Abstract
Multidrug-resistant (MDR) Gram-negative pathogens remain an unmet public health threat. In recent times, increased rates of resistance have been reported not only to commonly used antibiotics, but also to the last-resort antibiotics, such as polymyxins. More worryingly, despite the current trends in resistance, there is a lack of new antibiotics in the drug-discovery pipeline. Hence, it is imperative that new strategies are developed to preserve the clinical efficacy of the current antibiotics, particularly the last-line agents. Combining conventional antibiotics such as polymyxins with non-antibiotics (or adjuvants), has emerged as a novel and effective strategy against otherwise untreatable MDR pathogens. This review explores the available literature detailing the latest polymyxin/non-antibiotic combinations, their mechanisms of action, and potential avenues to advance their clinical application.
1. Introduction
Before the modern era of antibiotics, bacterial infections such as pneumonia, tuberculosis, and gastrointestinal infections were responsible for high fatality rates worldwide [1]. Following the introduction of penicillin, the world has witnessed the clinical approval of a plethora of antibiotic classes that have extensively decreased mortality rates, and improved human quality of life [2,3]. Regrettably, the misuse and excessive administration of antibiotics in healthcare and agriculture have led to the selection and proliferation of multi-drug resistant (MDR) strains, including MDR Gram-negative pathogens (e.g., Acinetobacter baumannii, Pseudomonas aeruginosa and K. pneumoniae) [1,3,4]. These MDR Gram-negative bacteria have been recently ranked by the World Health Organization as critical priority pathogens, owing to their prevalence behind most hospital-acquired infections (accounting for 70% since 2015) and the shortage of relevant effective antibiotics to treat them [5]. Presently in the United States, MDR infections have led to ~35,000 fatalities annually, and it is projected that by 2050, the annual mortality rate of antibiotic resistance (10 million) would supersede even that of cancer (8.2 million) [6,7]. Antibiotic resistance is achieved via genetic mutations and by horizontal gene transfer of resistance genes between bacteria, augmenting the pathogen’s viability in environmental niches previously perilous for survival [4,8]. Resistant pathogens utilize four mechanisms to counter antibiotic action: (i) Active expulsion of antibiotics via the overexpression of membrane efflux pumps, (ii) mutations in membrane porin structure or decreased porin expression to decrease antibiotic uptake, (iii) expression of antibiotic-degradative or inactivating enzymes, and (iv) structural modification of drug targets impeding antibiotic binding [9].
Exacerbating this troublesome situation is the decline of new antibiotic approvals over the past 30 years, largely because drugs used for treating chronic diseases (i.e., cancer and metabolic diseases) were deemed to have far better profitability compared to antibiotics; the latter exhibit therapeutic activity of short duration, and rapidly become obsolete when resistant pathogens become prevalent in the community over time [1,3,9]. Without effective antibiotics, invasive and immunosuppressive treatments would become prohibitive, and healthcare systems would find themselves extensively overwhelmed by the spread of MDR bacteria [1,4]. Hence, while prudent stewardship of antibiotics may mitigate the spread of antibiotic resistance, it remains paramount that we expand our armamentarium of alternative treatment options to compensate for the dwindling efficacies of various last-resort antibiotics, such as polymyxins and carbapenems, which are used to treat persistent Gram-negative infections [10]. Drug repurposing (also known as drug repositioning) has been receiving heightened attention as an effective strategy to reassess de-risked FDA-approved drugs and/or investigational drugs for new therapeutic purposes [11]. This serendipitous approach offers lower overall development costs, shorter development timelines, and extremely low safety risks, compared to the de novo discovery of new agents (e.g., antibiotics) [12,13]. For decades, combining conventional antibiotics with non-antibiotics has been successfully used to treat infections caused by problematic Gram-negative and Gram-positive pathogens, tuberculosis, malaria, and viruses [14].
In this review, we shed light on the progress of the last-resort antibiotics polymyxins and non-antibiotic combinations, in combating the MDR Gram-negative pathogens based on the available literature, highlighting these non-antibiotics as prospective adjuvants. Further perspectives on translating in vitro synergistic polymyxin/non-antibiotic combinations to the same effect in vivo, to yield favorable clinical outcomes, will be discussed as well.
2. Combination Strategies to Overcome Antibiotic Resistance
2.1. Antibiotic-Antibiotic Combination Therapy
Antibiotic–antibiotic combinations have been explored and used as a popular treatment regime, as necessitated by the severity of the blight of antibiotic resistance. The reason behind using combinations is that the general probability of developing resistance against multiple antibiotics simultaneously, is surmised to be minimal; therefore, this enables rapid eradication of resistant pathogens before resistance to either or both drugs used in combination can arise, as the resulting synergistic or additive antibacterial effect of the combined antibiotics exceeds that of either drug alone [15,16]. With different metabolic pathways and/or drug targets inhibited concurrently, it increases the spectrum of coverage and potentially reduces the dosing regimens required for each antibiotic involved [17,18]. One notable antibiotic combination that was approved by the FDA to treat MDR-tuberculosis infections was the bedaquiline, pretomanid and linezolid cocktail [19,20]. This combination exerts synergistic bactericidal activity via the simultaneous disruption of oxidative phosphorylation (bedaquiline), protein synthesis inhibition (linezolid), mycolic acid biogenesis inhibition and/or release of reactive nitric oxide (pretomanid) [21,22,23]. However, certain antibiotic combinations can have an unintended antagonistic effect; one antibiotic could indirectly induce resistance against a second antibiotic, and potentially result in super-infections due to the survival of resistant bacteria [24]. Furthermore, resistance can evolve against both antibiotics concurrently, owing to horizontal gene transfer events [25]. To illustrate, Vestergaard et. al., observed that the combination of ciprofloxacin and ceftazidime augmented resistance in Pseudomonas aeruginosa compared to either antibiotic used alone. The combination resulted in overexpression of the mexAB–oprM operon; thus, increasing the production of efflux pumps, resulting in increased efflux of drugs out of the bacterial cell, and lowering the intracellular concentration [26]. Aside from substantially increased monitoring, drug preparation and administration costs associated with antibiotic co-administration, toxic side effects can also be exacerbated following exposure to multiple antibiotics. The latter is exemplified by the combined use of aminoglycosides and cephalosporins, which augment nephrotoxicity [27,28]. Therefore, the development of antibiotic alternatives or the combined use of antibiotics with non-antibiotic drugs that reverse bacterial resistance, is of the utmost importance [15].
2.2. Drug Repurposing
Drug repurposing is a cost-efficient strategy involving the investigation of existing approved drugs and/or investigational drugs for new therapeutic purposes, such as antimicrobial therapy [11,29]. Through this strategy, failures in drug discovery are mitigated as, for example, certain drugs withdrawn because of safety concerns could be salvaged for treating alternate diseases. One notable example is the teratogen thalidomide, which was successfully repurposed as a first-line antineoplastic for multiple myeloma because its antiangiogenic activity was confirmed to be therapeutically advantageous by destroying blood vessels nourishing malignant tumors—despite being responsible for arrested fetal limb development [30]. Another drug that is successfully repurposed is cyclosporin A, an immunosuppressant for treating organ transplantation and autoimmune diseases, which displays antibiofilm activity against Mycobacterium tuberculosis, enabling its clinical application as an anti-tuberculosis therapeutic [31,32]. Moreover, as the drug candidates selected for repurposing have their pharmacokinetic/pharmacodynamic profiles established from prior discovery and preclinical stages, they have already passed most of the toxicological and pharmacokinetic safety hurdles, allowing drug developers to bypass approximately 40% of the overall cost of commercializing their candidates [33,34].
