Differential Role of Type 2 Diabetes as a Risk Factor for Tuberculosis in the Elderly versus Younger Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Study Population and Age Categories
2.2. Regional Registry for Tamaulipas, Mexico
2.3. Data Analysis
3. Results
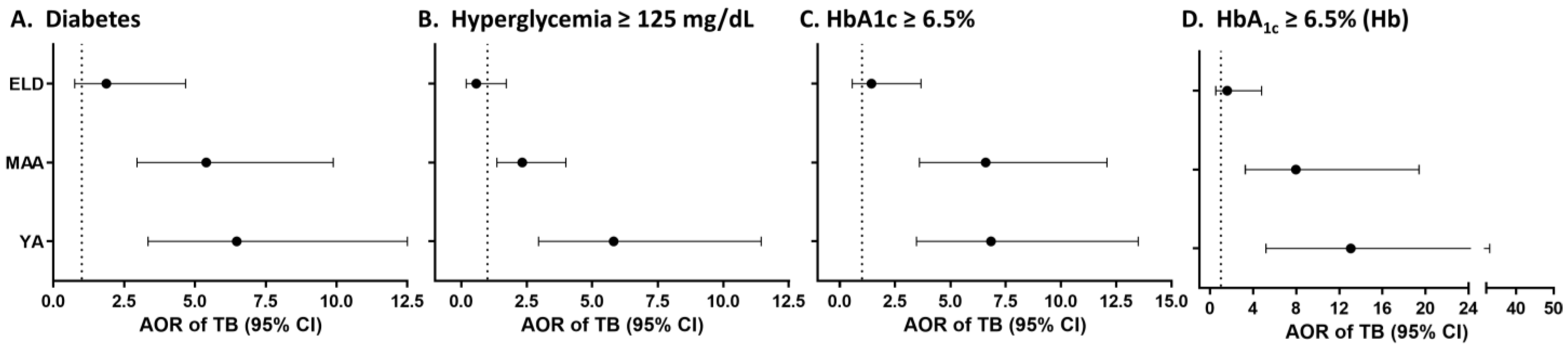
3.1. Strength of the Association between TB and T2D by Age
3.2. Validation Using Data from the TB Control Program for Tamaulipas, Mexico
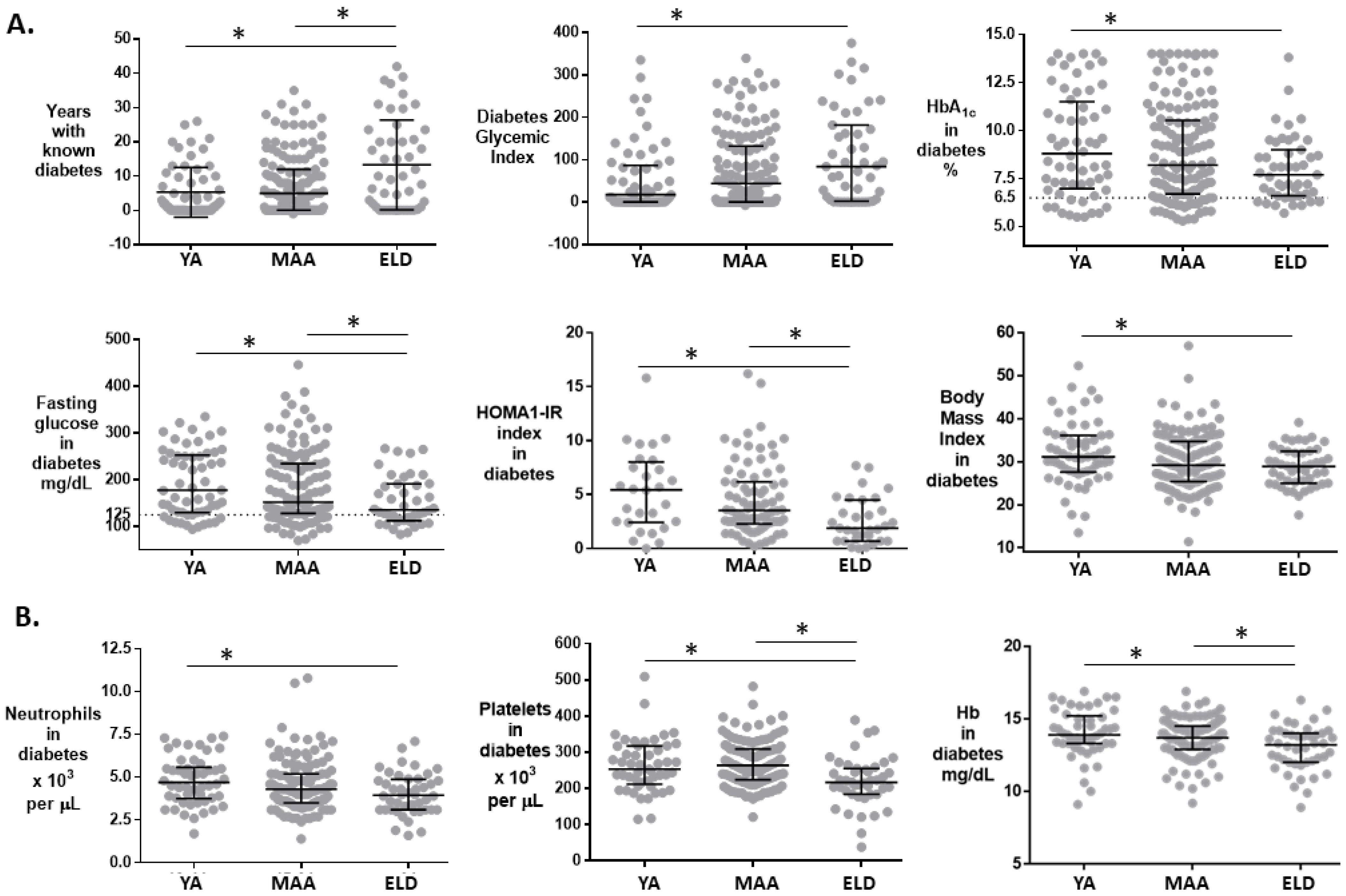
3.3. Differences in T2D Patients between ELD vs. YA/MAA
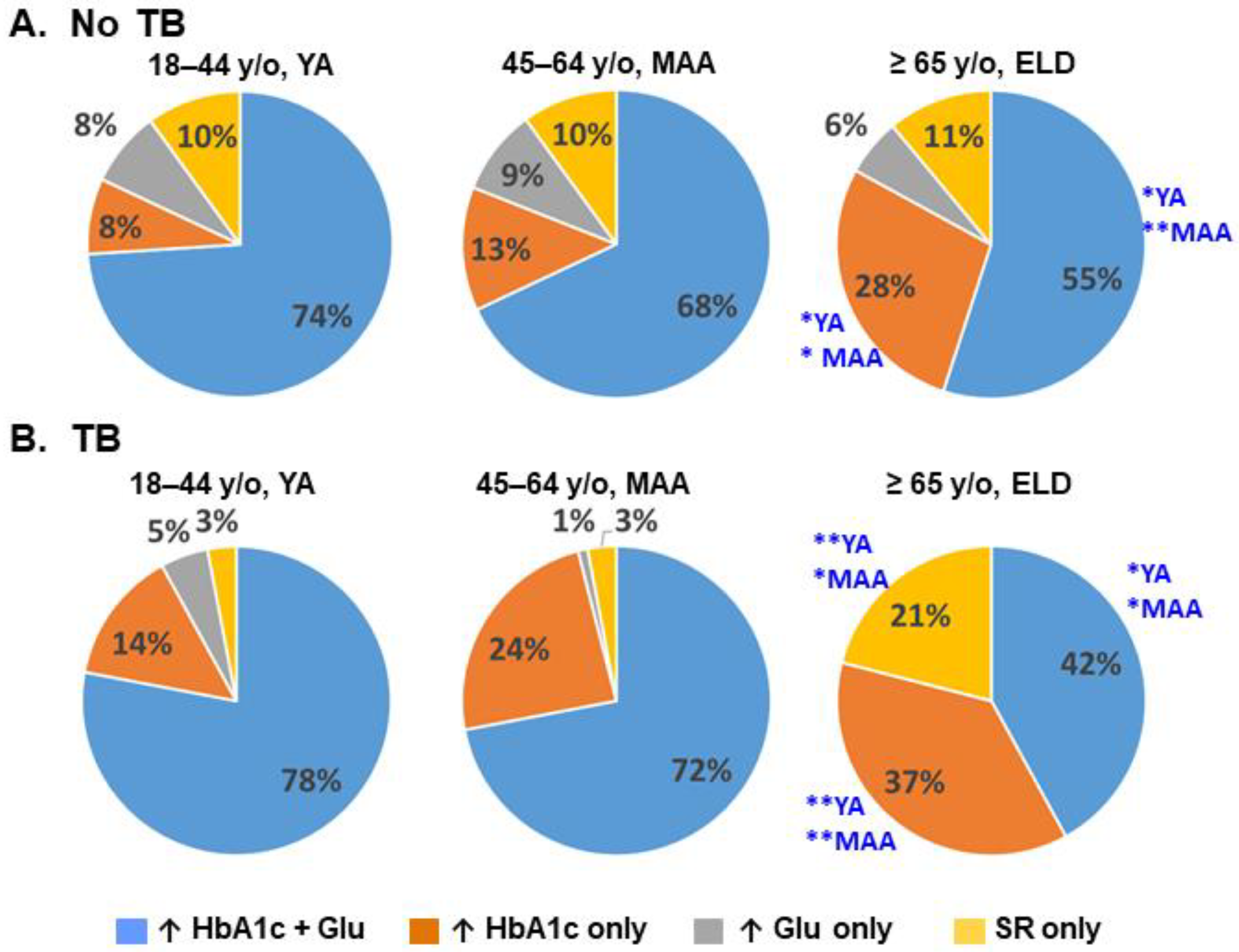
3.4. Relationships between Level of Glucose Control and TB Risk
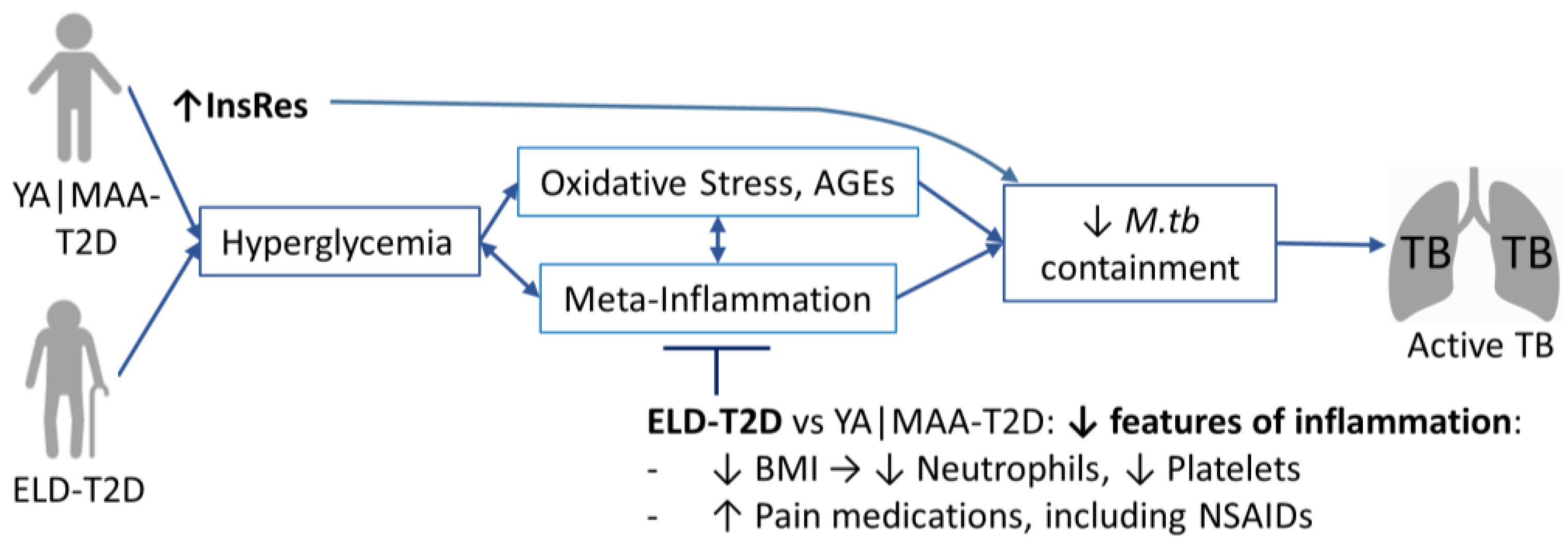
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadena, J.; Rathinavelu, S.; Lopez-Alvarenga, J.C.; Restrepo, B.I. The re-emerging association between tuberculosis and diabetes: Lessons from past centuries. Tuberculosis 2019, 116S, S89–S97. [Google Scholar] [CrossRef]
- Critchley, J.A.; Restrepo, B.I.; Ronacher, K.; Kapur, A.; Bremer, A.A.; Schlesinger, L.S.; Basaraba, R.; Kornfeld, H.; van Crevel, R. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 1: Epidemiology and Clinical Management. Chest 2017, 152, 165–173. [Google Scholar] [CrossRef]
