local microclimate determine the abundance of infected ticks, which affects LD prevalence. Numerous tick species are expanding beyond their historical distribution range and invading new regions, and the increase in the number of human cases of tick-borne disease is concomitant with such an expansion [32,33]. We showed that the typical habitats of uninfected/infected ticks were woodlands and grasslands in regions with mild climates, which tallies with the geographic range of LD transmission. These habitats provide sufficient humidity for the development and survival of ticks and vertebrate hosts. We demonstrated that ticks usually become active from spring to late summer, which is consistent with the peak incidence of LD in humans. LD in humans is also correlated with meteorological conditions that influence tick feeding and human behavior, such as temperature, humidity, and rainfall [19,34,35]. Ticks usually feed on blood meals in the summer, which is the same time that recreation by humans increases. In areas with a high incidence of ticks, the annual average temperature is stable at 6.85–16.85 °C, and spring vegetation is lush [19].
Understanding the distribution of ticks spcies can help in the prevention and diagnosis of LD. Equally important, differences among genospecies of B. burgdorferi s.l. are thought to cause variability in the clinical symptoms of LD in different geographic areas. We analyzed the geographic and genus distribution of ticks infected with B. burgdorferi s.l. Furthermore, our study contains data primarily on China, which are not generalizable to other large areas. I. persulcatus and I. granulatus were the most numerous and had a higher infection rate. Consistent with previous research, I.persulcatus is the prevalent vector in the southern forest zone on the Asian side of Eurasis, from the western border of Russia to its far eastern frontier bordering China, Korea, and Japan. In Southeast Asia and West Asia, tick infection rates are low and data collection is low, and more studies should be added. In conclusion, this meta-analysis is the first attempt to explain the B. burgdorferi infection of hard-body ticks in Asia. Our study can provide a reference for more comprehensive and in-depth investigations of ticks in Asia infected by B. burgdorferi spirochetes.
4. Materials and Methods
4.1. Search Strategy
In this meta-analysis, two independent investigators searched PubMed, Excerpta Medica Database (Embase), the China National Knowledge Infrastructure, and Wanfang databases to identify all relevant abstracts published until March 2021. The key search terms were “Ixodes” OR “Ixodidae” OR “Tick” AND “Borrelia” AND “Asia”. Titles and abstracts of articles retrieved from the literature search were screened independently by two investigators. The full text of potentially eligible studies were obtained and assessed further for final inclusion. A third investigator analyzed any inconsistent results to resolve discrepancies.
4.2. Literature Search and Data Extraction
Studies were considered eligible only if they: (i) were carried out within Asia; (ii) were a surveillance report or cross-sectional study, neither experimental studies nor review articles; (iii) the study object was Ixodidae; (iv) were written in English or Chinese.
The exclusion criteria were: (i) incomplete data; (ii) the study was a review, case report, or comment to editors (lacking primary data); (iii) the study was a repeated publication.
After training, two individuals reviewed the abstracts independently and identified articles for detailed assessment. In case of disagreement, the two parties discussed and resolved the issue or referred it to a third researcher for a final decision. Then, they extracted data from each included study and entered the results into a database. Data on the first author, year of publication, country, sample-collection sites, screening test used, sample size, and number of infections were extracted.
4.3. Quality of Evidence and Risk of Bias
The methodological quality of included studies was evaluated using the tool developed by Hoy and colleagues [36]. A score of 1 (“yes”) or 0 (“no”) was assigned for each item. Scores were summed across items to generate an overall quality score that ranged from 0 to 10 (Supplementary Information). Then, studies were classified as having a low (>8), moderate [6,7,8], or high (≤5) risk of bias. Four investigators independently assessed the methodological quality of one-quarter of included studies each, and all assessments were reviewed independently by a fifth investigator, with disagreements being resolved through consensus.
4.4. Data Analyses
Extracted data were entered into Excel 2016 within Office (Microsoft, Redmond, WA, USA). The meta-analysis was conducted using the “meta” package in R 3.1.3 (R Project for Statistical Computing, Vienna, Austria) to estimate the prevalence of B. burgdorferi in ticks from Ixodidae. We used valid classification lists of tick genera [37]. Our study was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [38]. The PRISMA checklist (Supplementary Information) was used as the basis for inclusion of relevant information.