Hence, drug repurposing is an attractive option for filling the current antibiotic discovery void [35]. However, repurposing selected drug candidates as antibacterial agents does bring about its own challenges. To illustrate this point, we would like to emphasize that antibiotics are typically administered at considerably higher dosages than non-antibiotic drugs; therefore, efficacy is determined at doses exceeding the prior effective concentrations for non-infectious diseases that the drug candidate was originally employed for therapeutically, thus posing toxicity risks. In addition, plasma protein binding often results in insufficient free drug concentrations at the infection site, impairing antibacterial activity [12]. A means to circumvent these drawbacks is combination therapy, of the non-antibiotic drugs of interest with existing antimicrobials, an approach that would prove advantageous due to the dose-sparing impact of synergistic combinations and/or the reversal of bacterial resistance [36].
2.3. Antibiotic and Non-Antibiotic/Adjuvant Combination Therapy
Certain co-administered non-antibiotic drugs despite being conventionally used to treat non-infectious diseases and possessing little to no in vitro antibacterial activity, remarkably are able to re-sensitize resistant pathogens to antibiotics [37,38]. It is purported that such non-antibiotics, designated adjuvants, potentiate antibiotic activity by interfering with the modes of resistance involving intrinsic mechanisms such as enzyme inhibition, obstructing efflux pumps, increasing membrane permeability, interfering with virulence signaling pathways, and biofilm formation (Figure 1) [39,40]. For these reasons non-antibiotic compounds could be repurposed for use in antibiotic/non-antibiotic combination therapies, to compensate for the severe attrition experienced in the antibiotic pipeline [13,33].
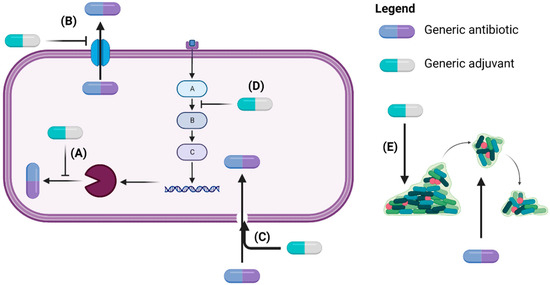
Figure 1.
Adjuvants exert anti-resistance mechanisms by inhibiting enzymes involved in antibiotic inactivation (A), blocking efflux pumps (B), disrupting membrane integrity to enable intracellular antibiotic access (C), interfering with signaling pathways leading to antibiotic resistance (D), and dispersing bacteria in biofilms to expose them to antibiotics (E) (Created with BioRender.com) [39,41].
Multiple compounds with adjuvant properties were since discovered and semi-synthetically developed, functioning as efflux pump inhibitors, membrane-permeabilizing peptides and antibiofilm agents (with brief examples outlined in Table 1). Nonetheless, the antibiotic/adjuvant combinations that have successfully entered clinical use, to date, are the β-lactam/β-lactamase inhibitor combinations and the siderophore-cephalosporin conjugate cefiderocol (Table 1). β-lactams are popularly prescribed antibiotics that exert bactericidal action by inhibiting peptidoglycan chain cross-linking [42]. Although the β-lactamase inhibitors themselves do not kill target bacteria, they augment β-lactam efficacy by competitively inhibiting β-lactamases, shielding the β-lactam from degradation [43]. Similarly, the catechol scaffold of cefiderocol is devoid of intrinsic antibacterial activity but increases cephalosporin uptake via the bacterial membrane iron transport pathways [44]. Hence, adjuvant strategies display promising potential in circumventing the constant need for new antibiotics to overcome resistance, by acting as decoys that retard the activity of resistance-conferring enzymes.

Table 1.
Examples of antibiotic/non-antibiotic combinations.
3. The Time-Honored ‘Magic Bullet’ Polymyxin Lipopeptide Antibiotics Are Rapidly Losing Their Caliber
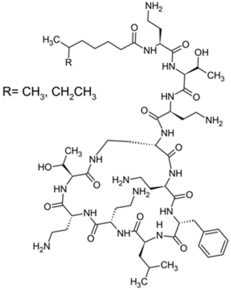
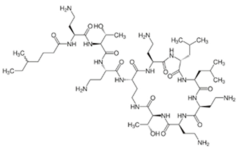
Polymyxins were discovered in the 1940s and introduced in the clinic as effective antibacterial agents against Gram-negative bacteria. However, their clinical use was reserved in the 1960s owing to their nephrotoxicity and neurotoxicity, and also better tolerated antibiotics from different classes were introduced [56]. In recent times, the use of polymyxins has experienced a resurgence as a treatment of last resort against difficult-to-treat MDR Gram-negative pathogens; however, alarming reports from clinics showed that there is a sharp upsurge in the polymyxin resistance particularly following polymyxin monotherapy [57,58,59]. This would warrant a necessity to develop an innovative strategy (e.g., combining polymyxins with nonantibiotics) to preserve the clinical effectiveness of polymyxin and halt the development of polymyxin resistance [60,61]. In terms of their chemical constitution, polymyxins are non-ribosomal cyclic lipopeptides extracted from the Gram-positive soil bacterium Paenibacillus polymyxa. Fundamentally, polymyxins are comprised of a polycationic ring with a short peptide extension to which a lipophilic chain is attached [57]. Of the five major polymyxin isoforms (A–E), only two (polymyxin B and polymyxin E aka. colistin) were clinically utilized in the 1950s [62]. Polymyxin B and colistin share nearly identical structures, only differing by an amino acid at position six in the peptide ring (Figure 2A), and as such share cross-resistance [57,62]. Polymyxins bind the lipid A component of LPS via electrostatic attractions, displacing the Ca2+ and Mg2+ ions required to stabilize the LPS leaflet. Thus, the polymyxin is anchored in the outer membrane via its lipophilic chain, causing membrane disruption. Subsequent polymyxin-mediated fusion of the inner leaflets of the outer membrane and the outer leaflet of the cytoplasmic membrane, results in osmotic lysis (Figure 2B) [62,63,64]. Other proposed secondary mechanisms leading to bacterial death include polymyxin inhibition of NADH oxidases involved in cell respiration, and the induction of reactive oxygen species (ROS) production [65]. However, owing to their membrane-disrupting activities, aside from nephrotoxicity polymyxins also cause neurotoxicity in the host, so their use is unduly restricted. Furthermore, the lack of properly established optimized regimens resulted in suboptimal drug exposure selecting for polymyxin-resistant strains [66].
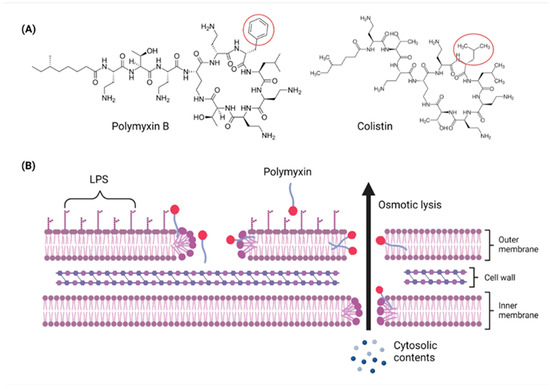
Figure 2.
Chemical structures and pharmacological mechanisms of polymyxins. (A) Structures of polymyxin B and colistin with the amino acid differences between them circled in red. (B) The bactericidal action of polymyxins against Gram-negative bacteria via targeting LPS on the outer membrane and perforating the inner membrane (Created with Biorender.com).
The Gram-negative bacterial outer membrane serves as the primary means of resistance against multiple antibiotic classes; any alteration in the outer membrane, such as increased hydrophobicity, mutations in porins, overexpression of efflux pumps, and other factors, significantly impedes antibiotic penetration [67]. The outer membrane is comprised of a phospholipid-rich inner leaflet, akin to the cytoplasmic inner membrane, and the LPS rich outer leaflet which faces the extracellular environment [68]. The LPS contains three key domains, namely the O-antigen, 2-keto-3-deoxyoctonoic acid (inner core), and lipid A, which possesses anionic phosphate moieties accentuating the outer membrane’s selective permeability [69].