- Awad, S.F.; Dargham, S.R.; Omori, R.; Pearson, F.; Critchley, J.A.; Abu-Raddad, L.J. Analytical Exploration of Potential Pathways by which Diabetes Mellitus Impacts Tuberculosis Epidemiology. Sci. Rep. 2019, 9, 8494. [Google Scholar] [CrossRef]
- Huangfu, P.; Ugarte-Gil, C.; Golub, J.; Pearson, F.; Critchley, J. The effects of diabetes on tuberculosis treatment outcomes: An updated systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 783–796. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Golden, S.H.; Cefalu, W.T. Diabetes and Aging: Unique Considerations and Goals of Care. Diabetes Care 2017, 40, 440–443. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Horsburgh, C.R., Jr. Prevention of tuberculosis in older adults in the United States: Obstacles and opportunities. Clin. Infect. Dis. 2013, 56, 1240–1247. [Google Scholar] [CrossRef]
- Ponce-De-Leon, A.; Garcia-Garcia Md Mde, L.; Garcia-Sancho, M.C.; Gomez-Perez, F.J.; Valdespino-Gomez, J.L.; Olaiz-Fernandez, G.; Rojas, R.; Ferreyra-Reyes, L.; Cano-Arellano, B.; Bobadilla, M.; et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care 2004, 27, 1584–1590. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Murray, M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008, 5, e181. [Google Scholar]