A forest plot and funnel plot were generated to judge the overall effect size and ascertain if publication bias was present. Heterogeneity among studies was assessed using Cochran’s Q test (reported as p-values), which is quantified by I2 values. If there was evidence of heterogeneity (I2 > 50%), infection rates were combined using a random-effects model; otherwise, infection rates were combined using a fixed-effects model. Unadjusted prevalence was recalculated on the basis of the information of crude numerators and denominators provided by individual studies. The pooled prevalence and its 95%CI of B. burgdorferi in Ixodidae were calculated with the Freeman–Tukey double arcsine transformation [39]. Publication bias was assessed using Egger’s test and funnel plots [40].
The effects of heterogeneity on seroprevalence estimates were examined by subgroup and meta-regression analyses. Such analyses were undertaken on different variables: country, tick genus, species-detection method, and time the research was done.
5. Conclusions
This meta-analysis is the first attempt to explain the B. burgdorferi infection of Ixodid ticks in Asia. The infection rate for each country and infection rate of different tick genera were analyzed: there were large differences between them. The literature is concentrates mainly on East Asia, and data are limited. Our study can provide a reference for a more comprehensive and in-depth investigations of ticks in Asia infected by B. burgdorferi spirochetes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens11020143/s1, Supplementary Material 1: Assessment of risk of bias; Supplementary Material 2: PRISMA checklist; Supplementary Material 3: Meta-analysis included articles; Supplementary Material 4: Study characteristic; Supplementary Material 5: Asia prevalence of B. burgdorferi in Ixodidae by subgroup(country); Supplementary Material 6: Asia prevalence of B. burgdorferi in Ixodidae by subgroup(genus).
Author Contributions
Conceptualization, F.B. and A.L.; methodology, F.B. and A.L.; software, Z.J., J.K., B.L., and Y.D.; validation, J.K., B.L., and Y.D.; formal analysis, Z.J.; investigation, M.J. and M.L.; data curation, Z.J., P.Y., Y.P., J.Y., S.W., W.C. and G.Z.; resources, Y.F.; writing—original draft preparation, Z.J., J.C.; writing—review and editing, All; visualization, M.L.; supervision, X.X., Y.Z., and X.S.; project administration, Z.J., F.B. and A.L.; All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (No. 32060180, 81860644, 81560596, and 31560051) and Yunnan Province Department of Science and Technology-Kunming Medical University Joint Fundation [No. 2019FE001 (-002) and 2017FE467 (-001)].
Institutional Review Board Statement
Due to the nature of the survey, formal approval from an Ethics Committee was not a requirement at the time of the survey.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this survey are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China and Yunnan Province Department of Science and Technology-Kunming Medical University Joint Fund Projects. The funding institutions had no involvement in the design of the study or review of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steere, A.C. Lyme disease. N. Engl. J. Med. 2001, 345, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Fingerle, V.; Pfister, H.W. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat. Rev. Neurol. 2015, 11, 446–456. [Google Scholar] [CrossRef]
- Yeung, C.; Baranchuk, A. Diagnosis and treatment of Lyme carditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Glickstein, L. Elucidation of Lyme arthritis. Nat. Rev. Immunol. 2004, 4, 143–152. [Google Scholar] [CrossRef]
- Sanchez, E.; Vannier, E.; Wormser, G.P.; Hu, L.T. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: A review. JAMA 2016, 315, 1767–1777. [Google Scholar] [CrossRef]
- Stanek, G.; Strle, F. Lyme borreliosis—From tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 2018, 42, 233–258. [Google Scholar] [CrossRef] [Green Version]
- Krieg, N.R.S.; Staley, J.T.; Brown, D.R.; Hedlund, B.P.; Paster, B.J.; Ward, N.L.; Ludwig, W.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology. In Vol. 4: The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes.2; Springer: Berlin/Heidelberg, Germany, 2011; pp. 484–531. [Google Scholar]
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Mysterud, A.; Easterday, W.R.; Stigum, V.M.; Aas, A.B.; Meisingset, E.L.; Viljugrein, H. Contrasting emergence of Lyme disease across ecosystems. Nat. Commun. 2016, 7, 11882. [Google Scholar] [CrossRef]