The primary resistance mechanisms employed by polymyxin-resistant strains include the synthesis of modified lipid A with anionic charges neutralized, preventing LPS binding by polymyxins. Succeeding events such as the cessation of LPS production, as well as the upregulation of genes expressing phospholipids and other OM components, augment polymyxin resistance [63,66]. Furthermore, a plasmid-borne mobile colistin resistance gene (mcr-1), a gene that was first reported in China, which upon expression culminates in the modification of lipid A with phosphoethanolamine, impairing polymyxin targeting [70]. The mcr-1 plasmid is readily transferrable via conjugation across Gram-negative species, which results in the global dissemination of mcr-1, therefore, substantially threatening polymyxin efficacy and raising public health concerns globally [71,72]. Hence, various research groups and our own have investigated combinations of several FDA-approved non-antibiotic drugs and natural products with polymyxins, to evaluate possible synergy against MDR/polymyxin-resistant Gram-negative pathogens and the repurposing potential of these non-antibiotics as polymyxin adjuvants. Instances of non-antibiotic drugs tested in combination with polymyxins are listed in Table 2.

Table 2.
Summary of the polymyxin/non-antibiotic combinations investigated.
4. Polymyxin/Non-Antibiotic Combinations
4.1. Antineoplastic Drugs
Our group firstly reported the antibacterial synergetic activity of three mainstream selective estrogen receptor modulators (SERMs), tamoxifen, raloxifene, and toremifene in combination with polymyxin B [73,74]. While conventionally used for breast cancer treatment, some SERMs were reported to exhibit direct antimicrobial properties via interference with cell wall synthesis (e.g., inhibition of wall teichoic acid synthesis in E. faecium and S. aureus by clomiphene) and membrane perforation (e.g., tamoxifen) [96,97]. Based on time-kill assays, polymyxin-resistant P. aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii strains were considerably re-sensitized to polymyxin B, in the presence of each SERM (≥2.0-log10 decrease in bacterial viability relative to either monotherapy at 24 h) [74]. Metabolomics analysis of the tamoxifen-polymyxin B combination against an MDR polymyxin-resistant cystic fibrosis P. aeruginosa (FADDI-PA006) strain revealed extensive perturbations in the fatty acid and glycerophospholipid synthetic pathways involved in membrane biogenesis; thus implying the combination exerts synergy via concerted damaging effect against the outer membrane [73].
Mitotane, an antineoplastic drug used for the treatment of adrenal cancers [98], displayed synergy with polymyxin B against polymyxin-resistant MDR A. baumannii, P. aeruginosa, and K. pneumoniae in vitro. It also prevented the development of polymyxin-resistance (i.e., regrowth) in the polymyxin-susceptible isolates, compared to monotherapy from time-kill assays [75]. The in vitro synergy was further confirmed in a mouse burn wound model, in which the combination treatment decreased wound infection by a polymyxin-resistant A. baumannii isolate, compared to either polymyxin B or mitotane monotherapy. Scanning electron microscopy and transmission electron microscopy (SEM/TEM) imaging of A. baumannii isolates ATCC™ 17978 and FADDI-AB225 treated with the combination, revealed the formation of aberrant cell clusters suggesting the combination disrupts binary fission. While outer membrane damage was observed in the polymyxin-susceptible ATCC™ 17978 following polymyxin monotherapy, the polymyxin-resistant FADDI-AB225 strain displayed membrane blebbing; thus, it was inferred that polymyxin B perforates the outer membrane sufficiently for the hydrophobic mitotane to diffuse across the outer membrane and exerts antibacterial activity against intracellular targets. Untargeted metabolomics analysis revealed significant disruptions in the TCA cycle and nucleotide metabolism in the metabolome of four A. baumannii isolates treated with the combination [76].
4.2. Antipsychotic and Antidepressant Agents
Phenothiazines are heterocyclic compounds clinically utilized as antipsychotics, that function as dopamine antagonists [99,100]. Over time, the antimicrobial characteristics of the phenothiazines were serendipitously discovered, such as chlorpromazine which was observed to exert anti-mycobacterial activity and thioridazine which potentiates first-line anti-tuberculosis antibiotics enabling rapid clearance of MDR/XDR (extensively drug-resistant) tuberculosis infections [99,101,102,103]. The proposed antibacterial mechanisms exerted by phenothiazines include augmenting complete phagocytosis of bacteria by macrophages, inducing abnormalities in binary fission, and inhibiting antibiotic efflux pumps [99,100,101]. The three phenothiazines, prochlorperazine, chlorpromazine, and thiethylperazine were investigated by our group [78]. Among the three, prochlorperazine showed superior synergy with polymyxin B, as evident from the rapid and extensive bactericidal activity (4.0–6.0-log10 CFU/mL decrease in bacterial inoculum at 4 and 8 h, relative to the untreated control) observed against all tested strains in the time-kill assays. SEM/TEM imaging revealed extensive membrane blebbing, cell shriveling, perforation, and vesicle formation following exposure of the polymyxin-resistant P. aeruginosa strain FADDI-PA070 to the polymyxin B/prochlorperazine combination, reflecting augmented damage to the outer membrane. This was further verified by metabolomics, which reveal considerable perturbations in glycerophospholipid, fatty acid, and LPS biosynthesis, following exposure to the combination. Additional pathways perturbed include acetyl-CoA, arginine, and proline metabolism, suggesting the combination also impairs bacterial respiration, growth, and biofilm formation [78].
Selective serotonin reuptake inhibitors (SSRI) function by blocking presynaptic axon terminal serotonin transporters, and are largely prescribed for major depressive and anxiety disorders [104]. Interestingly, various research groups have reported antimicrobial activity from SSRIs, such as femoxetine and paroxetine, that purportedly exert direct antibacterial activity via efflux pump inhibition [105]. One SSRI in particular, sertraline, was reported by Ayaz et. al., to exert concentration-dependent reversal of resistance towards ciprofloxacin, levofloxacin, norfloxacin, gentamicin, and moxifloxacin in the clinical isolates of S. aureus, E. coli, and P. aeruginosa [104]. Sertraline was observed to readily synergize with polymyxin B at lower concentrations in time-kill assays (>3.0-log10 CFU/mL decrease in bacterial viability) against polymyxin-resistant P. aeruginosa, A. baumannii, and K. pneumoniae strains [82]. Further SEM/TEM and metabolomics studies indicated the combination synergistic killing activity primarily involves the inhibition of amino-sugar, sugar-nucleotide metabolism, glycerophospholipid, and fatty acid metabolism causing disruption of the outer membrane integrity, cell wall synthesis, and respiration [82].
4.3. Antifungal Drugs
Caspofungin is an antifungal drug that operates by inhibiting the enzyme (1→3)-β-D-glucan synthase, destabilizing fungal cell wall integrity. Caspofungin serves as a therapeutic agent against Aspergillus and Candida fungal infections [106]. Intriguingly, caspofungin was observed to exert antibacterial activity by inhibiting biofilms [80]. Further untargeted metabolomics analysis by Hussein et. al. [79] revealed the synergistic combination of polymyxin B/caspofungin against K. pneumoniae acts by inhibiting multiple interconnected metabolic pathways, including bacterial envelope biosynthesis, the phosphotransferase system (involved in biofilm formation), ATP-binding cassette (ABC) transporter production. Another antifungal, miconazole, possesses broad spectrum fungicidal activity by penetrating the chitin fungal cell wall and permeabilizing the cell membrane to external noxious substances [107]. This membrane-disrupting ability of miconazole allows it to interfere with bacterial lipid membranes and accounts for its direct antibacterial activity [108]. The combination of polymyxin B/miconazole was reported to exert synergistic bacterial killing, purportedly through permeabilization of the outer membrane by polymyxin B, which in turn allows miconazole to access the periplasmic space and disrupt the inner cytoplasmic membrane, causing bacterial lysis [81].