- Kim, S.J.; Hong, Y.P.; Lew, W.J.; Yang, S.C.; Lee, E.G. Incidence of pulmonary tuberculosis among diabetics. Tuber. Lung Dis. 1995, 76, 529–533. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Camerlin, A.J.; Rahbar, M.H.; Wang, W.; Restrepo, M.A.; Zarate, I.; Mora-Guzman, F.; Crespo-Solis, J.G.; Briggs, J.; McCormick, J.B.; et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis cases. Bull. World Health Organ. 2011, 89, 352–359. [Google Scholar] [CrossRef]
- Scordo, J.M.; Aguillon-Duran, G.P.; Ayala, D.; Quirino-Cerrillo, A.P.; Rodriguez-Reyna, E.; Mora-Guzman, F.; Caso, J.A.; Ledezma-Campos, E.; Schlesinger, L.S.; Torrelles, J.B.; et al. A prospective cross-sectional study of tuberculosis in elderly Hispanics reveals that BCG vaccination at birth is protective whereas diabetes is not a risk factor. PLoS ONE 2021, 16, e0255194. [Google Scholar] [CrossRef]
- Kaplan, G.A.; Haan, M.N.; Wallace, R.B. Understanding changing risk factor associations with increasing age in adults. Annu. Rev. Public Health 1999, 20, 89–108. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice, C.; American Diabetes Association Professional Practice, C.; Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Pickup, J.C. Innate immunity, insulin resistance and type 2 diabetes. Diabetologia 2012, 55, 273–278. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Kleynhans, L.; Salinas, A.B.; Abdelbary, B.E.; Tshivhula, H.; Aguillon, G.; Kunsevi, C.; Salinas, G.; Malherbe, S.; Garcia-Viveros, M.; et al. Diabetes screen during tuberculosis contact investigations highlights opportunity for diabetes diagnosis and reveals metabolic differences between ethnic groups. Tuberculosis 2018, 113, 10–18. [Google Scholar] [CrossRef]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Meigs, J.B.; Bonadonna, R.C.; Muggeo, M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: The Bruneck study. Diabetes Care 2007, 30, 318–324. [Google Scholar] [CrossRef]
- Abdelbary, B.E.; Garcia-Viveros, M.; Ramirez-Oropesa, H.; Rahbar, M.H.; Restrepo, B.I. Tuberculosis-diabetes epidemiology in the border and non-border regions of Tamaulipas, Mexico. Tuberculosis 2016, 101S, S124–S134. [Google Scholar] [CrossRef]
- Lonnroth, K.; Jaramillo, E.; Williams, B.G.; Dye, C.; Raviglione, M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc. Sci. Med. 2009, 68, 2240–2246. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Fisher-Hoch, S.P.; Crespo, J.G.; Whitney, E.; Perez, A.; Smith, B.; McCormick, J.B.; Nuevo Santander Tuberculosis, T. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol. Infect. 2007, 135, 483–491. [Google Scholar] [CrossRef]
- INEGI. Censo de Poblacion y Vivienda 2010; Instituto Nacional de Estadística, Geografía e Informática: Ciudad de Mexico, Mexico, 2015.
