Survey on Limnic Gastropods: Relationships between Human Health and Conservation
Abstract
1. Introduction
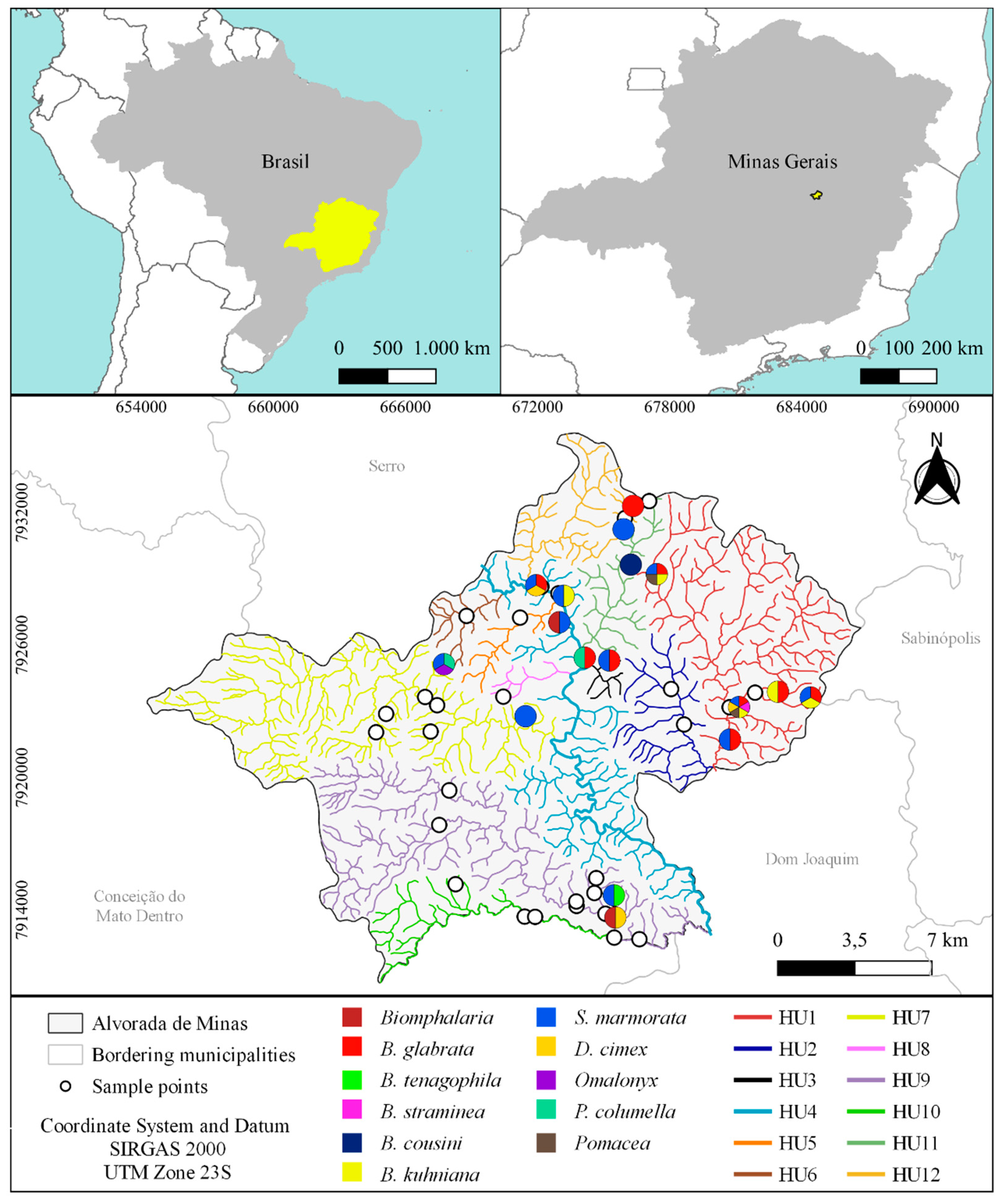
2. Materials and Methods
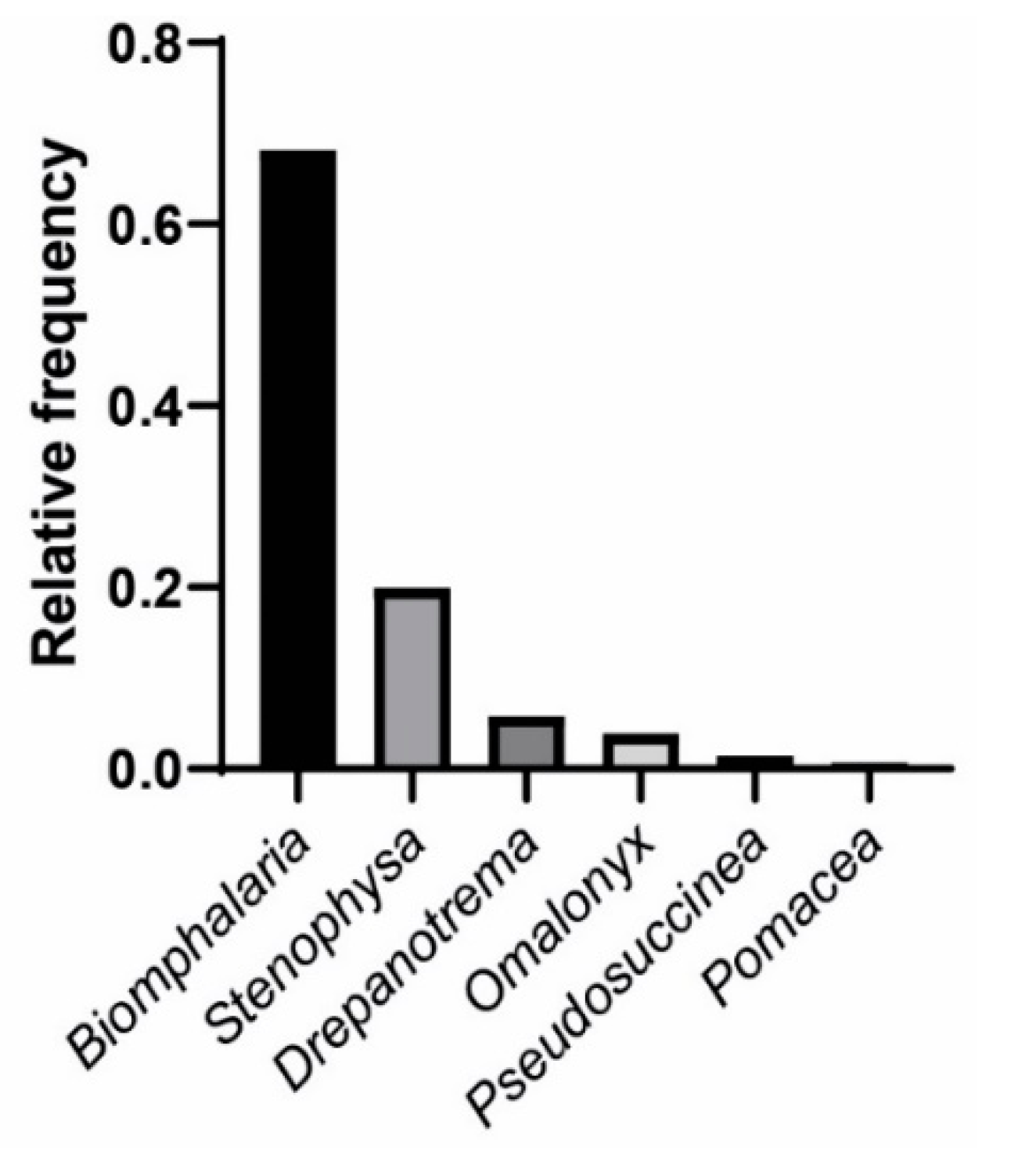
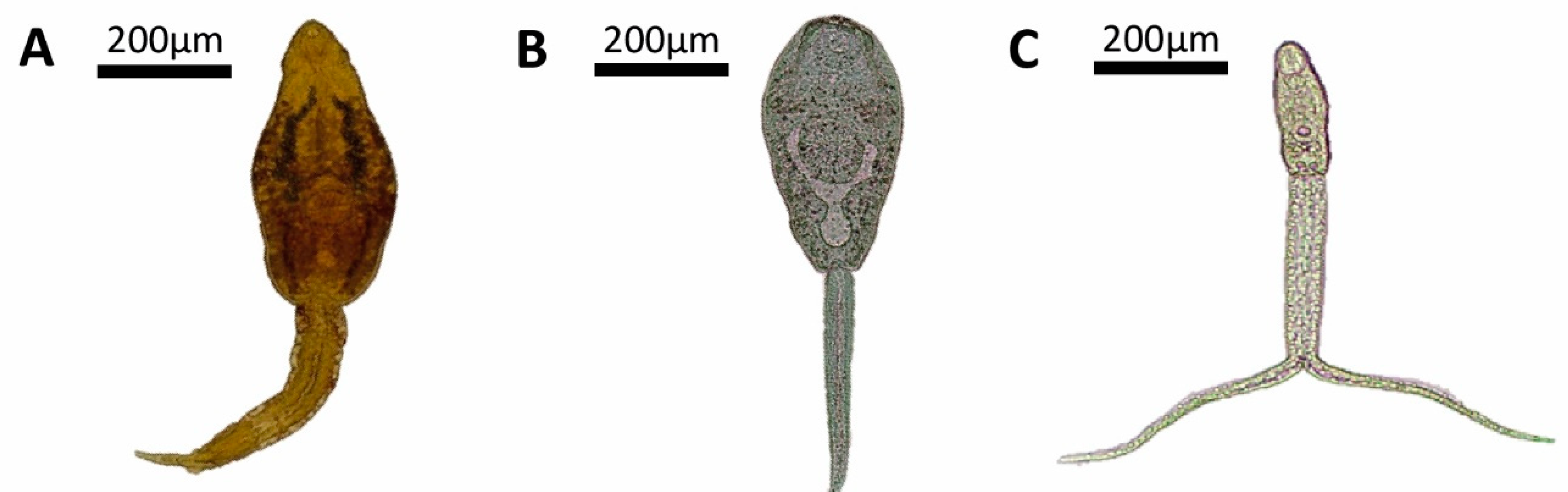
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Maltchik, L.; Stenert, C.; Kotzian, C.; Pereira, D. Responses of freshwater molluscs to environmental factors in Southern Brazil wetlands. Braz. J. Biol. 2010, 70, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Inda, L.A.; Consoni, J.M.; Konrad, H.G. Composição e abundância de espécies de moluscos do bentos marginal da microbacia do arroio Capivara, Triunfo, RS, Brasil. Biociências 2001, 9, 3–20. [Google Scholar]
- Queiroz, J.F.; Trivinho-Strixino, S.; Nascimento, V.M.D.C. Organismos Bentônicos Bioindicadores da qualidade das Águas da Bacia do Médio São Francisco; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2000. [Google Scholar]