4.4. Antiparasitic Drugs
Closantel exerts an anti-parasitic mechanisms of action via the disruption of oxidative phosphorylation and the inhibition of chitinase activity [109]. As monotherapy, closantel had no antibacterial effect (minimal inhibitory concentration, MIC ≥ 16 mg/L); however, in combination with polymyxin B, synergy and inhibition of polymyxin resistance against A. baumannii were achieved at therapeutic concentrations [88]. In the presence of closantel, the polymyxin-resistant A. baumannii strains (polymyxin MIC ≥ 4 mg/L) were considerably re-sensitized to polymyxin B concentrations at 2 mg/L and below, which coincides with the polymyxin MIC susceptibility breakpoints (≤2 mg/L) [110]. In addition, Domalaon et. al., investigated the combination of colistin/closantel, and with other related anthelmintic salicylanilides oxyclozanide and rafoxanide. The combinations displayed synergy against MDR P. aeruginosa, A. baumannii, K. pneumoniae, E. coli, and E. cloacae, and reversed resistance to colistin apparently via potentiating damage to the outer membrane [91].
4.5. Natural Products
Cannabinoids are secondary metabolites isolated from the plant Cannabis sativa, the same plant from which the illicit drug marijuana is derived [111]. Several cannabinoids are behind the psychotropic effects of marijuana; these are synthesized via the alkylation of olivetolic acid, producing cannabigerolic acid, a precursor to most cannabinoids [112]. Of all the cannabinoids discovered, cannabidiol is the main non-psychoactive ingredient of Cannabis sativa [113]. Reports of antibacterial activity exerted by cannabidiol date back to the 1950s, with an investigation conducted by Van Klingeren and Ten Ham [114], noting MICs of cannabidiol within the ranges of 1–5 μg/mL for Gram-positive Staphylococci and Streptococci. Additional investigations carried out by Blaskovich et. al. [113] revealed consistent MICs (1–4 μg/mL) of cannabidiol against many Gram-positive MDR Staphylococci (methicillin-resistant S. aureus, vancomycin-intermediate S. aureus, vancomycin-resistant S. aureus), vancomycin-resistant Enterococci (E. faecalis, E. faecium), Clostridioides (C. difficile), and Streptococci (S. pyogenes, S. pneumoniae) strains. Radiolabeled macromolecular synthesis assays in S. aureus RN42200 and bacterial cytological profiling, indicated cannabidiol exerts antibacterial activity via membrane permeabilization, as evident from the marked perturbations in lipid synthesis at sub-MIC levels.
However, cannabidiol was inactive against Gram-negative species tested, with the exceptions of Neisseria and Legionella isolates. Further investigations involving exposure of efflux pump E. coli and P. aeruginosa mutants revealed the inactivity of cannabidiol nonetheless, ruling out efflux pumps as a possible explanation for the cannabinoid’s general ineffectiveness against Gram-negative bacteria. This was also confirmed by Abichabki et. al., who noted the inactivity of cannabidiol against K. pneumoniae, A. baumannii, P. aeruginosa, and E. cloacae even, in the presence of efflux pump inhibitors such as curcumin [84]. However, cannabidiol was active against an E. coli lpxC cell membrane mutant, indicating that outer membrane LPS deficiency enables penetration of cannabidiol. This conclusion was further supported by the increased susceptibility of lipid A-deficient A. baumannii (ATCC™ 19606R) to cannabidiol (MIC >128 to 0.25 μg/mL), which in turn has an polymyxin MIC (>128 μg/mL) relative to its parent strain (0.25 μg/mL) [115]. Our group investigated polymyxin B and cannabidiol as another possible antibiotic/adjuvant combination for treating polymyxin-resistant Gram-negative infections [83]. Broth microdilution tests involving polymyxin B combined with cannabidiol (fixed at 256 μg/mL) against 13 different Gram-negative pathogens (52 strains in total), yielded observable antibacterial activity of the combination against 47 of the 52 strains. Subsequent checkerboard assays combining polymyxin B/cannabidiol verified the synergistic activity, as evident from the low polymyxin B concentrations (≤2 µg/mL, within the EUCAST “susceptible” breakpoint) enabling cannabidiol to exert antibacterial activity at a minimal effective antibiotic concentration (MEAC) ≤4 µg/mL. Moreover, the polymyxin B-cannabidiol combination substantially decreased the viability of four K. pneumoniae strains (>2.0-log10 CFU/mL decline) relative to polymyxin B monotherapy at several time points, verifying the combination’s synergistic bactericidal activity. Similarly, checkerboard and time-kill assays confirmed synergy against A. baumannii, P. aeruginosa, and K. pneumoniae, both polymyxin-resistant and polymyxin-susceptible isolates alike. Moreover, it was inferred from metabolomics analysis of A. baumannii ATCC™ 19606 that the polymyxin B/cannabidiol combination substantially disrupts cell envelope biogenesis through a time-dependent perturbation of amino–sugar and nucleotide–sugar metabolism, and the pentose phosphate pathway. Consequently, peptidoglycan and LPS synthesis are disrupted owing to decreased levels of core membrane lipid components, phosphatidylethanolamine, phosphatidic acid, sn-glycero-3-phosphoethanolanime, and sn-glycerol 3-phosphate.
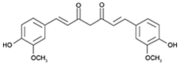
Curcumin, a phenolic compound isolated from the plant Curcuma longa, has been traditionally used for gastrointestinal treatments owing to its antioxidant and anti-inflammatory activities [116]. Intriguingly, this natural product was also reported to exhibit antimicrobial activity; various reported antibacterial mechanisms exerted by curcumin include membrane damage, efflux inhibition, biofilm disruption, and growth inhibition [117,118]. When combined with polymyxin B, considerable synergy was observed against polymyxin-resistant strains (as well as expanding the polymyxin activity spectrum coverage to Gram-positive bacteria) at reduced polymyxin MICs; the mechanisms purported to account for synergy involve outer membrane permeabilization by curcumin, allowing access to the inner cytoplasmic membrane by polymyxin B [85]. Furthermore, colistin in combination with curcumin was observed to reverse polymyxin-resistance in A. baumannii through outer membrane permeabilization by colistin, facilitating the entry of curcumin which triggers increased ROS production, severely impacting bacterial viability [95].
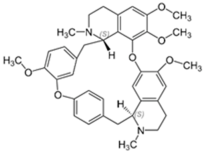
Tetrandrine is a bis-benzylisoquinoline alkaloid isolated from the herb Stephania tetrandra [119]. Although tetrandrine functions pharmacologically as a calcium channel blocker, it was also found to possess anti-inflammatory and antineoplastic properties through the inhibition of pro-inflammatory cytokine production and the induction of apoptosis in tumor cells, respectively [120]. Interestingly, tetrandrine exhibits antibacterial properties as observed from its ability to inhibit antibiotic efflux from MRSA and Mycobacterium tuberculosis [121,122]. Hence, when tested in combination with colistin against mcr-1-positive colistin-resistant Salmonella, tetrandrine was noted to enhance colistin activity by enhancing the permeability of the outer membrane through efflux inhibition sufficiently for colistin to act, as well as by disrupting oxidative phosphorylation (ATP production) and down-regulating mcr-1 expression [93].
4.6. Other Non-Antibiotic Drugs
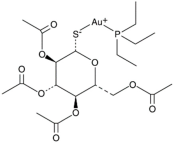
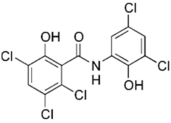
The cystic fibrosis transmembrane conductance regulator (CFTR) potentiator ivacaftor, commonly used to treat cystic fibrosis patients, exerts apparent antimicrobial activity via weak inhibition of DNA gyrase and topoisomerase IV enzymes, owing to certain structural similarities with quinolones. When combined with polymyxin B against P. aeruginosa, augmented damage against the outer membrane was observed via nitrocefin assay, SEM and TEM [87]. This was further substantiated by metabolomics analysis which revealed extensive phospholipid and LPS biosynthesis perturbations, following exposure to the polymyxin/ivacaftor combination [86]. Auranofin, an anti-rheumatic drug for treating arthritis, displays negligible anti-Gram-negative activity [123]; however, in combining with colistin, it results in extensive cell shrinkage and lysis as observed from SEM/TEM imaging, validating auranofin as a prospective adjuvant. In addition, the colistin/auranofin combination extensively counters colistin resistance in Gram-negative pathogens and substantially improves the survival rate in mouse peritoneal infection models [89].