- Instituto Nacional de Salud Pública. ENSANUT-Encuesta Nacional de Salud y Nutrición 2012. In Resultados Por Entidad Federativa; Instituto Nacional de Salud Pública: Tamaulipas, Mexico, 2013. [Google Scholar]
- Zocchetti, C.; Consonni, D.; Bertazzi, P.A. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int. J. Epidemiol. 1997, 26, 220–223. [Google Scholar] [CrossRef]
- Al-Rifai, R.H.; Pearson, F.; Critchley, J.A.; Abu-Raddad, L.J. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0187967. [Google Scholar] [CrossRef]
- Scordo, J.M.; Aguillon-Duran, G.P.; Ayala, D.; Quirino-Cerrillo, A.P.; Rodriguez-Reyna, E.; Joya-Ayala, M.; Mora-Guzman, F.; Ledezma-Campos, E.; Villafanez, A.; Schlesinger, L.S.; et al. Interferon Gamma Release Assays for Detection of Latent Mycobacterium tuberculosis in Elderly Hispanics. Int. J. Infect. Dis. 2021, 111, 85–91. [Google Scholar] [CrossRef]
- Calderon, R.I.; Arriaga, M.B.; Lopez, K.; Barreda, N.N.; Sanabria, O.M.; Froes Neto, J.F.; Araujo, D.N.; Lecca, L.; Andrade, B.B. High prevalence and heterogeneity of Dysglycemia in patients with tuberculosis from Peru: A prospective cohort study. BMC Infect. Dis. 2019, 19, 799. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.T. The global burden of tuberculosis: Results from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2018, 18, 261–284. [Google Scholar] [CrossRef]
- Liu, Q.; You, N.; Pan, H.; Shen, Y.; Lu, P.; Wang, J.; Lu, W.; Zhu, L.; Martinez, L. Glycemic Trajectories and Treatment Outcomes of Patients with Newly Diagnosed Tuberculosis: A Prospective Study in Eastern China. Am. J. Respir. Crit. Care Med. 2021, 204, 347–356. [Google Scholar] [CrossRef]
- Tan, K.S.; Lee, K.O.; Low, K.C.; Gamage, A.M.; Liu, Y.; Tan, G.Y.; Koh, H.Q.; Alonso, S.; Gan, Y.H. Glutathione deficiency in type 2 diabetes impairs cytokine responses and control of intracellular bacteria. J. Clin. Investig. 2012, 122, 2289–2300. [Google Scholar] [CrossRef]
- Ronacher, K.; van Crevel, R.; Critchley, J.A.; Bremer, A.A.; Schlesinger, L.S.; Kapur, A.; Basaraba, R.; Kornfeld, H.; Restrepo, B.I. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 2: Underlying Biologic Mechanisms. Chest 2017, 152, 174–180. [Google Scholar] [CrossRef]
- Martinez, N.; Ketheesan, N.; West, K.; Vallerskog, T.; Kornfeld, H. Impaired Recognition of Mycobacterium tuberculosis by Alveolar Macrophages From Diabetic Mice. J. Infect. Dis. 2016, 214, 1629–1637. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Schlesinger, L.S. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis 2013, 93, S10–S14. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Twahirwa, M.; Rahbar, M.H.; Schlesinger, L.S. Phagocytosis via Complement or Fc-Gamma Receptors Is Compromised in Monocytes from Type 2 Diabetes Patients with Chronic Hyperglycemia. PLoS ONE 2014, 9, e92977. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Beever, A.; Kachour, N.; Owens, J.; Sasaninia, K.; Kolloli, A.; Kumar, R.; Ramasamy, S.; Sisliyan, C.; Khamas, W.; Subbian, S.; et al. L-GSH Supplementation in Conjunction With Rifampicin Augments the Treatment Response to Mycobacterium tuberculosis in a Diabetic Mouse Model. Front. Pharmacol. 2022, 13, 879729. [Google Scholar] [CrossRef]
- Kewcharoenwong, C.; Prabowo, S.A.; Bancroft, G.J.; Fletcher, H.A.; Lertmemongkolchai, G. Glibenclamide Reduces Primary Human Monocyte Functions Against Tuberculosis Infection by Enhancing M2 Polarization. Front. Immunol. 2018, 9, 2109. [Google Scholar] [CrossRef]