- Giannelli, A.; Cantacessi, C.; Colella, V.; Dantas-Torres, F.; Otranto, D. Gastropod-Borne Helminths: A Look at the Snail–Parasite Interplay. Trends Parasitol. 2016, 32, 255–264. [Google Scholar] [CrossRef]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The Global Decline of Nonmarine Mollusks. Bioscience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Amaral, A.C.Z.; Ribeiro, C.V.; Mansur, M.C.D.; Paiva, A.W.E.; Mattews-Cascon, H.; Leite, F.P.P.; de Melo, G.A.S.; Coelho, P.A.; Buckup, L.; Ventura, C.R.R. A situação de ameaça dos invertebrados aquáticos do Brasil. Livro Vermelho Fauna Bras. Ameaçada Extinção 2008, 1, 156–165. [Google Scholar]
- Dos Santos, S.B.; Thiengo, S.C.; Fernandez, M.A.; Miyahira, I.C.; Gonçalves, I.C.B.; Ximenes, R.D.F.; Mansur, M.C.D.; Pereira, D. Espécies de moluscos límnicos invasores no Brasil. Moluscos Límnicos Invasores No Bras. Biol. Prevenção Controle 2012, 25–49. [Google Scholar]
- Miyahira, I.C.; Santos, S.B.; Mansur, M.C.D.; Carneiro, J.B. Freshwater mussels in Brazil: Past, present and future, at least, we hope they have one. Am. Conchol. 2012, 40, 16–18. [Google Scholar]
- Metzger, J.P.; Casatti, L. Do diagnóstico à conservação da biodiversidade: O estado da arte do programa BIOTA/FAPESP. Biota Neotrop. 2006, 6. [Google Scholar] [CrossRef]