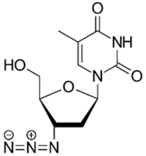
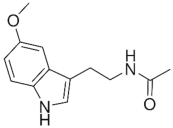
Zidovudine is a nucleoside/nucleotide reverse transcriptase inhibitor that acts by incorporating into the elongating viral DNA strand, and slowing down the progression of HIV infection [124]. Intriguingly, antibacterial activity was also observed from zidovudine, likewise by acting as a DNA-chain terminator and arresting bacterial DNA replication [125]. When combined with polymyxin B, zidovudine extensively potentiated polymyxin activity against K. pneumoniae, ≥4.0-log10 CFU/mL in time-kill assay; ≥3.0-log10 CFU/mL in murine thigh infection model [77]. Similarly, the colistin/zidovudine combination was observed to reverse colistin resistance in time-kill assay (>3.0-log10 CFU/mL) with the absence of regrowth in the combination 24 h post-exposure, and displayed in vivo synergy against NDM-1 K. pneumoniae and mcr-1-positive E. coli in murine peritoneal infection models [90,126].
Melatonin, a hormone secreted by the pineal gland in the brain, functions to regulate circadian rhythms and blood pressure, thus is used medically to treat sleep disorders. Astonishingly, melatonin was reported to exert bacteriostatic (growth-inhibiting) activity, via the perturbation of key metabolic pathways and the expression of genes involved in cell division. Furthermore, the combination of colistin with melatonin substantially permeabilizes the outer membrane and inhibits efflux pump activity, hence re-sensitizing polymyxin-resistant Gram-negative pathogens to polymyxins [127]. Additional transcriptomics analysis involving mcr-1-positive E. coli reveals significant disruptions in the expression of genes involved in LPS modifications and efflux pump production, in the presence of the colistin–melatonin combination [94].
5. Perspectives and Future Directions
This review highlights the promising clinical approach of combining non-antibiotic FDA-approved drugs or natural products with polymyxins, for treating MDR Gram-negative infections. Considering the majority of the FDA-approved non-antibiotic drugs are already well-characterized pharmacokinetically and toxicologically, it would enable them to forgo clinical trials as information from prior safety studies can help guide design of future studies focused on evaluating their applicability as antibacterial adjuvants [12]. Consequently, our ability to translate these adjuvants in combination with polymyxins to the bedside, could potentially be accelerated due to reduced developmental costs by the leapfrogging of extensive prior preclinical development insights [34,128]. Moreover, as these non-antibiotic drugs do not exert direct inhibitory activity against specific bacterial molecular targets, evolutionary pressure on bacteria to evolve resistance would likely be eschewed [38,129]. Hence, non-antibiotic drugs represent a valuable novel reservoir of anti-infective adjuvants for combination therapy.
In general, the polymyxin/non-antibiotic combinations covered in this review function synergistically by augmenting penetrative damage against the outer membrane causing bacterial lysis. Alternatively, the outer membrane may be permeabilized sufficiently (either by the polymyxin or the adjuvant) for the combination to access the inner membrane, leading to either perforation of the inner membrane (and lysis) or diffusion across the membrane, substantially disrupting vital metabolic pathways (i.e., respiration, DNA replication, cell envelope maintenance) and/or repressing plasmid-mediated polymyxin resistance (Figure 3). In consequence, effective polymyxin concentrations for bacterial killing are decreased, reversing polymyxin resistance, and potentially reducing polymyxin dosing regimens required for the maximum clearance of pathogens. This would improve the polymyxin therapeutic index, since nephrotoxicity and neurotoxicity side effects are likely to be reduced [130].
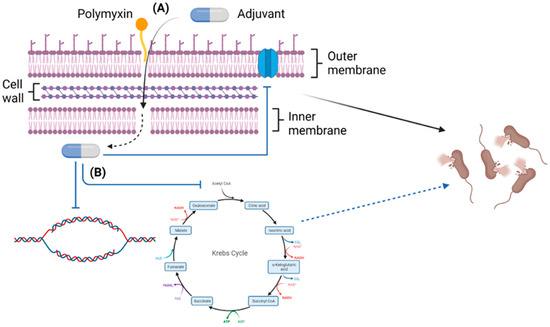
Figure 3.
An overview of the general synergistic mechanisms of polymyxin/non-antibiotic combinations (Created with BioRender.com). (A) The combinations function via augmented membrane perforation, and (B) disruption of vital metabolic pathways, DNA replication, and gene expression contributing to resistance.
In closing, this contemporary review provides detailed information about a potentially central hub for several novel combinations, which could be utilized as promising antibacterial therapies to bridge the antibiotic development gap. In the last decade, this innovative therapeutic approach has emerged as an area of great interest in the anti-infectives field and should be pursued to fruition vigorously.
Author Contributions
Conceptualization, A.K.J.J. and M.H.; A.K.J.J.; investigation, A.K.J.J.; resources, A.K.J.J.; data curation, A.K.J.J.; writing—original draft preparation, M.H.; G.G.R.; J.L.; writing—review and editing, T.V.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
G.G.R., T.V., and J.L. are supported by the National Institute of Allergy and Infectious Diseases, award number R01AI146241. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
J.L. is an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellow, and T.V. is an Australian NHMRC Industry Career Development Level 2 Research Fellow.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Pandemics throughout history. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist 2018, 11, 1645–1658. [Google Scholar]
- Venter, H. Reversing resistance to counter antimicrobial resistance in the World Health Organisation’s critical priority of most dangerous pathogens. Biosci. Rep. 2019, 39, BSR20180474. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Morris, S.; Cerceo, E. Trends, epidemiology, and management of multi-drug resistant gram-negative bacterial infections in the hospitalized setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular engineering of antimicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021, 13, 35–69. [Google Scholar] [CrossRef]
- Matlock, A.; Garcia, J.A.; Moussavi, K.; Long, B.; Liang, S.Y. Advances in novel antibiotics to treat multidrug-resistant gram-negative bacterial infections. Intern. Emerg. Med. 2021, 16, 2231–2241. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.K.; Reyes-Ortega, F.; Velkov, T.; Li, J. Antibiotic–non-antibiotic combinations for combating extremely drug-resistant Gram-negative ‘superbugs’. Essays Biochem. 2017, 61, 115–125. [Google Scholar] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Ahmed, A.; Azim, A.; Gurjar, M.; Baronia, A.K. Current concepts in combination antibiotic therapy for critically ill patients. Indian J. Crit. Care Med. 2014, 18, 310–314. [Google Scholar]
- Wang, N.; Luo, J.; Deng, F.; Huang, Y.; Zhou, H. Antibiotic Combination Therapy: A Strategy to Overcome Bacterial Resistance to Aminoglycoside Antibiotics. Front. Pharmacol. 2022, 13, 839808. [Google Scholar] [CrossRef]
- Grande, V.; Kumar, A. Optimizing antimicrobial therapy of sepsis and septic shock: Focus on Antibiotic Combination Therapy. Semin. Respir. Crit. Care Med. 2015, 36, 154–166. [Google Scholar] [CrossRef]