- Kewcharoenwong, C.; Saenwongsa, W.; Willcocks, S.J.; Bancroft, G.J.; Fletcher, H.A.; Lertmemongkolchai, G. Glibenclamide alters interleukin-8 and interleukin-1beta of primary human monocytes from diabetes patients against Mycobacterium tuberculosis infection. Tuberculosis 2020, 123, 101939. [Google Scholar] [CrossRef]
- Herrera, M.T.; Gonzalez, Y.; Hernandez-Sanchez, F.; Fabian-San Miguel, G.; Torres, M. Low serum vitamin D levels in type 2 diabetes patients are associated with decreased mycobacterial activity. BMC Infect. Dis. 2017, 17, 610. [Google Scholar] [CrossRef]
- Piergallini, T.J.; Turner, J. Tuberculosis in the elderly: Why inflammation matters. Exp. Gerontol. 2018, 105, 32–39. [Google Scholar] [CrossRef]
- Wang, W.; Du, Z.; Ni, M.; Wang, Z.; Liang, M.; Sheng, H.; Zhang, A.; Yang, J. Aspirin enhances the clinical efficacy of anti-tuberculosis therapy in pulmonary tuberculosis in patients with type 2 diabetes mellitus. Infect. Dis. 2020, 52, 721–729. [Google Scholar] [CrossRef]
- Fox, K.A.; Kirwan, D.E.; Whittington, A.M.; Krishnan, N.; Robertson, B.D.; Gilman, R.H.; Lopez, J.W.; Singh, S.; Porter, J.C.; Friedland, J.S. Platelets Regulate Pulmonary Inflammation and Tissue Destruction in Tuberculosis. Am. J. Respir. Crit. Care Med. 2018, 198, 245–255. [Google Scholar] [CrossRef]
- Dorhoi, A.; Iannaccone, M.; Maertzdorf, J.; Nouailles, G.; Weiner, J., III; Kaufmann, S.H. Reverse Translation in Tuberculosis: Neutrophils Provide Clues for Understanding Development of Active Disease. Front. Immunol. 2014, 5, 36. [Google Scholar] [CrossRef]
- Kroesen, V.M.; Groschel, M.I.; Martinson, N.; Zumla, A.; Maeurer, M.; van der Werf, T.S.; Vilaplana, C. Non-Steroidal Anti-inflammatory Drugs As Host-Directed Therapy for Tuberculosis: A Systematic Review. Front. Immunol. 2017, 8, 772. [Google Scholar] [CrossRef]
- Canan, C.H.; Gokhale, N.S.; Carruthers, B.; Lafuse, W.P.; Schlesinger, L.S.; Torrelles, J.B.; Turner, J. Characterization of lung inflammation and its impact on macrophage function in aging. J. Leukoc. Biol. 2014, 96, 473–480. [Google Scholar] [CrossRef]
- Mavros, Y.; Simar, D.; Singh, M.A. Glucose Tranporter-4 expression in monocytes: A systematic review. Diabetes Res. Clin. Pract. 2009, 84, 123–131. [Google Scholar] [CrossRef]
- Fischer, H.J.; Sie, C.; Schumann, E.; Witte, A.K.; Dressel, R.; van den Brandt, J.; Reichardt, H.M. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J. Immunol. 2017, 198, 1910–1920. [Google Scholar] [CrossRef]
- Walrand, S.; Guillet, C.; Boirie, Y.; Vasson, M.P. Insulin differentially regulates monocyte and polymorphonuclear neutrophil functions in healthy young and elderly humans. J. Clin. Endocrinol. Metab. 2006, 91, 2738–2748. [Google Scholar] [CrossRef]
- Shi, L.; Jiang, Q.; Bushkin, Y.; Subbian, S.; Tyagi, S. Biphasic Dynamics of Macrophage Immunometabolism during Mycobacterium tuberculosis Infection. mBio 2019, 10, e02550-18. [Google Scholar] [CrossRef]
- Boillat-Blanco, N.; Ramaiya, K.L.; Mganga, M.; Minja, L.T.; Bovet, P.; Schindler, C.; Von Eckardstein, A.; Gagneux, S.; Daubenberger, C.; Reither, K.; et al. Transient Hyperglycemia in Patients With Tuberculosis in Tanzania: Implications for Diabetes Screening Algorithms. J. Infect. Dis. 2016, 213, 1163–1172. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004, 328, 1519. [Google Scholar] [CrossRef]
- Burusie, A.; Enquesilassie, F.; Addissie, A.; Dessalegn, B.; Lamaro, T. Effect of smoking on tuberculosis treatment outcomes: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0239333. [Google Scholar] [CrossRef]


| All (≥18 y/o) | YA (18–44 y/o) | MAA (45–64 y/o) | ELD (≥65 y/o) | YA vs. MAA vs. ELD | |
|---|---|---|---|---|---|