- Pinto, H.A.; De Melo, A.L. Larvas de trematódeos em moluscos do Brasil: Panorama e perpectivas após um século de estudos. Rev. Patol. Trop. 2014, 42. [Google Scholar] [CrossRef]
- Pinto, H.A.; De Melo, A.L. A checklist of cercariae (Trematoda: Digenea) in molluscs from Brazil. Zootaxa 2013, 3666, 449. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da saúde (MS). Vigilância e Controle de Moluscos de Importância Epidemiológica; 2008. Available online: https://www.arca.fiocruz.br/handle/icict/40259 (accessed on 4 October 2022)ISBN 9788533414389.
- Cantanhede, S.P.D.; Fernandez, M.A.; de Mattos, A.C.; Montresor, L.C.; Silva-Souza, N.; Thiengo, S.C. Freshwater gastropods of the Baixada Maranhense Microregion, an endemic area for schistosomiasis in the State of Maranhão, Brazil: I—Qualitative study. Rev. Soc. Bras. Med. Trop. 2014, 47, 79–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiff, C. Why reinvent the wheel? Lessons in schistosomiasis control from the past. PLoS Negl. Trop. Dis. 2017, 11, e0005812. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.M.Z.; Caldeira, R.L. Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect. Dis. Poverty 2016, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, Q.; Tchuenté, L.-A.T.; Bergquist, R.; Sacko, M.; Utzinger, J.; Lin, D.-D.; Yang, K.; Zhang, L.-J.; Wang, Q.; et al. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infect. Dis. 2016, 16, 376–383. [Google Scholar] [CrossRef]
- Rekha, K.; Anbalagan, S.; Dinakaran, S. Distributional ecology of snails (Gastropoda: Mollusca) in seasonal ponds of Tamil Nadu, South India. Acta Ecol. Sin. 2021, 41, 410–415. [Google Scholar] [CrossRef]
- Olivier, L.; Schneiderman, M. A method for estimating the density of aquatic snail populations. Exp. Parasitol. 1956, 5, 109–117. [Google Scholar] [CrossRef]
- Lobato, P. Lymnaea Viatrix: A Study of Tropotypic Specimens (Mollusca: Lymnaeidae). Rev. Bras. Biol. 1976, 36, 419–428. [Google Scholar]
- Molluscabase.org. Molluscabase—Lymnaea Columella Say, 1817. Available online: https://www.molluscabase.org/aphia.php?p=taxdetails&id=724461 (accessed on 30 May 2022).