- Nimmo, C.; Naidoo, K.; O’Donnell, M. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 2376. [Google Scholar]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef]
- Manjunatha, U.; Boshoff, H.I.; Barry, C.E. The mechanism of action of PA-824: Novel insights from transcriptional profiling. Commun. Integr. Biol. 2009, 2, 215–218. [Google Scholar] [CrossRef]
- Zahedi Bialvaei, A.; Rahbar, M.; Yousefi, M.; Asgharzadeh, M.; Samadi Kafil, H. Linezolid: A promising option in the treatment of Gram-positives. J. Antimicrob. Chemother. 2017, 72, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, J.P.; Gruber, G.; Dick, T. Re-understanding the mechanisms of action of the anti-mycobacterial drug bedaquiline. Antibiotics 2019, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; McGrath, B.J. Combination antimicrobial therapy for bacterial infections. Drugs 1996, 52, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskaya, V.; Baasov, T. Dual-acting hybrid antibiotics: A promising strategy to combat bacterial resistance. Expert Opin. Drug Discov. 2010, 5, 883–902. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Paulander, W.; Marvig, R.L.; Clasen, J.; Jochumsen, N.; Molin, S.; Jelsbak, L.; Ingmer, H.; Folkesson, A. Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2016, 47, 48–55. [Google Scholar] [CrossRef]
- Mannion, J.C.; Bloch, R.; Popovich, N.G. Cephalosporin-aminoglycoside synergistic nephrotoxicity: Fact or fiction? Drug Intell. Clin. Pharm. 1981, 15, 248–256. [Google Scholar] [CrossRef]
- Thiyagarajan, D.; Das, G.; Ramesh, A. Amphiphilic Cargo-Loaded Nanocarrier Enhances Antibiotic Uptake and Perturbs Efflux: Effective Synergy for Mitigation of Methicillin-Resistant Staphylococcus aureus. ChemMedChem 2017, 12, 1125–1132. [Google Scholar] [CrossRef]
- Strittmatter, S.M. Overcoming drug development bottlenecks with repurposing: Old drugs learn new tricks. Nat. Med. 2014, 20, 590–591. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Thalidomide in multiple myeloma. Oncology 2000, 14 (Suppl. S13), 11–16. [Google Scholar]
- Kumar, A.; Alam, A.; Grover, S.; Pandey, S.; Tripathi, D.; Kumari, M.; Rani, M.; Singh, A.; Akhter, Y.; Ehtesham, N.Z.; et al. Peptidyl-prolyl isomerase-B is involved in Mycobacterium tuberculosis biofilm formation and a generic target for drug repurposing-based intervention. npj Biofilms Microbiomes 2019, 5, 3. [Google Scholar] [CrossRef]
- An, Q.; Li, C.; Chen, Y.; Deng, Y.; Yang, T.; Luo, Y. Repurposed drug candidates for antituberculosis therapy. Eur. J. Med. Chem. 2020, 192, 112175. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Sadeghi, H.; Adeli, I.; Mousavi, T.; Daniali, M.; Nikfar, S.; Abdollahi, M. Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Life 2021, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef] [PubMed]
- Konreddy, A.K.; Rani, G.U.; Lee, K.; Choi, Y. Recent drug-repurposing-driven advances in the discovery of novel antibiotics. Curr. Med. Chem. 2019, 26, 5363–5388. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Bernal, P.; Molina-Santiago, C.; Daddaoua, A.; Llamas, M.A. Antibiotic adjuvants: Identification and clinical use. Microb. Biotechnol. 2013, 6, 445. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Melander, C. The challenge of overcoming antibiotic resistance: An adjuvant approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef]
- Gill, E.E.; Franco, O.L.; Hancock, R.E. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem. Biol. Drug Des. 2015, 85, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, I.; Favini-Stabile, S.; Dessen, A. Resistance to antibiotics targeted to the bacterial cell wall. Protein Sci. 2014, 23, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.J.; Melander, C. Overcoming resistance to β-lactam antibiotics. J. Org. Chem. 2013, 78, 4207–4213. [Google Scholar] [CrossRef]
- Masters, C.; Selkoe, D. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006262. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs 2019, 79, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.-A. Imipenem/cilastatin/relebactam: A review in Gram-negative bacterial infections. Drugs 2021, 81, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Drawz, S.M.; Bonomo, R.A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Li, X.; He, S.; Yu, M.; Wu, M.; Vederas, J.C. Synthesis of Tridecaptin–Antibiotic Conjugates with in Vivo Activity against Gram-Negative Bacteria. J. Med. Chem. 2015, 58, 9779–9785. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Pagès, J.M.; Lee, V.J. Inhibitors of bacterial efflux pumps as adjuvants in antibiotic treatments and diagnostic tools for detection of resistance by efflux. Recent Pat. Antiinfect. Drug Discov. 2006, 1, 157–175. [Google Scholar] [CrossRef]
- Berry, L.; Domalaon, R.; Brizuela, M.; Zhanel, G.G.; Schweizer, F. Polybasic peptide-levofloxacin conjugates potentiate fluoroquinolones and other classes of antibiotics against multidrug-resistant Gram-negative bacteria. Medchemcomm 2019, 10, 517–527. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T.; et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 2001, 45, 105–116. [Google Scholar] [CrossRef]
- Bruhn, D.F.; Scherman, M.S.; Liu, J.; Scherbakov, D.; Meibohm, B.; Böttger, E.C.; Lenaerts, A.J.; Lee, R.E. In vitro and in vivo Evaluation of Synergism between Anti-Tubercular Spectinamides and Non-Classical Tuberculosis Antibiotics. Sci. Rep. 2015, 5, 13985. [Google Scholar] [CrossRef] [PubMed]
- Aboelenin, A.M.; Hassan, R.; Abdelmegeed, E.S. The effect of EDTA in combination with some antibiotics against clinical isolates of gram negative bacteria in Mansoura, Egypt. Microb. Pathog. 2021, 154, 104840. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Dhouib, R.; Fairfull-Smith, K.E.; Totsika, M. Nitroxide Functionalized Antibiotics Are Promising Eradication Agents against Staphylococcus aureus Biofilms. Antimicrob. Agents Chemother. 2019, 64, e01685-19. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Hawas, S.; Harris, J.; Totsika, M.; Fairfull-Smith, K.E. Isothiazolone-Nitroxide Hybrids with Activity against Antibiotic-Resistant Staphylococcus aureus Biofilms. ACS Omega 2022, 7, 5300–5310. [Google Scholar] [CrossRef] [PubMed]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure−Activity Relationships of Polymyxin Antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Wang, J.; Thompson, P.E.; Li, J. Teaching ‘Old’ Polymyxins New Tricks: New-Generation Lipopeptides Targeting Gram-Negative ‘Superbugs’. ACS Chem. Biol. 2014, 9, 1172–1177. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Bergen, P.J.; Bulman, Z.P.; Saju, S.; Bulitta, J.B.; Landersdorfer, C.; Forrest, A.; Li, J.; Nation, R.L.; Tsuji, B.T. Polymyxin combinations: Pharmacokinetics and pharmacodynamics for rationale use. Pharmacotherapy 2015, 35, 34–42. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Nation, R.L.; Tsuji, B.T. Synergistic combinations of polymyxins. Int. J. Antimicrob. Agents 2016, 48, 607–613. [Google Scholar]