| n = 913 | n = 455 | n = 333 | n = 125 | p Value | |
| Sociodemographics | |||||
| Male sex | 294 (32.1%) | 155 (33.8%) | 106 (31.7%) | 33 (26.4%) | 0.263 |
| Education, High school or higher | 354 (38.6%) | 242 (52.8%) | 99 (29.6%) | 13 (10.4%) | <0.001 |
| Current or past smoker | 260 (28.4%) | 131 (28.6%) | 96 (28.7%) | 33 (26.4%) | 0.868 |
| TB-related variables | |||||
| Recent TB exposure | 676 (73.7%) | 363 (79.3%) | 246 (73.7%) | 67 (53.6%) | <0.001 |
| Past TB | 23 (2.5%) | 11 (2.4%) | 8 (2.4%) | 4 (3.2%) | 0.872 |
| Latent TB infection | 507 (55.3%) | 238 (52%) | 191 (57.2%) | 78 (62.4%) | 0.093 |
| BCG vaccination | 821 (89.5%) | 420 (91.7%) | 290 (86.8%) | 111 (88.8%) | 0.136 |
| Type 2 diabetes and other conditions | |||||
| Type 2 diabetes | 242 (26.4%) | 60 (13.1%) | 135 (40.4%) | 47 (37.6%) | <0.001 |
| Overweight/obese, BMI ≥ 25 | 705 (76.9%) | 354 (77.3%) | 263 (78.7%) | 88 (70.4%) | 0.184 |
| Central obesity (M ≥ 0.90 M; F ≥ 0.86) | 712 (77.6%) | 328 (71.6%) | 282 (84.4%) | 102 (81.6%) | <0.001 |
| High cholesterol (200 mg/dL) | 153 (16.7%) | 42 (9.2%) | 85 (25.5%) | 26 (20.8%) | <0.001 |
| High LDL (100 mg/dL) | 388 (42.3%) | 147 (32.1%) | 180 (53.9%) | 61 (48.8%) | <0.001 |
| Low HDL (40 M, 50 F, mg/dL) | 655 (71.4%) | 349 (76.2%) | 221 (66.2%) | 85 (68.0%) | 0.006 |
| High Triglycerides (150 mg/dL) | 268 (29.2%) | 113 (24.7%) | 117 (35.0%) | 38 (30.4%) | 0.006 |
| Macrovascular diseases | 234 (25.5%) | 41 (9.0%) | 114 (34.1%) | 79 (63.2%) | <0.001 |
| Microvascular diseases | 212 (23.1%) | 58 (12.7%) | 98 (29.3%) | 56 (44.8%) | <0.001 |
| Anti-inflammatory medications | 171 (18.7%) | 66 (14.4%) | 65 (19.5%) | 40 (32%) | <0.001 |
| All (≥18 y/o) | YA (18–44 y/o) | MAA (45–64 y/o) | ELD (≥65 y/o) | YA vs. MAA vs. ELD | |
|---|---|---|---|---|---|
| n = 243 | n = 105 | n = 97 | n = 41 | p Value | |
| Sociodemographics | |||||
| Male sex | 151 (62.1%) | 58 (55.2%) | 67 (69.1%) | 26 (63.4%) | 0.067 |
| Education, High school or higher | 63 (25.9%) | 35 (33.3%) | 25 (25.8%) | 3 (7.3%) | 0.006 |
| Current or past smoker | 101 (41.6%) | 37 (35.2%) | 46 (47.4%) | 18 (43.9%) | 0.223 |
| TB-related variables | |||||
| Recent TB exposure | N/A | ||||
| Past TB | 18 (7.4%) | 8 (7.6%) | 5 (5.2%) | 5 (12.2%) | 0.467 |
| Latent TB infection | N/A | ||||
| BCG vaccination | 200 (82.3%) | 91 (86.7%) | 81 (83.5%) | 28 (68.3%) | 0.025 |
| Type 2 diabetes and other conditions | |||||
| Type 2 diabetes | 122 (50.2%) | 36 (34.3%) | 67 (69.1%) | 19 (46.3%) | <0.001 |
| Overweight/obese, BMI ≥ 25 | 63 (25.9%) | 24 (22.9%) | 34 (35.1%) | 5 (12.2%) | 0.010 |
| Central obesity (M ≥ 0.90 M; F ≥ 0.86) | 149 (61.3%) | 53 (50.5%) | 66 (68%) | 30 (73.2%) | 0.013 |
| High cholesterol (200 mg/dL) | 10 (4.1%) | 4 (3.8%) | 4 (4.1%) | 2 (4.9%) | 0.948 |
| High LDL (100 mg/dL) | 38 (15.6%) | 17 (16.2%) | 15 (15.5%) | 6 (14.6%) | 0.955 |
| Low HDL (40 M, 50 F, mg/dL) | 178 (73.3%) | 81 (77.1%) | 70 (72.2%) | 27 (65.9%) | 0.268 |
| High Triglycerides (150 mg/dL) | 22 (9.1%) | 7 (6.7%) | 13 (13.4%) | 2 (4.9%) | 0.221 |
| Macrovascular diseases | 53 (21.8%) | 13 (12.4%) | 23 (23.7%) | 17 (41.5%) | <0.001 |
| Microvascular diseases | 88 (36.2%) | 24 (22.9%) | 49 (50.5%) | 15 (36.6%) | <0.001 |
| Anti-inflammatory medications | 73 (30%) | 31 (29.5%) | 28 (28.9%) | 14 (34.2%) | 0.625 |
| All (≥18 y/o) | YA (18–44 y/o) | MAA (45–64 y/o) | ELD (≥65 y/o) | YA vs. MAA vs. ELD | |
|---|---|---|---|---|---|
| n = 242 | n = 60 | n = 135 | n = 47 | p Value | |
| Sociodemographics and TB | |||||
| Male sex | 71 (29.5%) | 25 (41.7%) | 35 (26.1%) | 11 (23.4%) | 0.054 |