- de Simone, L.R.L. Land and Freshwater Mollusks of Brazil: An Illustrated Inventory on the Brazilian Malacofauna, Including Neighbor Regions of the South America, Respect to the Terrestrial and Freshwater Ecosystem; Museu de Zoologia, Universidade de São Paulo: São Paulo, Brazil, 2006. [Google Scholar]
- Paraense, W.L. Physa Marmorata Guilding, 1828 (Pulmonata: Physidae). Mem. Inst. Oswaldo Cruz 1986, 81, 459–469. [Google Scholar] [CrossRef]
- Núñez, V. Revisión de dos especies de Physidae. Rev. Mex. Biodivers. 2011, 82, 93–108. [Google Scholar] [CrossRef]
- Deslandes, N. Técnica de dissecação e exame de planorbídeos. Rev. Serv. Espec. Saude Publica 1951, 4, 371–382. [Google Scholar]
- Paraense, W.L. Estado atual da sistemática dos planorbídeos brasileiros (Mollusca, Gastropoda). Arq. Mus. Nac. Rio Jan. 1975, 55, 105–128. [Google Scholar]
- Vidigal, T.H.; Caldeira, R.L.; Simpson, A.J.; Carvalho, O.S. Further studies on the molecular systematics of Biomphalaria snails from Brazil. Mem. Inst. Oswaldo Cruz 2000, 95, 57–66. [Google Scholar] [CrossRef]
- Caldeira, R.L.; Teodoro, T.M.; Jannotti-Passos, L.K.; Lira-Moreira, P.M.; Goveia, C.D.O.; Carvalho, O.D.S. Characterization of South American Snails of the Genus Biomphalaria (Basommatophora: Planorbidae) and Schistosoma mansoni (Platyhelminthes: Trematoda) in Molluscs by PCR-RFLP. Biomed Res. Int. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, R.E. Effect of light and temperature on shedding of Schistosoma mansoni cercariae. Eff. Light Temp. Shedding Schistosoma Mansom Cercariae 1946, 7–16. [Google Scholar]
- Kostadinova, A.; Gibson, D.I. The systematics of the echinostomes. In Echinostomes as Experimental Models for Biological Research; Fried, B., Graczyk, T.K., Eds.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 31–57. [Google Scholar]
- Ellenberg, D.; Mueller-Dombois, D. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; W. H. Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Scott, A.J.; Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: Hoboken, NJ, USA, 1991; Volume 47, ISBN 9780470582473. [Google Scholar]
- Ter Braak, C.J.F.; Looman, C.W.N. Weighted averaging, logistic regression and the Gaussian response model. Vegetatio 1986, 65, 3–11. [Google Scholar] [CrossRef]
- Medeiros, C.; Scholte, R.G.C.; D’Ávila, S.; Caldeira, R.L.; Carvalho, O.D.S. Distribuição espacial de Lymnaeidae (Mollusca, Basommatophora), hospedeiros intermediários de Fasciola hepatica Linnaeus, 1758 (Trematoda, Digenea) no Brasil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 235–252. [Google Scholar] [CrossRef]
- Palasio, R.G.S.; Xavier, I.G.; Chiaravalotti-Neto, F.; Tuan, R. Diversity of Biomphalaria spp. freshwater snails and associated mollusks in areas with schistosomiasis risk, using molecular and spatial analysis tools. Biota Neotrop. 2019, 19. [Google Scholar] [CrossRef]
- Coelho, P.R.S.; Ker, F.T.O.; Araújo, A.D.; Guimarães, R.J.P.S.; Negrão-Corrêa, D.A.; Caldeira, R.L.; Geiger, S.M. Identification of Risk Areas for Intestinal Schistosomiasis, Based on Malacological and Environmental Data and on Reported Human Cases. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Carvalho, O.D.S.; de Mendonça, C.L.F.; Marcelino, J.M.D.R.; Passos, L.K.J.; Fernandez, M.A.; Leal, R.D.S.; Caldeira, R.L.; Scholte, R.G.C.; Carmo, E.H.; Mesquita, S.G.; et al. Distribuição geográfica dos hospedeiros intermediários do Schistosoma mansoni nos estados do Paraná, Minas Gerais, Bahia, Pernambuco e Rio Grande do Norte, 2012–2014. Epidemiol. Serv. Saude Rev. Sist. Unico Saude Bras. 2018, 27, e2017343. [Google Scholar] [CrossRef]