- Ayoub Moubareck, C. Polymyxins and Bacterial Membranes: A Review of Antibacterial Activity and Mechanisms of Resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Bates, N.C.; Hancock, R.E. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 1986, 29, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Azad, M.A.K.; Velkov, T.; Zhou, Q.; Li, J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol. Rev. 2021, 73, 679–728. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Clifton, L.A.; Skoda, M.W.; Daulton, E.L.; Hughes, A.V.; Le Brun, A.P.; Lakey, J.H.; Holt, S.A. Asymmetric phospholipid: Lipopolysaccharide bilayers; a Gram-negative bacterial outer membrane mimic. J. R. Soc. Interface 2013, 10, 20130810. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an ‘old’ class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Hassan, J.; Eddine, R.Z.; Mann, D.; Li, S.; Deng, X.; Saoud, I.P.; Kassem, I.I. The Mobile Colistin Resistance Gene, mcr-1.1, Is Carried on IncX4 Plasmids in Multidrug Resistant E. coli Isolated from Rainbow Trout Aquaculture. Microorganisms 2020, 8, 1636. [Google Scholar] [CrossRef]
- Gao, R.; Hu, Y.; Li, Z.; Sun, J.; Wang, Q.; Lin, J.; Ye, H.; Liu, F.; Srinivas, S.; Li, D. Dissemination and mechanism for the MCR-1 colistin resistance. PLoS Pathog. 2016, 12, e1005957. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Han, M.-L.; Zhu, Y.; Schneider-Futschik, E.K.; Hu, X.; Zhou, Q.T.; Lin, Y.-W.; Anderson, D.; Creek, D.J.; Hoyer, D. Mechanistic insights from global metabolomics studies into synergistic bactericidal effect of a polymyxin B combination with tamoxifen against cystic fibrosis MDR Pseudomonas aeruginosa. Comput. Struct. Biotechnol. J. 2018, 16, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.H.; Schneider, E.K.; Elliott, A.G.; Han, M.; Reyes-Ortega, F.; Morris, F.; Blaskovich, M.A.; Jasim, R.; Currie, B.; Mayo, M. From breast cancer to antimicrobial: Combating extremely resistant Gram-negative “superbugs” using novel combinations of polymyxin B with selective estrogen receptor modulators. Microb. Drug Resist. 2017, 23, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Wang, J.; Doi, Y.; Velkov, T.; Bergen, P.J.; Li, J. Novel polymyxin combination with antineoplastic mitotane improved the bacterial killing against polymyxin-resistant multidrug-resistant gram-negative pathogens. Front. Microbiol. 2018, 9, 721. [Google Scholar] [CrossRef]
- Tran, T.B.; Bergen, P.J.; Creek, D.J.; Velkov, T.; Li, J. Synergistic killing of polymyxin B in combination with the antineoplastic drug mitotane against polymyxin-susceptible and-resistant Acinetobacter baumannii: A metabolomic study. Front. Pharmacol. 2018, 9, 359. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Abdul Rahim, N.; Zhao, J.; Han, M.-L.; Yu, H.H.; Wickremasinghe, H.; Chen, K.; Wang, J.; Paterson, D.L.; Zhu, Y. Novel polymyxin combination with the antiretroviral zidovudine exerts synergistic killing against NDM-producing multidrug-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2019, 63, e02176-18. [Google Scholar] [CrossRef]
- Hussein, M.; Hu, X.; Paulin, O.K.; Crawford, S.; Zhou, Q.T.; Baker, M.; Schneider-Futschik, E.K.; Zhu, Y.; Li, J.; Velkov, T. Polymyxin B combinations with FDA-approved non-antibiotic phenothiazine drugs targeting multi-drug resistance of Gram-negative pathogens. Comput. Struct. Biotechnol. J. 2020, 18, 2247–2258. [Google Scholar] [CrossRef]
- Hussein, M.; Wong, L.J.; Zhao, J.; Rees, V.E.; Allobawi, R.; Sharma, R.; Rao, G.G.; Baker, M.; Li, J.; Velkov, T. Unique mechanistic insights into pathways associated with the synergistic activity of polymyxin B and caspofungin against multidrug-resistant Klebsiella pneumoniae. Comput. Struct. Biotechnol. J. 2022, 20, 1077–1087. [Google Scholar] [CrossRef]
- Fernandes, L.; Fortes, B.N.; Lincopan, N.; Ishida, K. Caspofungin and polymyxin B reduce the cell viability and total biomass of mixed biofilms of carbapenem-resistant Pseudomonas aeruginosa and Candida spp. Front. Microbiol. 2020, 11, 573263. [Google Scholar] [CrossRef]
- Pietschmann, S.; Hoffmann, K.; Voget, M.; Pison, U. Synergistic effects of miconazole and polymyxin B on microbial pathogens. Vet. Res. Commun. 2009, 33, 489–505. [Google Scholar] [CrossRef]
- Hussein, M.; Schneider-Futschik, E.K.; Paulin, O.K.; Allobawi, R.; Crawford, S.; Zhou, Q.T.; Hanif, A.; Baker, M.; Zhu, Y.; Li, J. Effective strategy targeting polymyxin-resistant gram-negative pathogens: Polymyxin B in combination with the selective serotonin reuptake inhibitor sertraline. ACS Infect. Dis. 2020, 6, 1436–1450. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Allobawi, R.; Levou, I.; Blaskovich, M.A.; Rao, G.G.; Li, J.; Velkov, T. Mechanisms Underlying Synergistic Killing of Polymyxin B in Combination with Cannabidiol against Acinetobacter baumannii: A Metabolomic Study. Pharmaceutics 2022, 14, 786. [Google Scholar] [CrossRef] [PubMed]
- Abichabki, N.; Zacharias, L.V.; Moreira, N.C.; Bellissimo-Rodrigues, F.; Moreira, F.L.; Benzi, J.R.; Ogasawara, T.; Ferreira, J.C.; Ribeiro, C.M.; Pavan, F.R. Potential cannabidiol (CBD) repurposing as antibacterial and promising therapy of CBD plus polymyxin B (PB) against PB-resistant gram-negative bacilli. Sci. Rep. 2022, 12, 6454. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.W.; Sharili, A.S.; La Ragione, R.M.; Wareham, D.W. In Vitro Antibacterial Activity of Curcumin–Polymyxin B Combinations against Multidrug-Resistant Bacteria Associated with Traumatic Wound Infections. J. Nat. Prod. 2016, 79, 1702–1706. [Google Scholar] [CrossRef]
- Allobawi, R.; Ghelani, D.P.; Schneider-Futschik, E.K. Metabolomic description of ivacaftor elevating polymyxin B mediated antibacterial activity in cystic fibrosis Pseudomonas aeruginosa. ACS Pharmacol. Transl. Sci. 2020, 3, 433–443. [Google Scholar] [CrossRef]
- Schneider, E.K.; Azad, M.A.; Han, M.-L.; Zhou, Q.; Wang, J.; Huang, J.X.; Cooper, M.A.; Doi, Y.; Baker, M.A.; Bergen, P.J. An “unlikely” pair: The antimicrobial synergy of polymyxin B in combination with the cystic fibrosis transmembrane conductance regulator drugs KALYDECO and ORKAMBI. ACS Infect. Dis. 2016, 2, 478–488. [Google Scholar] [CrossRef]
- Tran, T.B.; Cheah, S.E.; Yu, H.H.; Bergen, P.J.; Nation, R.L.; Creek, D.J.; Purcell, A.; Forrest, A.; Song, J.; Velkov, T.; et al. Anthelmintic closantel enhances bacterial killing of polymyxin B against multidrug-resistant Acinetobacter baumannii. J. Antibiot. 2016, 69, 415–421. [Google Scholar] [CrossRef]
- Feng, X.; Liu, S.; Wang, Y.; Zhang, Y.; Sun, L.; Li, H.; Wang, C.; Liu, Y.; Cao, B. Synergistic Activity of Colistin Combined With Auranofin Against Colistin-Resistant Gram-Negative Bacteria. Front. Microbiol. 2021, 12, 676414. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Coates, A. Azidothymidine produces synergistic activity in combination with colistin against antibiotic-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e01630-18. [Google Scholar] [CrossRef]
- Domalaon, R.; Okunnu, O.; Zhanel, G.G.; Schweizer, F. Synergistic combinations of anthelmintic salicylanilides oxyclozanide, rafoxanide, and closantel with colistin eradicates multidrug-resistant colistin-resistant Gram-negative bacilli. J. Antibiot. 2019, 72, 605–616. [Google Scholar] [CrossRef]