- Földvári, G.; Farkas, R.; Lakos, A. Borrelia spielmanii erythema migrans, Hungary. Emerg. Infect. Dis. 2005, 11, 1794–1795. [Google Scholar] [CrossRef] [PubMed]
- Collares-Pereira, M.; Couceiro, S.; Franca, I.; Kurtenbach, K.; Schäfer, S.M.; Vitorino, L.; Gonçalves, L.; Baptista, S.; Vieira, M.L.; Cunha, C. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 2004, 42, 1316–1318. [Google Scholar] [CrossRef] [Green Version]
- Stanek, G.; Fingerle, V.; Hunfeld, K.P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O'Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerar, T.; Strle, F.; Stupica, D.; Ruzic-Sabljic, E.; McHugh, G.; Steere, A.C.; Strle, K. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto ttrains from Europe and the United States. Emerg. Infect. Dis. 2016, 22, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Rauter, C.; Hartung, T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Appl. Environ. Microbiol. 2005, 71, 7203–7216. [Google Scholar] [CrossRef] [Green Version]
- Masuzawa, T. Terrestrial distribution of the Lyme borreliosis agent Borrelia burgdorferi sensu lato in East Asia. Jpn. J. Infect. Dis. 2004, 57, 229–235. [Google Scholar] [PubMed]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Peña, A.; Cutler, S.; Potkonjak, A.; Vassier-Tussaut, M.; Van Bortel, W.; Zeller, H.; Fernández-Ruiz, N.; Mihalca, A.D. An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int. J. Health Geogr. 2018, 17, 41–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Fang, Q.Q.; Keirans, J.E.; Durden, L.A. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J. Parasitol. 2003, 89, 452–457. [Google Scholar] [CrossRef]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Sun, Y.; Zhan, W.; Jiang, J.F.; Wang, Q.; Zhang, B.; Ji, P.; et al. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell 2020, 182, 1328–1340.e13. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.A.; Zeidner, N.S.; Beard, C.B.; Dolan, M.C.; Dietrich, G.; Piesman, J. Kinetics of Borrelia burgdorferi infection in larvae of refractory and competent tick vectors. J. Med. Entomol. 2006, 43, 61–67. [Google Scholar] [CrossRef]
- Sun, T.; Pan, W.; Song, Y.; Zhang, J.; Wang, J.; Dai, J. Functional characterization of two defensins, HlDFS1 and HlDFS2, from the hard tick Haemaphysalis longicornis. Parasites Vectors 2017, 10, 455. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X.; et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet Health 2020, 4, e320–e329. [Google Scholar] [CrossRef]
- Kugeler, K.J.; Farley, G.M.; Forrester, J.D.; Mead, P.S. Geographic distribution and expansion of human lyme disease, United States. Emerg. Infect. Dis. 2015, 21, 1455–1457. [Google Scholar] [CrossRef]
- Bisanzio, D.; Fernández, M.P.; Martello, E.; Reithinger, R.; Diuk-Wasser, M.A. Current and future spatiotemporal patterns of Lyme disease reporting in the northeastern United States. JAMA Netw. Open 2020, 3, e200319. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.M.; Eisen, R.J.; Monaghan, A.; Mead, P. Meteorological influences on the seasonality of Lyme disease in the United States. Am. J. Trop. Med. Hyg. 2014, 90, 486–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fingerle, V.; Michel, H.; Hettche, G.; Hizo-Teufel, C.; Wilske, B. Borrelia burgdorferi s.l. OspA-types are widespread in Bavaria but show distinct local patterns. Int. J. Med. Microbiol. 2004, 293, 165–166. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Horak, I.G.; Camicas, J.L.; Keirans, J.E. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): A world list of valid tick names. Exp. Appl. Acarol. 2002, 28, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 7, e1000097. [Google Scholar]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Hinckley, A.F.; Connally, N.P.; Meek, J.I.; Johnson, B.J.; Kemperman, M.M.; Feldman, K.A.; White, J.L.; Mead, P.S. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis. 2014, 59, 676–681. [Google Scholar] [CrossRef]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in reported vectorborne disease cases—United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Mac, S.; Bahia, S.; Simbulan, F.; Pullenayegum, E.M.; Evans, G.A.; Patel, S.N.; Sander, B. Long-term sequelae and health-related quality of life associated with Lyme disease: A systematic review. Clin. Infect. Dis. 2020, 71, 440–452. [Google Scholar] [CrossRef]
- Barker, S.C.; Walker, A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 2014, 1–144. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).