- Fürst, T.; Duthaler, U.; Sripa, B.; Utzinger, J.; Keiser, J. Trematode Infections: Liver and lung flukes. Infect. Dis. Clin. N. Am. 2012, 26, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.D.S.; Soares, L.R.M.; Barçante, T.A. Occurrence of Fasciola hepatica (Linnaeus, 1758) infection in Brazilian cattle of Minas Gerais, Brazil. Rev. Bras. Parasitol. Veterinária 2009, 18, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, M.; Charlier, J.; Lauwers, L.; Vercruysse, J.; Van Huylenbroeck, G.; Van Meensel, J. Conceptual framework for analysing farm-specific economic effects of helminth infections in ruminants and control strategies. Prev. Vet. Med. 2013, 109, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Dracz, R.M.; Ribeiro, V.M.A.; Pereira, C.A.D.J.; Lima, W.D.S. Occurrence of Fasciola hepatica (Linnaeus, 1758) in capybara (Hydrochoerus hydrochaeris) (Linnaeus, 1766) in Minas Gerais, Brazil. Rev. Bras. Parasitol. Veterinária 2016, 25, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.P.N.; Costa, A.L.O.; Becattini, R.M.; Ferreira, M.A.N.D.; Paixão, H.P.R.D.; Coscarelli, D.; Vidigal, T.H.D.A.; Lima, W.D.S.; Pereira, C.A.D.J. Integrative taxonomy: Combining molecular and morphological characteristics to identify Lymnaea (Galba) cubensis, intermediate host of Fasciola hepatica. Rev. Bras. Parasitol. Veterinária 2021, 30. [Google Scholar] [CrossRef]
- Costa, C.H.C.; Romijn, P.C.; Pinheiro, J.G.; Kimura, L.M.S.; Nascimento, R.F.; Magalhães, H.; Gonçalves, W.M.; Bruno, L.M.P.; Bruno, A.M.P.; Carvalho, L.; et al. Desenvolvimento sustentável e vigilância ambiental em propriedades rurais de agricultura familiar, com a equação de situações de risco da ocorrência de doenças infectocontagiosas no noroeste do Estado do Rio de Janeiro. In Conhecimento, Tecnologia e Inovação para o Fortalecimento da Agricultura Familiar: Contribuicoes das Organizacoes Estaduais de Pesquisa Agropecuaria; Ministério do Desenvolvimento Agrário: Brasília, Brazil, 2014; pp. 241–246. [Google Scholar]
- Serra-Freire, N.M. Fasciolose no Brasil: Aspectos epidemiológicos. In Tópicos em Malacologia—Ecos do XIX EBRAM; Fernandez, M.A., Santos, S.B., Pimenta, A.D., Thiengo, S.C., Eds.; Technical Books Editora: Rio de Janeiro, Brasil, 2011; pp. 208–220. [Google Scholar]
- Molluscabase.org. Molluscabase—Physa Marmorata Guilding, 1828. Available online: https://www.molluscabase.org/aphia.php?p=taxdetails&id=848621 (accessed on 24 April 2022).
- Taylor, D.W. Introduction to Physidae (Gastropoda: Hygrophila); biogeography, classification, morphology. Rev. Biol. Trop. 2003, 265–287. [Google Scholar]
- ICMBio. Instituto Chico Mendes de Conservação da Biodiversidade. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção—Volume I, 1st ed.; Instituto Chico Mendes de Conservação da Biodiversidade/Ministério do Meio Ambiente: Brasília, Brazil, 2018; ISBN 9788561842796.
- ICMBio. Instituto Chico Mendes de Conservação da Biodiversidade. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção—Volume V—Anfíbios, 1st ed.; Instituto Chico Mendes de Conservação da Biodiversidade/Ministério do Meio Ambiente: Brasília, Brazil, 2018; Volume V.
- ICMBio. Instituto Chico Mendes de Conservação da Biodiversidade. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção—Volume VI—Peixes, 1st ed.; Instituto Chico Mendes de Conservação da Biodiversidade/Ministério do Meio Ambiente: Brasília, Brazil, 2018.