- Ayerbe-Algaba, R.; Gil-Marqués, M.L.; Miró-Canturri, A.; Parra-Millán, R.; Pachón-Ibáñez, M.E.; Jiménez-Mejías, M.E.; Pachón, J.; Smani, Y. The anthelmintic oxyclozanide restores the activity of colistin against colistin-resistant Gram-negative bacilli. Int. J. Antimicrob. Agents 2019, 54, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Liu, S.; Liu, P.; Luo, X.; Zhao, J.; Yan, F.; Pan, Y.; Liu, J.; Zhai, Y.; Hu, G. Synergistic antibacterial activity of tetrandrine combined with colistin against MCR-mediated colistin-resistant Salmonella. Biomed. Pharmacother. 2022, 149, 112873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Y.; Yang, K.; Tong, Z.; Shi, J.; Li, R.; Xiao, X.; Ren, W.; Hardeland, R.; Reiter, R.J. Melatonin overcomes MCR-mediated colistin resistance in Gram-negative pathogens. Theranostics 2020, 10, 10697. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, P.; Capalash, N. Curcumin alleviates persistence of Acinetobacter baumannii against colistin. Sci. Rep. 2018, 8, 11029. [Google Scholar] [CrossRef] [PubMed]
- Miró-Canturri, A.; Ayerbe-Algaba, R.; Vila-Domínguez, A.; Jiménez-Mejías, M.E.; Pachón, J.; Smani, Y. Repurposing of the tamoxifen metabolites to combat infections by multidrug-resistant Gram-negative bacilli. Antibiotics 2021, 10, 336. [Google Scholar] [CrossRef]
- Al-Janabi, A.A.H.S. Repurposing of Tamoxifen Against the Oral Bacteria. Turk. J. Pharm. Sci. 2021, 18, 68. [Google Scholar] [CrossRef]
- Lo Iacono, M.; Puglisi, S.; Perotti, P.; Saba, L.; Petiti, J.; Giachino, C.; Reimondo, G.; Terzolo, M. Molecular mechanisms of mitotane action in adrenocortical cancer based on in vitro studies. Cancers 2021, 13, 5255. [Google Scholar] [CrossRef]
- Grimsey, E.M.; Piddock, L.J. Do phenothiazines possess antimicrobial and efflux inhibitory properties? FEMS Microbiol. Rev. 2019, 43, 577–590. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Kristiansen, J.E.; Molnar, J.; Amaral, L. Role of phenothiazines and structurally similar compounds of plant origin in the fight against infections by drug resistant bacteria. Antibiotics 2013, 2, 58–72. [Google Scholar] [CrossRef]
- Amaral, L.; Viveiros, M.; Molnar, J. Antimicrobial activity of phenothiazines. In Vivo 2004, 18, 725–732. [Google Scholar]
- Amaral, L.; Viveiros, M.; Kristiansen, J.E. Phenothiazines: Potential alternatives for the management of antibiotic resistant infections of tuberculosis and malaria in developing countries. Trop. Med. Int. Health 2001, 6, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Viveiros, M. Thioridazine: A non-antibiotic drug highly effective, in combination with first line anti-tuberculosis drugs, against any form of antibiotic resistance of Mycobacterium tuberculosis due to its multi-mechanisms of action. Antibiotics 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Subhan, F.; Ahmed, J.; Khan, A.-U.; Ullah, F.; Ullah, I.; Ali, G.; Syed, N.-I.-H.; Hussain, S. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J. Biol. Res. Thessalon. 2015, 22, 4. [Google Scholar] [CrossRef]
- Hellio, C.; Pons, A.M.; Beaupoil, C.; Bourgougnon, N.; Le Gal, Y. Antibacterial, antifungal and cytotoxic activities of extracts from fish epidermis and epidermal mucus. Int. J. Antimicrob. Agents 2002, 20, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Ueda, T.; Uchida, M.; Nakamura, T. Action mechanism of idarubicin (4-demethoxydaunorubicin) as compared with daunorubicin in leukemic cells. Int. J. Hematol. 1993, 57, 121–130. [Google Scholar] [PubMed]
- Sreedhara Swamy, K. Studies on the mechanism of action of miconazole: Effect of miconazole on respiration and cell permeability of Candida albicans. Antimicrob. Agents Chemother. 1974, 5, 420–425. [Google Scholar] [CrossRef][Green Version]
- Vanden Bossche, H.; Marichal, P.; Willemsens, G.; Bellens, D.; Gorrens, J.; Roels, I.; Coene, M.-C.; Le Jeune, L.; Janssen, P. Saperconazole: A selective inhibitor of the cytochrome P-450-dependent ergosterol synthesis in Candida albicans, Aspergillus fumigatus and Trichophyton mentagrophytes. Mycoses 1990, 33, 335–352. [Google Scholar] [CrossRef]
- Tabatabaei, S.A.; Soleimani, M.; Mansouri, M.R.; Mirshahi, A.; Inanlou, B.; Abrishami, M.; Pakrah, A.R.; Masarat, H. Closantel; a veterinary drug with potential severe morbidity in humans. BMC Ophthalmol. 2016, 16, 207. [Google Scholar] [CrossRef]
- Li, J.; Rayner, C.R.; Nation, R.L.; Owen, R.J.; Spelman, D.; Tan, K.E.; Liolios, L. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2006, 50, 2946–2950. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Karas, J.A.; Wong, L.J.; Paulin, O.K.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The antimicrobial activity of cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Van Klingeren, B.; Ten Ham, M. Antibacterial activity of Δ9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.F.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; Michael, F.S.; Cox, A.D.; et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, Y.; Duan, W.; Wang, D.; Fu, M.; Wang, X. Ionic liquid-based ultrasound-assisted extraction of fangchinoline and tetrandrine from Stephaniae tetrandrae. J. Sep. Sci. 2009, 32, 3550–3554. [Google Scholar] [CrossRef]
- Li, D.-G.; Wang, Z.-R.; Lu, H.-M. Pharmacology of tetrandrine and its therapeutic use in digestive diseases. World J. Gastroenterol. 2001, 7, 627. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Han, S.-H.; Lee, S.-H.; Kim, Y.-G.; Park, C.-B.; Kang, O.-H.; Keum, J.-H.; Kim, S.-B.; Mun, S.-H.; Seo, Y.-S. The mechanism of antibacterial activity of tetrandrine against Staphylococcus aureus. Foodborne Pathog. Dis. 2012, 9, 686–691. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Xu, K.; Ji, Z.; Li, L. Tetrandrine reverses drug resistance in isoniazid and ethambutol dual drug-resistant Mycobacterium tuberculosis clinical isolates. BMC Infect. Dis. 2015, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R., Jr.; Schultz, P.G. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; St Clair, M.H.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3′-Azido-3′-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef] [PubMed]
- Doléans-Jordheim, A.; Bergeron, E.; Bereyziat, F.; Ben-Larbi, S.; Dumitrescu, O.; Mazoyer, M.-A.; Morfin, F.; Dumontet, C.; Freney, J.; Jordheim, L. Zidovudine (AZT) has a bactericidal effect on enterobacteria and induces genetic modifications in resistant strains. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Coates, A. Combination Comprising Zidovudine and Polymyxin. U.S. Patent No. 9,757,427, 12 September 2017. [Google Scholar]
- He, F.; Wu, X.; Zhang, Q.; Li, Y.; Ye, Y.; Li, P.; Chen, S.; Peng, Y.; Hardeland, R.; Xia, Y. Bacteriostatic potential of melatonin: Therapeutic standing and mechanistic insights. Front. Immunol. 2021, 12, 683879. [Google Scholar] [CrossRef]
- Chong, C.R.; Sullivan, D.J., Jr. New uses for old drugs. Nature 2007, 448, 645–646. [Google Scholar] [CrossRef]
- Harris, T.L.; Worthington, R.J.; Hittle, L.E.; Zurawski, D.V.; Ernst, R.K.; Melander, C. Small molecule downregulation of PmrAB reverses lipid A modification and breaks colistin resistance. ACS Chem. Biol. 2014, 9, 122–127. [Google Scholar] [CrossRef]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 2009, 48, 1724–1728. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).