| Education, High school or higher | 70 (29.1%) | 29 (48.3%) | 38 (28.4%) | 3 (6.4%) | <0.001 |
| Current smoker | 30 (12.4%) | 9 (3.7%) | 18 (7.4%) | 3 (1.2%) | 0.359 |
| NSAID use | 54 (24.2%) | 7 (13%) | 32 (26.0%) | 15 (32.6%) | 0.057 |
| BCG vaccination | 216 (89.3%) | 53 (88.3%) | 120 (89.6%) | 43 (91.5%) | 0.868 |
| Latent TB infection | 139 (57.7%) | 32 (53.3%) | 80 (59.7%) | 27 (57.5%) | 0.708 |
| Diabetes history | |||||
| Family history of diabetes | 197 (81.7%) | 52 (86.7%) | 111 (82.8%) | 34 (72.3%) | 0.145 |
| Self-reported diabetes | 179 (74.3%) | 40 (66.7%) | 103 (76.9%) | 36 (76.6%) | 0.298 |
| Years with diabetes | 4 (12.9) | 1.8 (9.3) | 5 (11.9) | 10 (23.29) | 0.003 |
| Glucose management | |||||
| Hyperglycemia, 2 levels at 125 mg/dL | 182 (75.5%) | 49 (81.7%) | 104 (77.6%) | 29 (61.7%) | 0.041 |
| HbA1c (%) | 8.1 (3.8) | 8.8 (4.3) | 8.2 (3.8) | 7.7 (2.4) | 0.106 |
| Glycemic Index (HbA1c * T2D Years) | 37.6 (125.6) | 17.8 (84.5) | 43.9 (131.2) | 84.0 (179.8) | 0.025 |
| Insulin Levels (mU/L) | 9.2 (12.0) | 12.7 (13.5) | 8.5 (10.9) | 7.7 (12.8) | 0.203 |
| HOMA-IR index | 3.3 (4.3) | 5.4 (5.4) | 3.5 (3.8) | 1.8 (3.7) | 0.002 |
| Diabetes medications in past month | |||||
| Any | 156 (65.2%) | 31 (51.7%) | 92 (68.2%) | 33 (70.2%) | 0.056 |
| Insulin | 38 (15.8%) | 8 (13.3%) | 20 (14.8%) | 10 (21.3%) | 0.487 |
| Metformin | 123 (51%) | 24 (40.0%) | 76 (56.3%) | 23 (48.9%) | 0.106 |
| Sulfonylureas | 83 (34.3%) | 18 (18.3%) | 49 (36.3%) | 23 (48.9%) | 0.003 |
| Metformin + Sulfonylureas | 65 (26.9%) | 9 (15%) | 40 (26.6%) | 16 (34%) | 0.048 |
| Diabetes-associated conditions | |||||
| Body-mass index | 29.6 (8.6) | 31.2 (8.5) | 29.2 (9.3) | 28.9 (7.5) | 0.032 |
| Central obesity (M ≥ 0.90 M; F ≥ 0.86) | 212 (88%) | 52 (86.7%) | 123 (91.8%) | 37 (78.7%) | 0.012 |
| Macrovascular diseases | 109 (45.2%) | 15 (25.0%) | 61 (45.5%) | 33 (70.2%) | <0.001 |
| Microvascular diseases | 113 (46.9%) | 26 (43.3%) | 61 (45.5%) | 26 (55.3%) | 0.418 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restrepo, B.I.; Scordo, J.M.; Aguillón-Durán, G.P.; Ayala, D.; Quirino-Cerrillo, A.P.; Loera-Salazar, R.; Cruz-González, A.; Caso, J.A.; Joya-Ayala, M.; García-Oropesa, E.M.; et al. Differential Role of Type 2 Diabetes as a Risk Factor for Tuberculosis in the Elderly versus Younger Adults. Pathogens 2022, 11, 1551. https://doi.org/10.3390/pathogens11121551
Restrepo BI, Scordo JM, Aguillón-Durán GP, Ayala D, Quirino-Cerrillo AP, Loera-Salazar R, Cruz-González A, Caso JA, Joya-Ayala M, García-Oropesa EM, et al. Differential Role of Type 2 Diabetes as a Risk Factor for Tuberculosis in the Elderly versus Younger Adults. Pathogens. 2022; 11(12):1551. https://doi.org/10.3390/pathogens11121551
Chicago/Turabian StyleRestrepo, Blanca I., Julia M. Scordo, Génesis P. Aguillón-Durán, Doris Ayala, Ana Paulina Quirino-Cerrillo, Raúl Loera-Salazar, America Cruz-González, Jose A. Caso, Mateo Joya-Ayala, Esperanza M. García-Oropesa, and et al. 2022. "Differential Role of Type 2 Diabetes as a Risk Factor for Tuberculosis in the Elderly versus Younger Adults" Pathogens 11, no. 12: 1551. https://doi.org/10.3390/pathogens11121551
APA StyleRestrepo, B. I., Scordo, J. M., Aguillón-Durán, G. P., Ayala, D., Quirino-Cerrillo, A. P., Loera-Salazar, R., Cruz-González, A., Caso, J. A., Joya-Ayala, M., García-Oropesa, E. M., Salinas, A. B., Martinez, L., Schlesinger, L. S., Torrelles, J. B., & Turner, J. (2022). Differential Role of Type 2 Diabetes as a Risk Factor for Tuberculosis in the Elderly versus Younger Adults. Pathogens, 11(12), 1551. https://doi.org/10.3390/pathogens11121551