- ICMBio. Instituto Chico Mendes de Conservação da Biodiversidade. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção—Volume VII—Invertebrados, 1st ed.; Instituto Chico Mendes de Conservação da Biodiversidade/Ministério do Meio Ambiente: Brasília, Brazil, 2018.
- De Souza, M.A.A.; de Souza, L.A.; Machado-Coelho, G.L.L.; de Melo, A.L. Levantamento malacológico e mapeamento das áreas de risco para transmissão da esquistossomose mansoni no Município de Mariana, Minas Gerais, Brasil. Rev. Ciências Médicas Biológicas 2006, 5, 132–139. [Google Scholar] [CrossRef]
- Miranda, G.S.; Rodrigues, J.G.M.; Lira, M.G.S.; Nogueira, R.A.; Gomes, G.C.C.; Miranda, B.S.; De Araújo, A.; Silva-Souza, N. Moluscos límnicos como hospedeiros de trematódeos digenéticos de uma região metropolitana da ilha do Maranhão, Brasil. Sci. Plena 2016, 12. [Google Scholar] [CrossRef]
- Famakinde, D. Treading the Path towards Genetic Control of Snail Resistance to Schistosome Infection. Trop. Med. Infect. Dis. 2018, 3, 86. [Google Scholar] [CrossRef]
- Basuki, K.; Toledo, R. Biomphalaria Snails and Larval Trematodes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2011; Volume 53, ISBN 978-1-4419-7027-5. [Google Scholar]
- Mc Cullough, F.S. The Role of Mollusciciding in Schistosomiasis Control; WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Dai, J.; Wang, W.; Liang, Y.; Li, H.; Guan, X.; Zhu, Y. A novel molluscicidal formulation of niclosamide. Parasitol. Res. 2008, 103, 405–412. [Google Scholar] [CrossRef]
- WHO. WHO Guideline on Control and Elimination of Human Schistosomiasis; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Field Use of Molluscicides in Schistosomiasis Control Programmes: An Operational Manual for Programme Managers; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Instituto Estadual de Florestas (IEF). Áreas Prioritárias: Estratégias para a Conservação da Biodiversidade e dos Ecossistemas de Minas Gerais; IEF: Belo Horizonte, Brazil, 2021; 162p.



| Limnic Environments (Sampled Habitats) | Presence of Limnic Gastropods (Habitats with Mollusks) | p-Value | |
|---|---|---|---|
| Lentic | 29 | 15 | 0.02338 |
| Lotic | 16 | 1 | |
| Total | 45 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, P.R.S.; Ker, F.T.O.; Araujo, A.D.; Pinto, H.A.; Negrão-Corrêa, D.A.; Caldeira, R.L.; Geiger, S.M. Survey on Limnic Gastropods: Relationships between Human Health and Conservation. Pathogens 2022, 11, 1533. https://doi.org/10.3390/pathogens11121533
Coelho PRS, Ker FTO, Araujo AD, Pinto HA, Negrão-Corrêa DA, Caldeira RL, Geiger SM. Survey on Limnic Gastropods: Relationships between Human Health and Conservation. Pathogens. 2022; 11(12):1533. https://doi.org/10.3390/pathogens11121533
Chicago/Turabian StyleCoelho, Paulo R. S., Fabricio T. O. Ker, Amanda D. Araujo, Hudson A. Pinto, Deborah A. Negrão-Corrêa, Roberta L. Caldeira, and Stefan M. Geiger. 2022. "Survey on Limnic Gastropods: Relationships between Human Health and Conservation" Pathogens 11, no. 12: 1533. https://doi.org/10.3390/pathogens11121533
APA StyleCoelho, P. R. S., Ker, F. T. O., Araujo, A. D., Pinto, H. A., Negrão-Corrêa, D. A., Caldeira, R. L., & Geiger, S. M. (2022). Survey on Limnic Gastropods: Relationships between Human Health and Conservation. Pathogens, 11(12), 1533. https://doi.org/10.3390/pathogens11121533









